Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles
Abstract
:1. Introduction
2. Classes of Nitrogen-Containing Macrocycles—Synthesis
3. Nitrogen-Containing Macrocycles—Applications
3.1. Selective Ion Detection
3.2. Gelation Properties
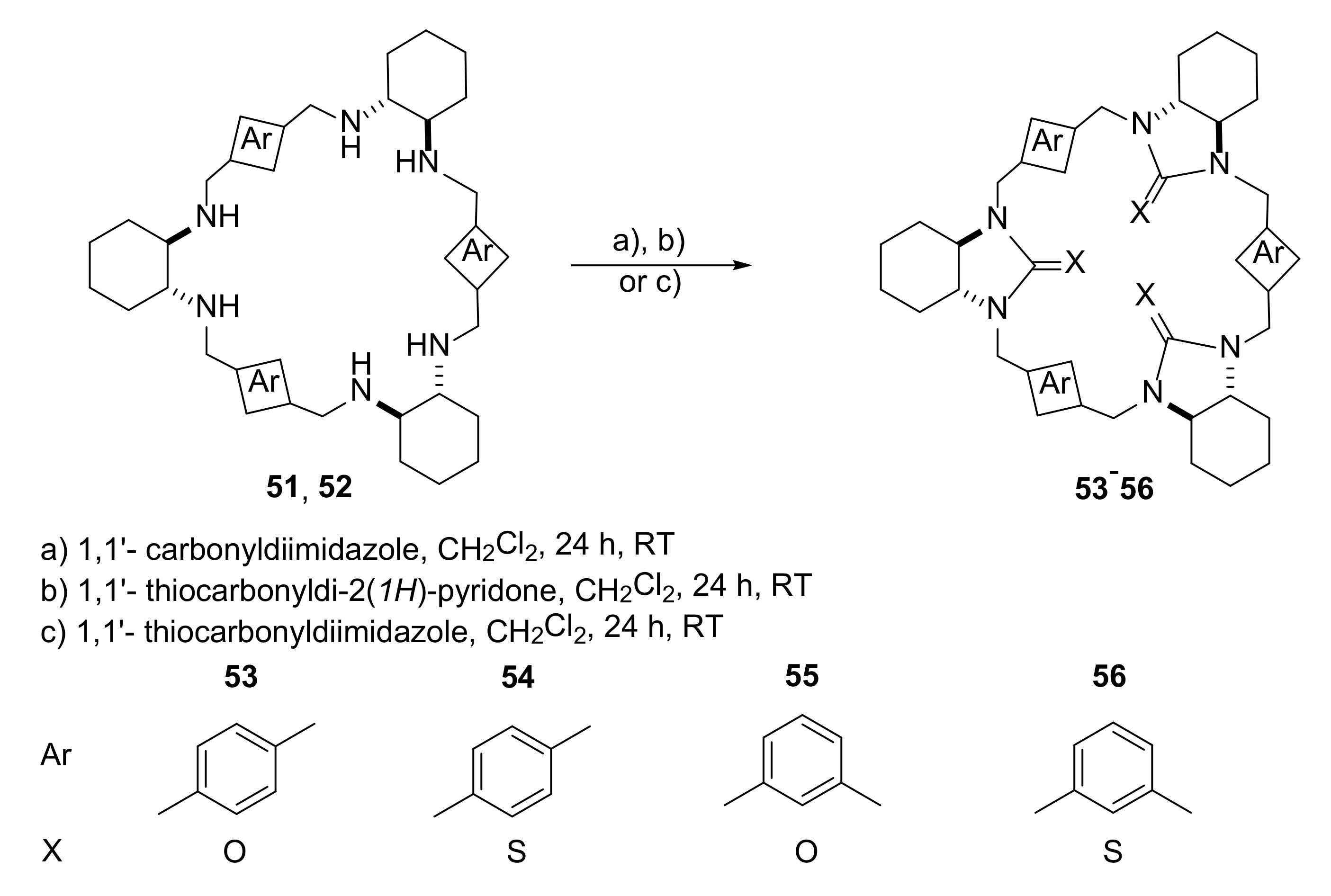
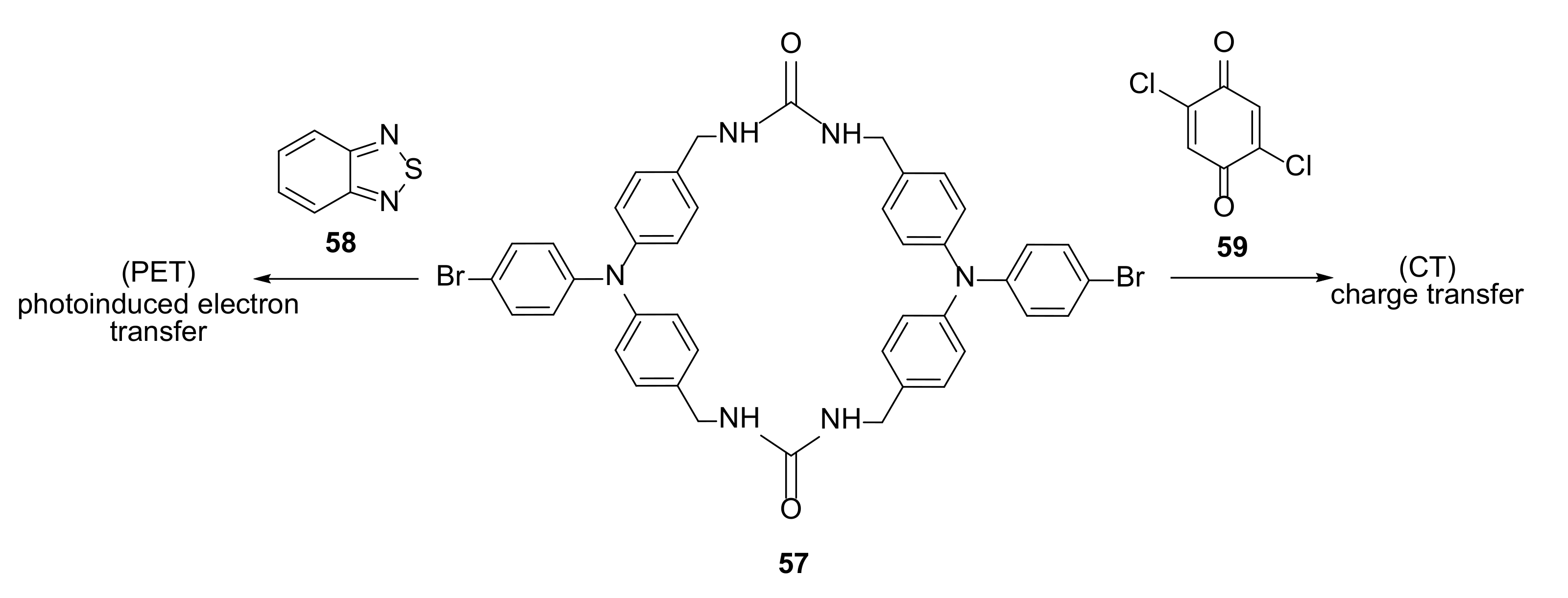
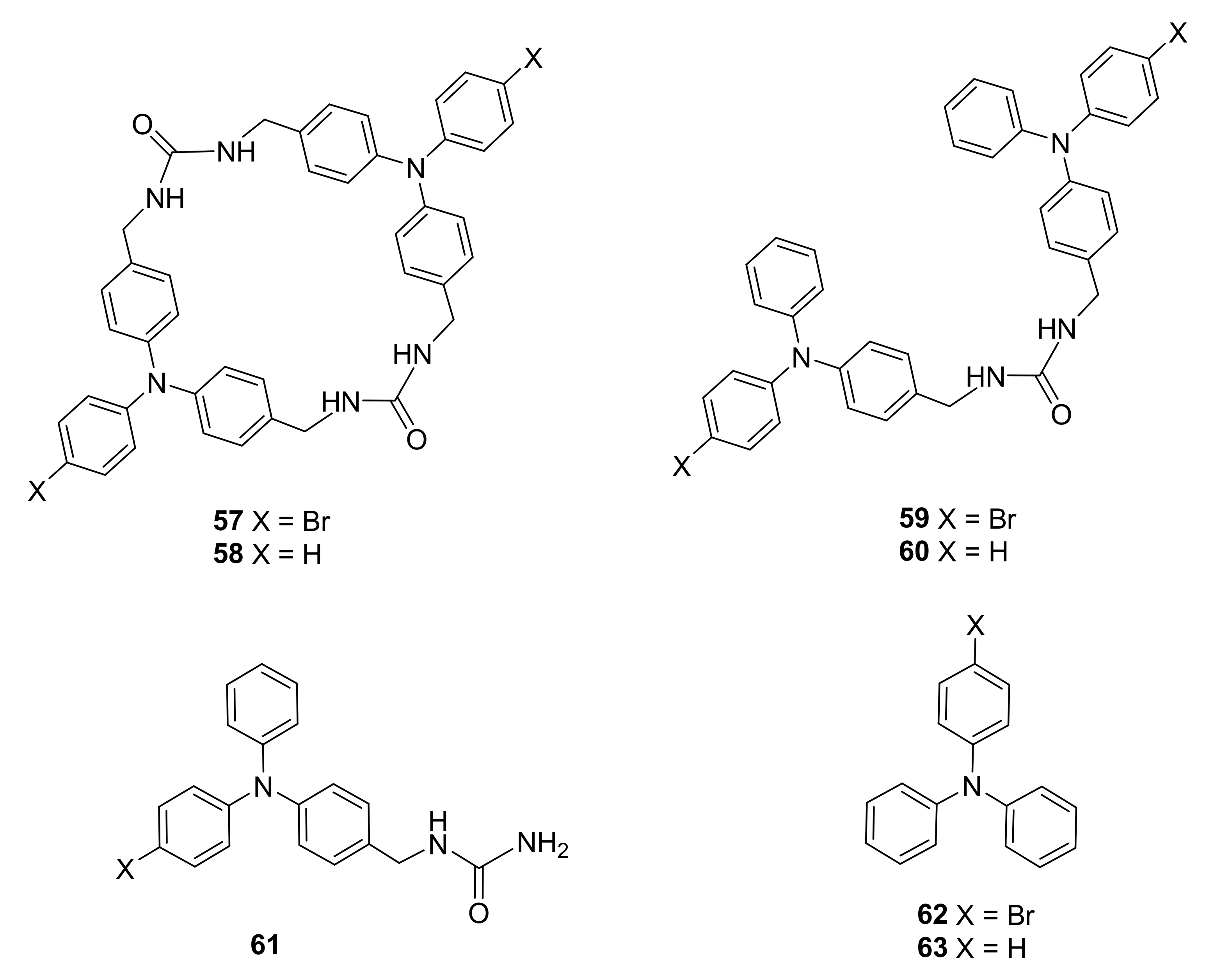
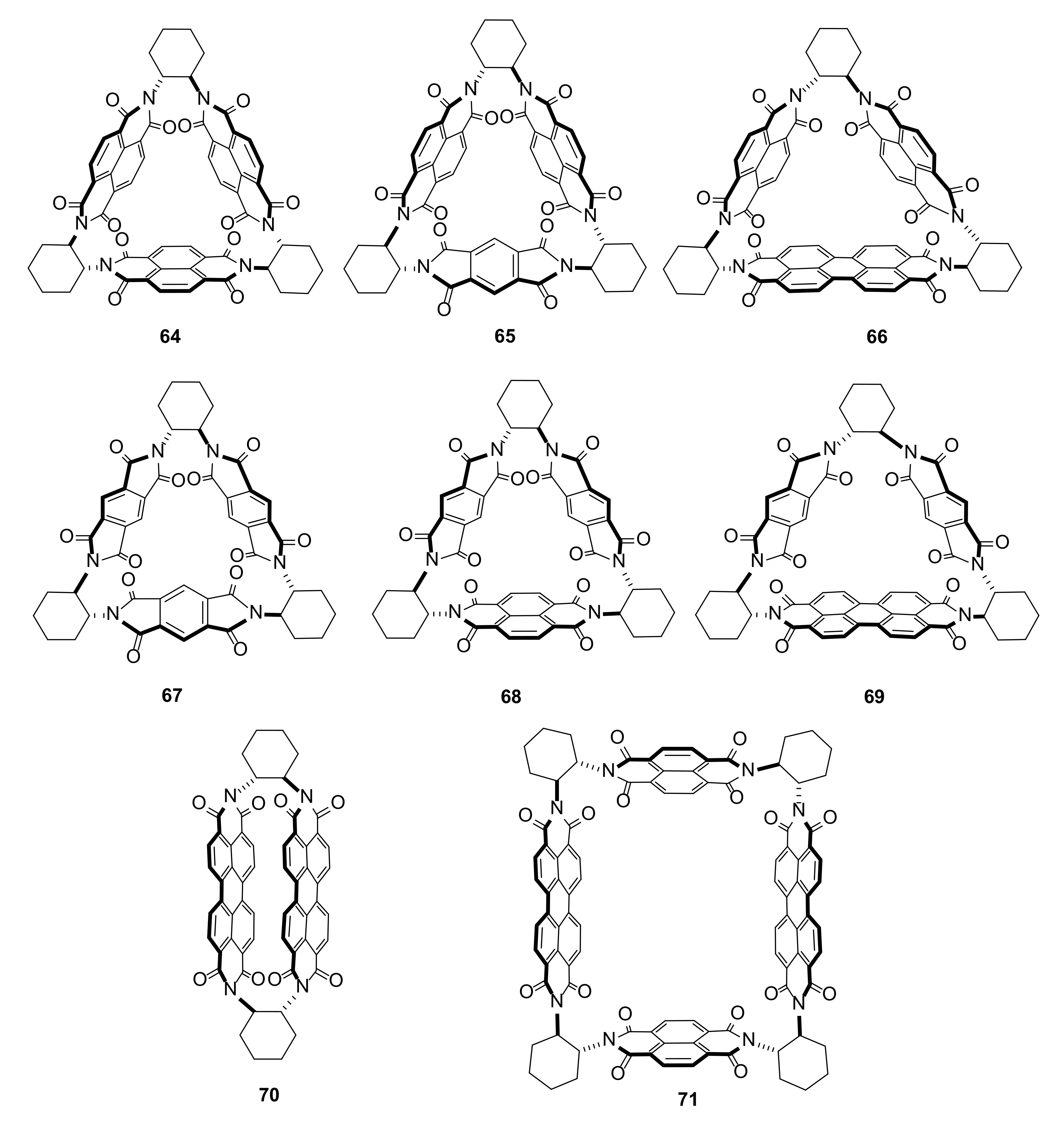
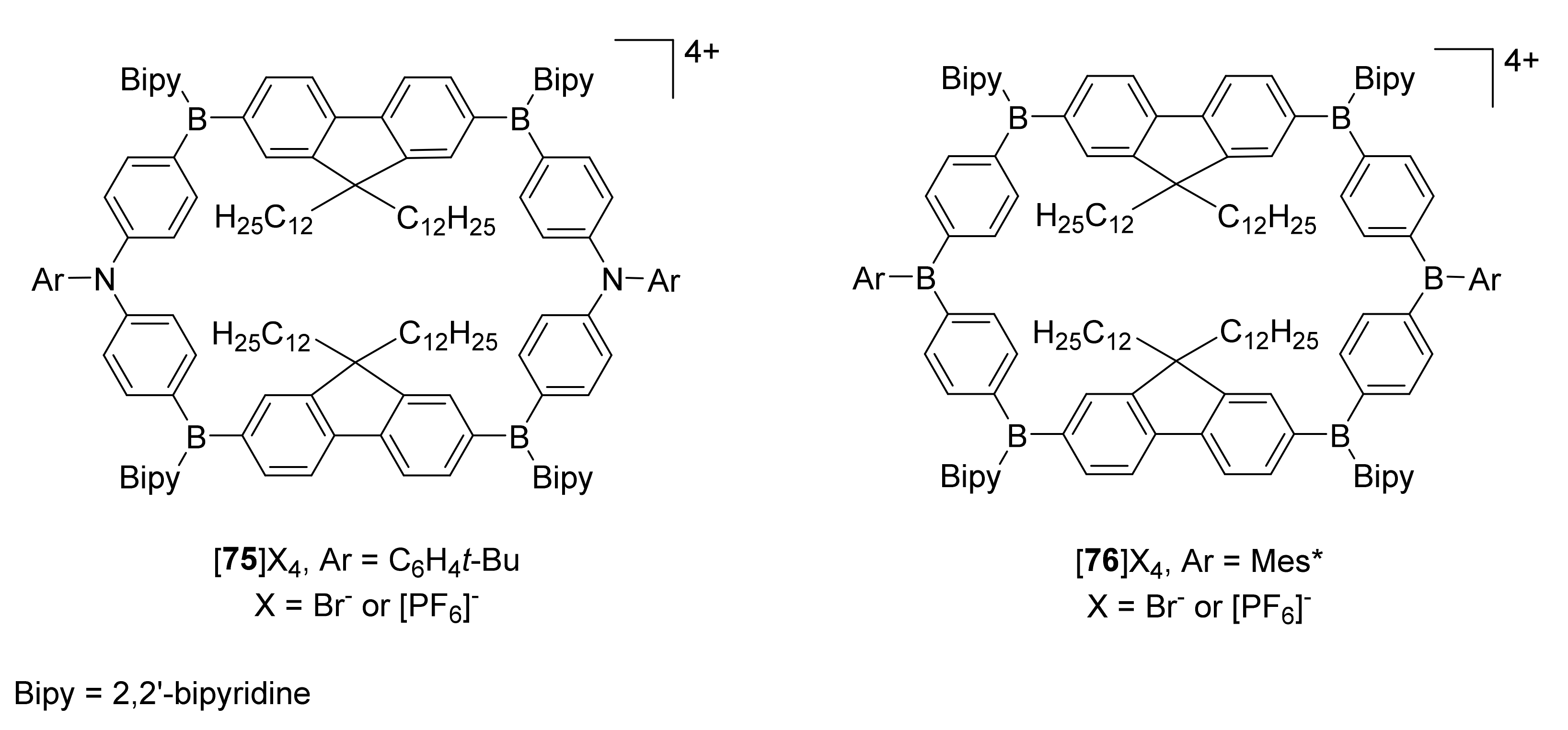
3.3. Electron-Transfer Properties
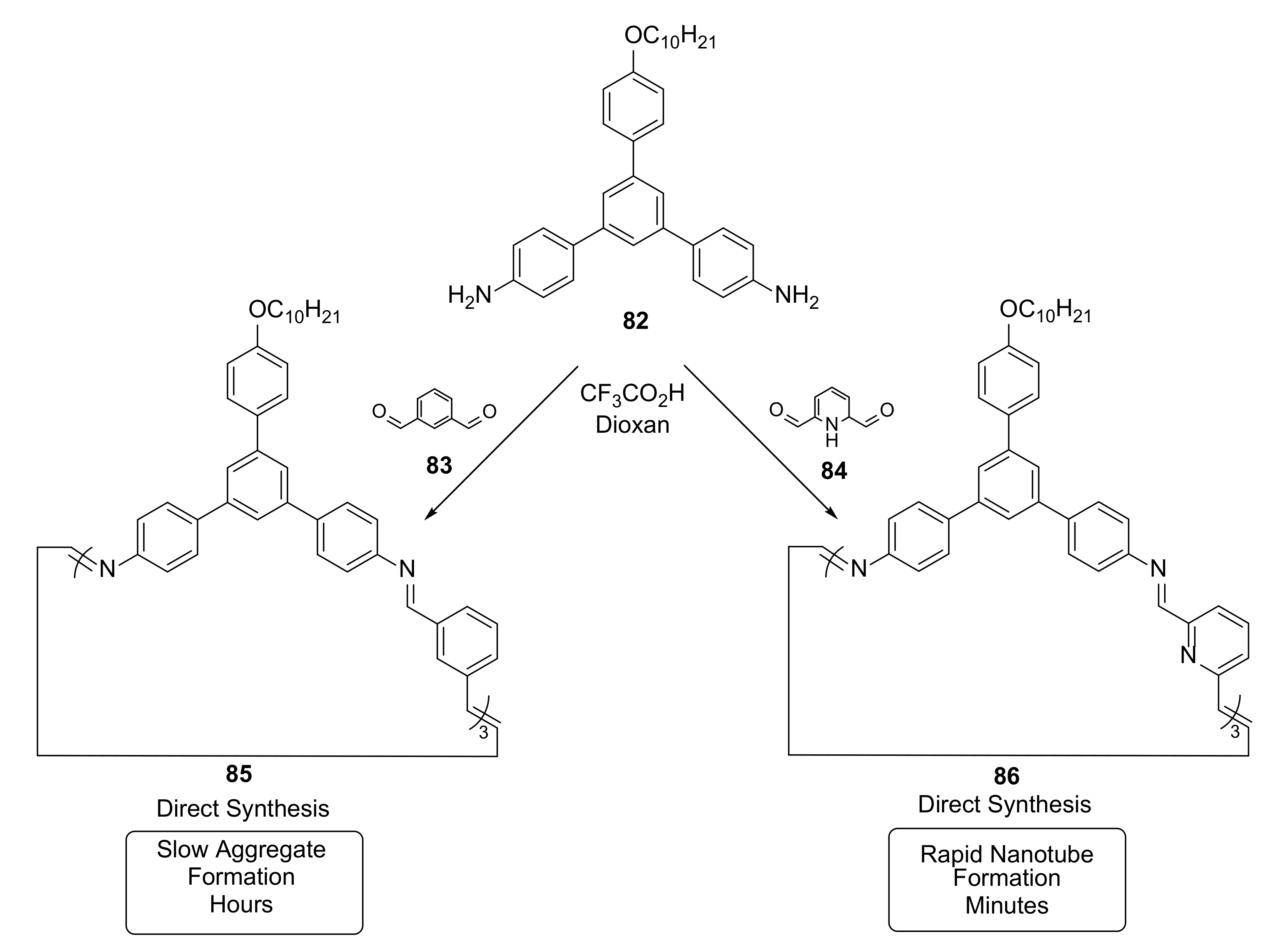
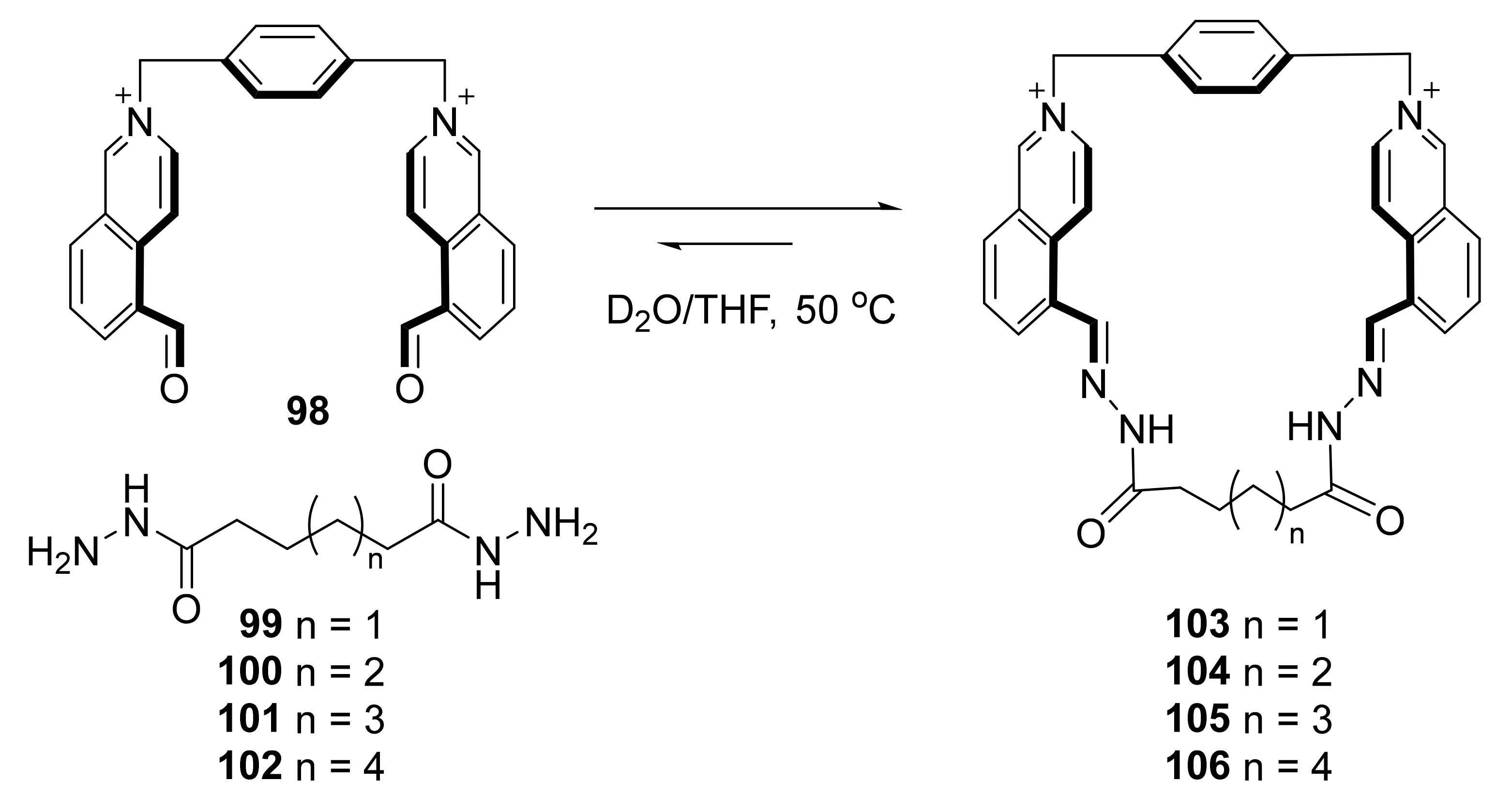
3.4. Imine Bond Exchange
3.5. Host–Guest Interactions with Organic Molecules
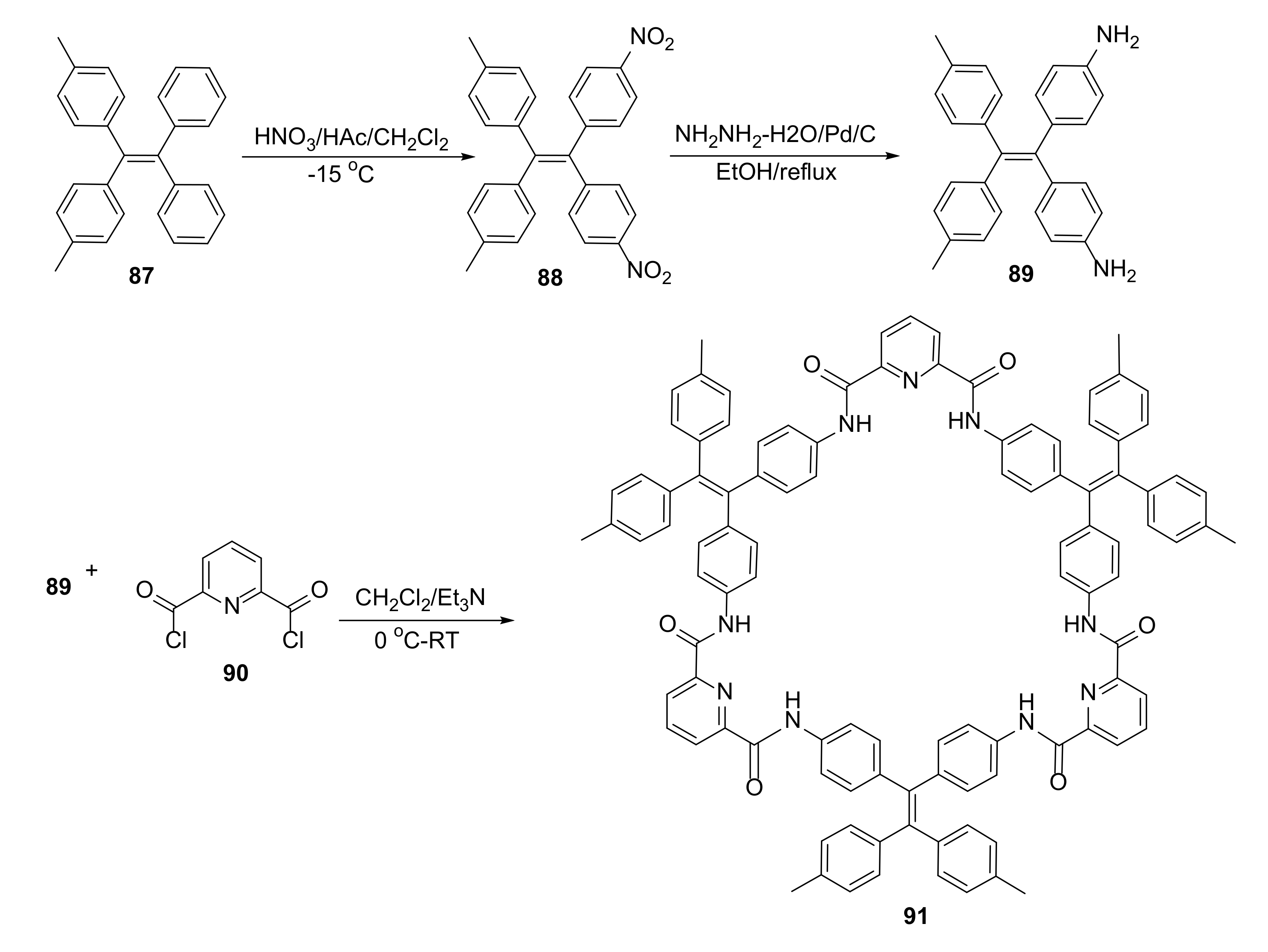
3.6. Ligand Exchange
3.7. Macrocycle Size and Shape Control
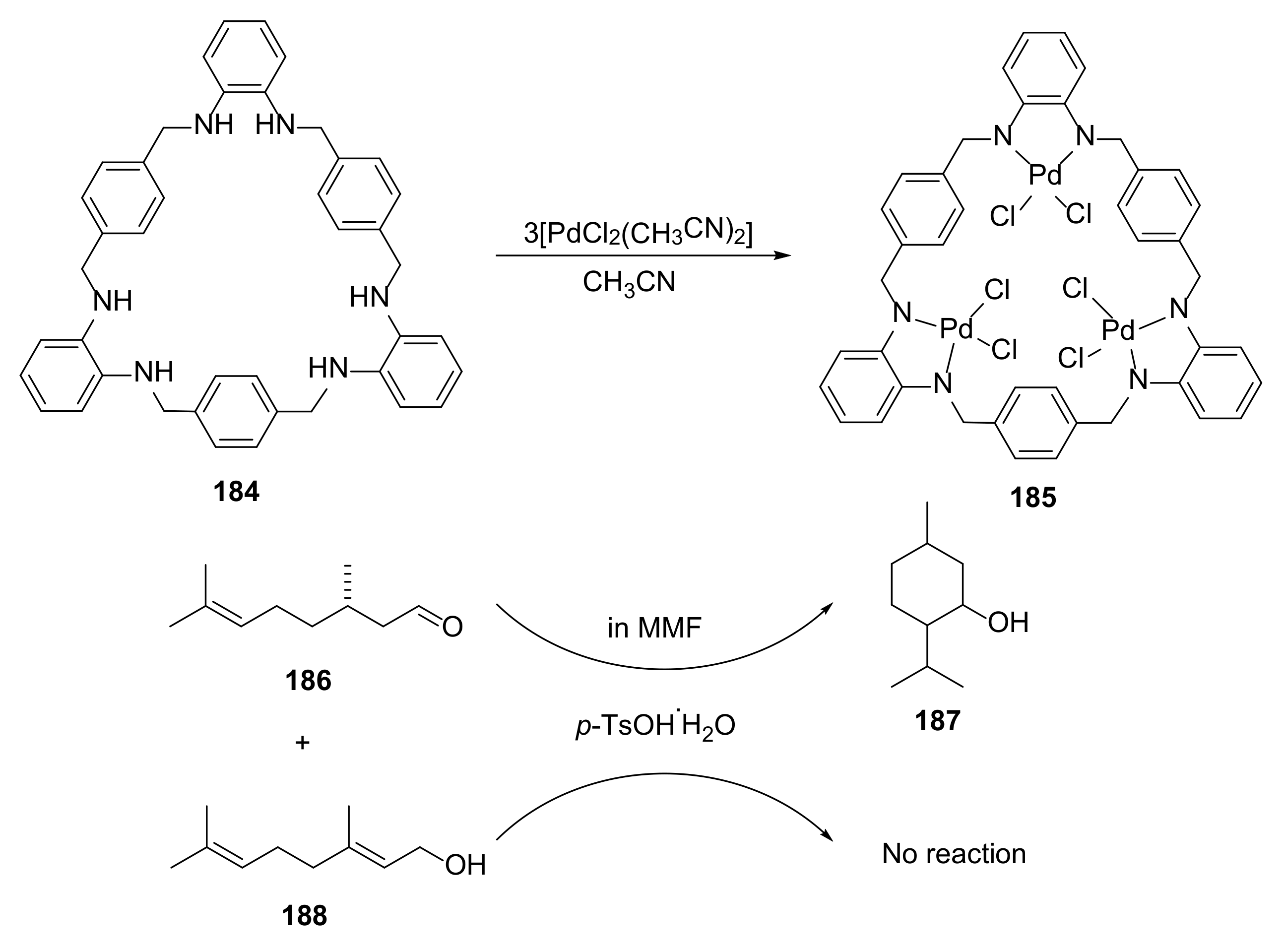
3.8. Other Properties
3.9. Catalysis
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Homberg, A.; Lacour, J. From reactive carbenes to chiral polyether macrocycles in two steps–Synthesis and applications made easy? Chem. Sci. 2020, 11, 6362–6369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisova, N.E.; Reshetova, M.D.; Ustynyuk, Y.A. Metal-Free Methods in the Synthesis of Macrocyclic Schiff Bases. Chem. Rev. 2007, 107, 46–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lin, W. Chiral Macrocycles. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2004; Volume 1, pp. 863–875. [Google Scholar]
- Srimurugan, S.; Suresh, P.; Babu, B.; Pati, H.N. Chiral Macrocyclic Schiff Bases: An Overview. Mini-Rev. Org. Chem. 2008, 5, 228–242. [Google Scholar] [CrossRef]
- Kwit, M.; Grajewski, J.; Skowronek, P.; Zgorzelak, M.; Gawroński, J. One-Step Construction of the Shape Persistent, Chiral but Symmetrical Polyimine Macrocycles. Chem. Rec. 2019, 19, 213–237. [Google Scholar] [CrossRef]
- Ji, X.; Ahmed, M.; Long, L.; Khashab, N.M.; Huang, F.; Sessler, J.L. Adhesive supramolecular polymeric materials constructed from macrocycle-based host–Guest interactions. Chem. Soc. Rev. 2019, 48, 2682–2697. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Shionoya, M. Novel Porous Crystals with Macrocycle-Based Well-Defined Molecular Recognition Sites. Acc. Chem. Res. 2020, 53, 632–643. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host−Guest Interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Lisowski, J. Metal Complexes of large imine and amine macrocycles. Wiadomości Chemiczne 2021, 75, 603–629. [Google Scholar]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef]
- Chaudhry, M.T.; Akine, S.; MacLachlan, M.J. Contemporary macrocycles for discrete polymetallic complexes: Precise control over structure and function. Chem. Soc. Rev. 2021, 50, 10713–10732. [Google Scholar] [CrossRef]
- Hodge, P. Entropically Driven Ring-Opening Polymerization of Strainless Organic Macrocycles. Chem. Rev. 2014, 114, 2278–2312. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Wysiecka, E.; Łukasik, N.; Biernat, J.F.; Luboch, E. Azo group(s) in selected macrocyclic compounds. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 189–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yawer, M.A.; Havel, V.; Sindelar, V.A. Bambusuril Macrocycle that Binds Anions in Water with High Affinity and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hay, B.P.; Young, N.J.; Yang, X.; Sessler, J.L. A pyrrole-based triazolium-phane with NH and cationic CH donor groups as a receptor for tetrahedral oxyanions that functions in polar media. Chem. Sci. 2013, 4, 1560–1567. [Google Scholar] [CrossRef] [Green Version]
- Lichosyt, D.; Dydio, P.; Jurczak, J. Azulene-Based Macrocyclic Receptors for Recognition and Sensing of Phosphate Anions. Chem. Eur. J. 2016, 22, 1–9. [Google Scholar] [CrossRef]
- Dabrowa, K.; Ulatowski, F.; Lichosyt, D.; Jurczak, J. Catching the chloride: Searching for non-Hofmeister selectivit behavior in systematically varied polyamide macrocyclic receptors. Org. Biomol. Chem. 2017, 15, 5927–5943. [Google Scholar] [CrossRef] [Green Version]
- Niedbała, P.; Jurczak, J. One-Pot Parallel Synthesis of Unclosed Cryptands-Searching for Selective Anion Receptors via Static Combinatorial Chemistry Techniques. ACS Omega 2020, 5, 26271–26277. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Cao, N.; Yang, C.; Li, H. A Kinetically Stable Macrocycle Self-Assembled in Water. Org. Lett. 2018, 20, 2356–2359. [Google Scholar] [CrossRef]
- Paurević, M.; Dandić, A.; Gajdošik, M.Š.; Vidović, B.; Perdih, F.; Balić, T. Efficient synthesis of new 17-, 18-, 19- and 20-membered N2O2-donor macrocycles by NaBH4 reduction and metal picrate extraction studiem. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 87–98. [Google Scholar] [CrossRef]
- Dey, A.; Chand, S.; Alimi, L.O.; Ghosh, M.; Cavallo, L.; Khashab, N.M. From Capsule to Helix: Guest-Induced Superstructures of Chiral Macrocycle Crystals. J. Am. Chem. Soc. 2020, 142, 15823–15829. [Google Scholar] [CrossRef]
- Gajewy, J.; Szymkowiak, J.; Kwit, M. Fine tuning of molecular and supramolecular properties of simple trianglimines the role of the functional group. RSC Adv. 2016, 6, 53358–53369. [Google Scholar] [CrossRef]
- Wang, Z.; Nour, H.F.; Roch, L.M.; Guo, M.; Li, W.; Baldridge, K.K.; Sue, A.C.-H.; Olson, M.A. [3 + 3] Cyclocondensation of Disubstituted Biphenyl Dialdehydes: Access to Inherently Luminescent and Optically Active Hexa-substituted C3-Symmetric and Asymmetric Trianglimine Macrocycles. J. Org. Chem. 2017, 82, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hachiken, S. Enantioselective Henry reaction catalyzed by trianglamine–Cu(OAc)2 complex under solvent-free conditions. Tetrahedron Lett. 2008, 49, 2533–2536. [Google Scholar] [CrossRef]
- Prusinowska, N.; Szymkowiak, J.; Kwit, M. Enantiopure Tertiary Urea and Thiourea Derivatives of Trianglamine Macrocycle: Structural Studies and Metallogeling Properties. J. Org. Chem. 2018, 83, 1167–1175. [Google Scholar] [CrossRef]
- Islam, M.F.; Sindt, A.J.; Hossain, M.S.; Ayare, P.J.; Smith, M.D.; Vannucci, A.K.; Garashchuk, S.; Shimizu, L.S. Assembled triphenylamine bis-urea macrocycles: Exploring photodriven electron transfer from host to guests. Phys. Chem. Chem. Phys. 2021, 23, 23953–23960. [Google Scholar] [CrossRef]
- Sindt, A.J.; DeHaven, B.A.; Goodlett, D.W.; Hartel, J.O.; Ayare, P.J.; Du, Y.; Smith, M.D.; Mehta, A.K.; Brugh, A.M.; Forbes, M.D.E.; et al. Guest Inclusion Modulates Concentration and Persistence of Photogenerated Radicals in Assembled Triphenylamine Macrocycles. J. Am. Chem. Soc. 2020, 142, 502–511. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Stoddart, J.F. Molecular Triangles: A New Class of Macrocycles. Acc. Chem. Res. 2021, 54, 2027–2039. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Wu, Y.; Cao, D.; Sun, J.; Schneebeli, S.T.; Nassar, M.S.; Mirkin, C.A.; Stoddart, J.F. Assembly of Supramolecular Nanotubes from Molecular Triangles and 1,2-Dihalohydrocarbons. J. Am. Chem. Soc. 2014, 136, 16651–16660. [Google Scholar] [CrossRef]
- Chen, D.; Avestro, A.-J.; Chen, Z.; Sun, J.; Wang, S.; Xiao, M.; Erno, Z.; Algaradah, M.M.; Nassar, M.S.; Amine, K.; et al. A Rigid Naphthalenediimide Triangle for Organic Rechargeable Lithium-Ion Batteries. Adv. Mater. 2015, 27, 2907–2912. [Google Scholar] [CrossRef]
- Mohan Nalluri, S.K.; Zhou, J.; Cheng, T.; Liu, Z.; Nguyen, M.T.; Chen, T.; Patel, H.A.; Krzyaniak, M.D.; Goddard, W.A., III; Wasielewski, M.R.; et al. Discrete Dimers of Redox-Active and Fluorescent Perylene Diimide-Based Rigid Isosceles Triangles in the Solid State. J. Am. Chem. Soc. 2019, 141, 1290–1303. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.T.; Krzyaniak, M.D.; Owczarek, M.; Ferris, D.P.; Wasielewski, M.R.; Stoddart, J.F. A Boat-Shaped Tetracationic Macrocycle with a Semiconducting Organic Framework. Angew. Chem. 2017, 129, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Jung, H.; Joo, S.H.; Sun, Q.K.; Li, C.; Noh, H.J.; Oh, I.; Kim, Y.J.; Kwak, S.K.; Yoo, J.W.; et al. Catalyst- and Solvent-Free Synthesis of Chemically Stable Aza-Bridged Bis(phenanthroline) Macrocycle-Linked Covalent Organic Framework. Angew. Chem. Int. Ed. 2021, 60, 17191–17197. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, D.; Baser-Kirazli, N.; Lalancette, R.A.; Jäkle, F. Electrochromic polycationic organoboronium macrocycles with multiple redox states. Angew. Chem. Int. Ed. Engl. 2021, 60, 17942–17946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, S.; Hou, X.; Wu, J. 1,6-Anthrazoline-Linked p-Conjugated Macrocycles and Two-Dimensional Polymer via Friedländer Synthesis. Angew. Chem. Int. Ed. 2021, 60, 25323–25327. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.J.; Evans, A.M.; Castano, I.; Li, R.L.; Dichtel, W.R. Supramolecular polymerization provides nonequilibrium product distributions of imine-linked macrocycles. Chem. Sci. 2020, 11, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.-B.; Wang, J.-H.; Li, B.; Zhang, C.; Tan, B.; Zheng, Y.-S. Porous Interdigitatio Molecular Cage from Tetraphenylethylene Trimeric Macrocycles That Showed Highly Selective Adsorption of CO2 and TNT Vapor in Air. Org. Lett. 2018, 20, 321–324. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Kanehashi, S.; Minegishi, K.; Wang, S.-P.; Cheng, J.; Ogino, K.; Li, S. One-pot synthesis of conjugated triphenylamine macrocycles and their complexation with fullerenes. RSC Adv. 2021, 11, 33431–33437. [Google Scholar] [CrossRef]
- Soto, M.A.; MacLachlan, M.J. Disabling Molecular Recognition through Reversible Mechanical Stoppering. Org. Lett. 2019, 21, 1744–1748. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.-Y.; Jiao, T.; Zhu, H.; Huang, F.; Li, H. Controllable Self-Assembly of Macrocycles in Water for Isolating Aromatic Hydrocarbon Isomers. J. Am. Chem. Soc. 2018, 140, 5955–5961. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A.; Martinez-Cuezva, A.; Marin-Luna, M.; Bautista, D.; Berna, J. Effective Encapsulation of C60 by Metal–Organic Frameworks with Polyamide Macrocyclic Linkers. Angew. Chem. Int. Ed. 2021, 60, 10814–10819. [Google Scholar] [CrossRef]
- Chaudhry, M.T.; Soto, M.A.; Lelj, F.; MacLachlan, M.J. Diverse binding of cationic guests by highly substituted [3 + 3] Schiff-base macrocycles. Org. Chem. Front. 2021, 8, 1437–1446. [Google Scholar] [CrossRef]
- Tominaga, M.; Mizuno, K.; Yamamoto, H.; Hyodo, T.; Yamaguchi, K. Co-Inclusion of cyclic ethers and chloroform by a macrocycle with benzophenone-3,3′,4,4′-tetracarboxylic diimide units. Cryst. Eng. Comm. 2020, 22, 2964–2969. [Google Scholar] [CrossRef]
- Perez-Martinez, J.; Morales, F.; Martinez-Cuezva, A.; Alajarin, M.; Berna, J. Synthesis of an Adamantane-Based Tetralactam and Its Association with Dicarboxamides. Proceedings 2020, 41, 65. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Dhamija, A.; Hwang, I.-C.; Lee, H.; Ko, Y.H.; Kim, K. One-pot Synthesis of a Truncated Cone-shaped Porphyrin Macrocycle and Its Self-assembly into Permanent Porous Material. Chem. Asian J. 2021, 16, 3209–3212. [Google Scholar] [CrossRef]
- Carta, V.; Mehr, S.H.M.; MacLachlan, M.J. Controlling Ligand Exchange through Macrocyclization. Inorg. Chem. 2018, 57, 3243–3253. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Dong, Y.; Wang, J.; Yu, Y.; Zhou, Y.; Sun, Y.; Zhang, C.; Chenga, H.; Zhao, F. Carbon dioxid as a sustainable resource for macrocyclic oligourea. Green Chem. 2016, 18, 2528–2533. [Google Scholar] [CrossRef]
- Grajewski, J.; Piotrowska, K.; Zgorzelak, M.; Janiak, A.; Biniek-Antosiak, K.; Rychlewska, U.; Gawronski, J. Introduction of axial chirality at a spiro carbon atom in the synthesis of pentaerythritol–imine macrocycles. Org. Biomol. Chem. 2018, 16, 981–987. [Google Scholar] [CrossRef]
- Zgorzelak, M.; Grajewski, J. Axial chirality inversion at a spiro carbon leads to efficient synthesis of polyimine macrocycle. J. Mol. Struct. 2020, 1202, 127336–127341. [Google Scholar] [CrossRef]
- Saito, Y.; Satake, M.; Mori, R.; Okayasu, M.; Masu, H.; Tominaga, M.; Katagiri, K.; Yamaguchi, K.; Kikkawa, S.; Hikawa, H.; et al. Synthesis and chiroptical properties of cylindrical macrocycles comprising two calix[3]aramide moieties. Org. Biomol. Chem. 2020, 18, 230–236. [Google Scholar] [CrossRef]
- Ziacha, K.; Jurczak, J. Mirror symmetry breaking upon spontaneous crystallization from a dynamic combinatorial library of macrocyclic imines. Chem. Commun. 2015, 51, 4306–4309. [Google Scholar] [CrossRef]
- Zgorzelak, M.; Grajewski, J.; Gawroński, J.; Kwit, M. Solvent-assisted synthesis of a shape-persistent chiral polyaza gigantocycle characterized by a very large internal cavity and extraordinarily high amplitude of the ECD exciton couplet. Chem. Commun. 2019, 55, 2301–2304. [Google Scholar] [CrossRef] [PubMed]
- Paćkowski, T.; Gregoliński, J.; Ślepokura, K.; Lisowski, J. 6 + 6 Macrocycles derived from 2,6-diformylpyridine and trans-1,2-diaminocyclohexane. Tetrahedron Lett. 2018, 59, 3669–3673. [Google Scholar] [CrossRef]
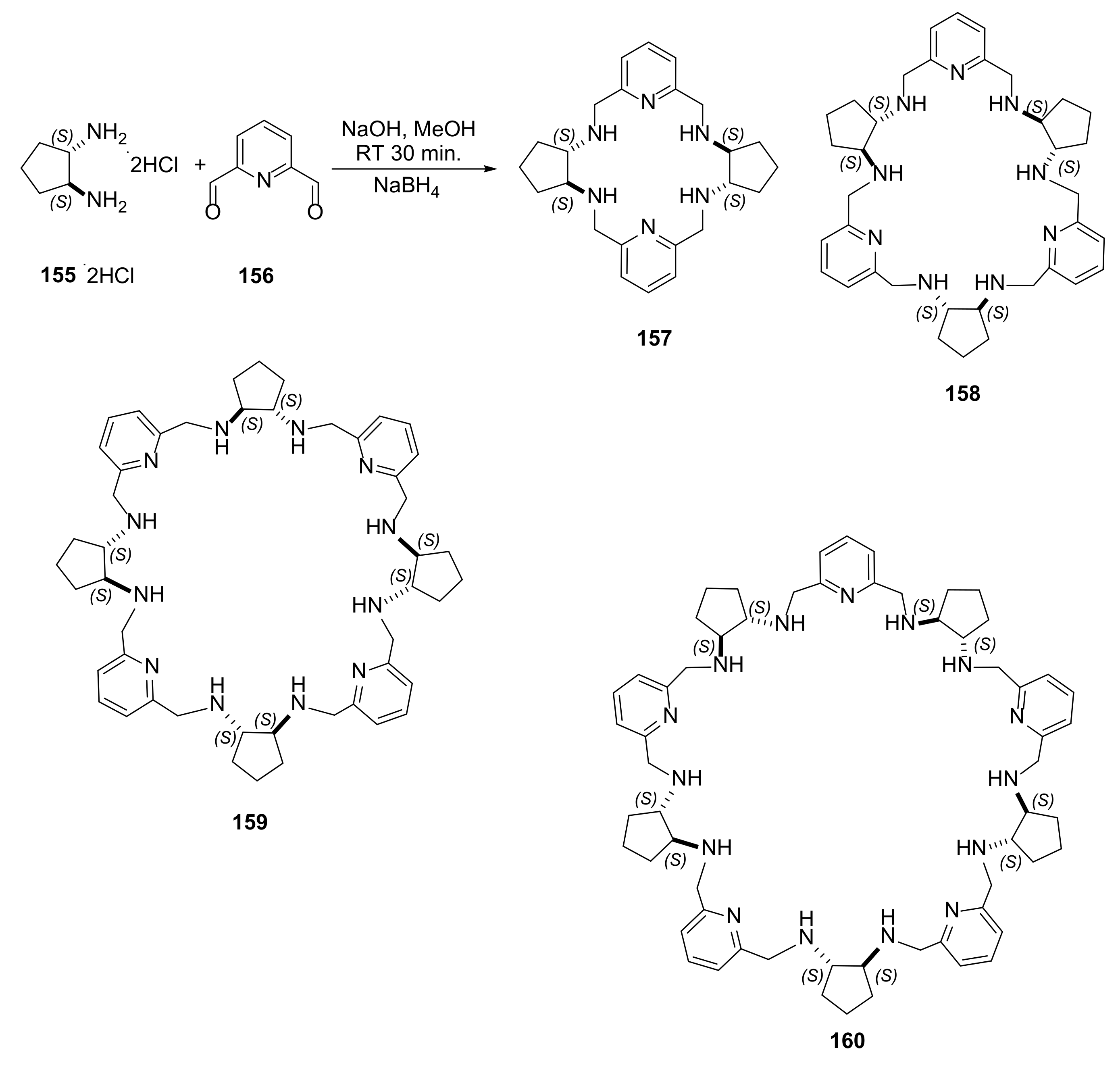
- Gregolinski, J.; Ślepokura, K.; Bil, A.; Lisowski, J. A new synthetic strategy leading to homochiral macrocycles derived from 2,6-diformylpyridine and (1S,2S)-trans-1,2-diaminocyclopentane. Eur. J. Org. Chem. 2020, 35, 5714–5728. [Google Scholar] [CrossRef]
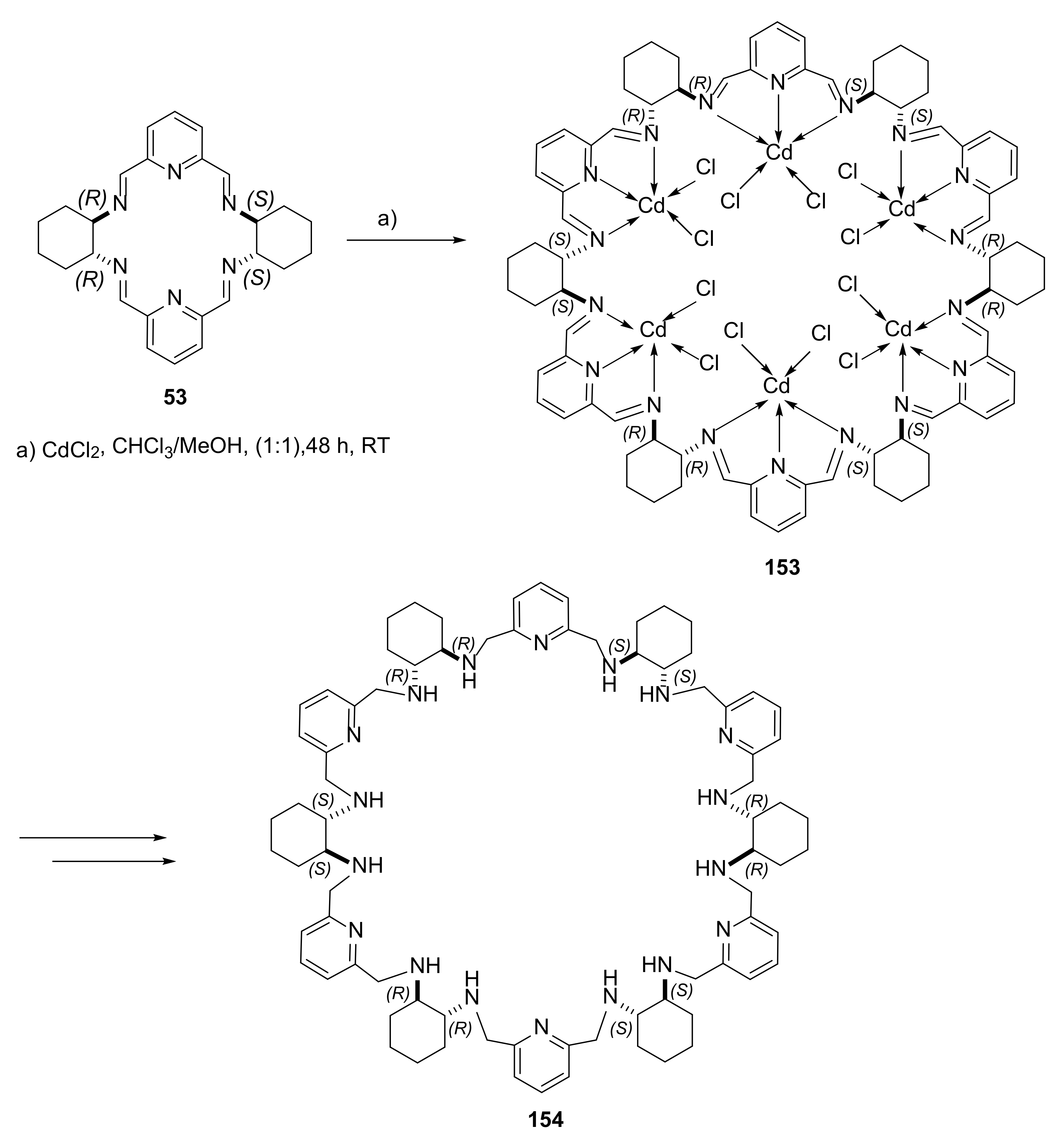
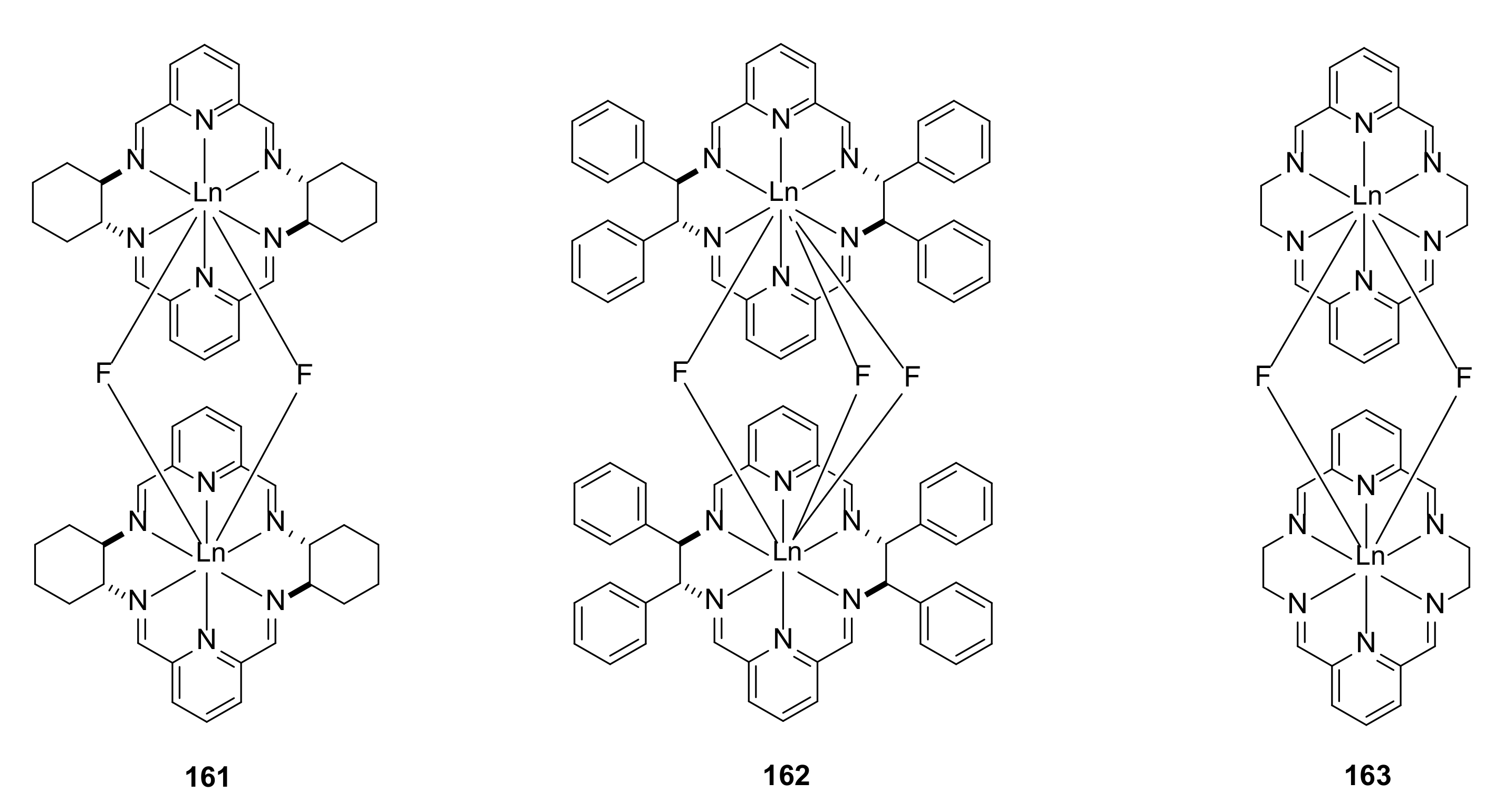
- Ślepokura, K.; Cabreros, T.A.; Muller, G.; Lisowski, J. Sorting Phenomena and Chirality Transfer in Fluoride-Bridged Macrocyclic Rare Earth Complexes. Inorg. Chem. 2021, 60, 18442–18454. [Google Scholar] [CrossRef] [PubMed]
- Bereta, T.; Mondal, A.; Ślepokura, K.; Peng, Y.; Powell, A.K.; Lisowski, J. Trinuclear and Hexanuclear Lanthanide(III) Complexes of the Chiral 3+3 Macrocycle: X-ray Crystal Structures and Magnetic Properties. Inorg. Chem. 2019, 58, 4201–4213. [Google Scholar] [CrossRef] [Green Version]
- Carta, V.; Soto, M.A.; MacLachlan, M.J. Ligand-modulated ring-expansion. Chem. Commun. 2019, 55, 1245–1248. [Google Scholar] [CrossRef]
- Beldjoudi, Y.; Narayanan, A.; Roy, I.; Pearson, T.J.; Cetin, M.M.; Nguyen, M.T.; Krzyaniak, M.D.; Alsubaie, F.M.; Wasielewski, M.R.; Stupp, S.I.; et al. Supramolecular Tessellations by a Rigid Naphthalene Diimide Triangle. J. Am. Chem. Soc. 2019, 141, 17783–17795. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, T.; He, L.; Gong, B. One-Pot Formation of Aromatic Tetraurea. Macrocycles. Org. Lett. 2012, 14, 2504–2507. [Google Scholar] [CrossRef]
- Sim, Y.; Leon, F.; Ganguly, R.; Díaz, J.; Clegg, J.K.; García, F. Synergic Topological- and Size-Control on Phosphazane Chemistry: First Unfolded Hybrid Tetrameric Macrocycle. Chem. Rxiv. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Raskatov, J.A.; Hargrove, A.E.; So, A.Y.; Dervan, P.B. Pharmacokinetics of Py-Im Polyamides Depend on Architecture: Cyclic versus Linear. J. Am. Chem. Soc. 2012, 134, 7995–7999. [Google Scholar] [CrossRef] [Green Version]
- Gawroński, J.; Kołbon, H.; Kwit, M.; Katrusiak, A. Designing Large Triangular Chiral Macrocycles: Efficient [3 + 3] Diamine−Dialdehyde Condensations Based on Conformational Bias. J. Org. Chem. 2000, 65, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhao, C.; Chen, L.; Little, M.A.; Chong, S.Y.; Clowes, R.; McKie, K.; Roper, M.G.; Day, G.M.; Liu, M.; et al. Inherent Ethyl Acetate Selectivity in a Trianglimine Molecular Solid. Chem. Eur. J. 2021, 27, 10589–10594. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Clowes, R.; Little, M.A.; Liu, M.; Cooper, A.I. Creating porosity in a trianglimine macrocycle by heterochiral pairing. Chem. Commun. 2021, 57, 6141–6144. [Google Scholar] [CrossRef] [PubMed]
- Dickmeis, M.; Cinar, H.; Ritter, H. Macrocyclic and Polymeric Oxaziridine-Derivatives. Macromol. Rapid Commun. 2013, 34, 263–268. [Google Scholar] [CrossRef]
- Garci, A.; Beldjoudi, Y.; Kodaimati, M.S.; Hornick, J.E.; Nguyen, M.T.; Cetin, M.M.; Stern, C.L.; Roy, I.; Weiss, E.A.; Stoddart, J.F. Mechanical-Bond-Induced Exciplex Fluorescence in an Anthracene-Based Homo[2]catenane. J. Am. Chem. Soc. 2020, 142, 7956–7967. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; He, W.; Hayashi, R.; Lin, Y.; Shionoya, M. Site-selective binding of terpenoids within a confined space of metal–macrocycle framework: Substrate-specific promotion or inhibition of cyclization reactions. Org. Chem. Front. 2021, 8, 4071–4077. [Google Scholar] [CrossRef]
- Chen, Z.; Sahli, B.J.; MacLachlan, M.J. Self-Assembly of Extended Head-to-Tail Triangular Pt3 Macrocycles into Nanotubes. Inorg. Chem. 2017, 56, 5383–5391. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grajewski, J. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles. Molecules 2022, 27, 1004. https://doi.org/10.3390/molecules27031004
Grajewski J. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles. Molecules. 2022; 27(3):1004. https://doi.org/10.3390/molecules27031004
Chicago/Turabian StyleGrajewski, Jakub. 2022. "Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles" Molecules 27, no. 3: 1004. https://doi.org/10.3390/molecules27031004
APA StyleGrajewski, J. (2022). Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles. Molecules, 27(3), 1004. https://doi.org/10.3390/molecules27031004





