Variation of Saponins in Sanguisorba officinalis L. before and after Processing (Paozhi) and Its Effects on Colon Cancer Cells In Vitro
Abstract
1. Introduction
2. Results
2.1. Optimization of Chromatographic and Mass Spectrometric Conditions
2.2. Identification of Compounds in Sraw and Sprocessed
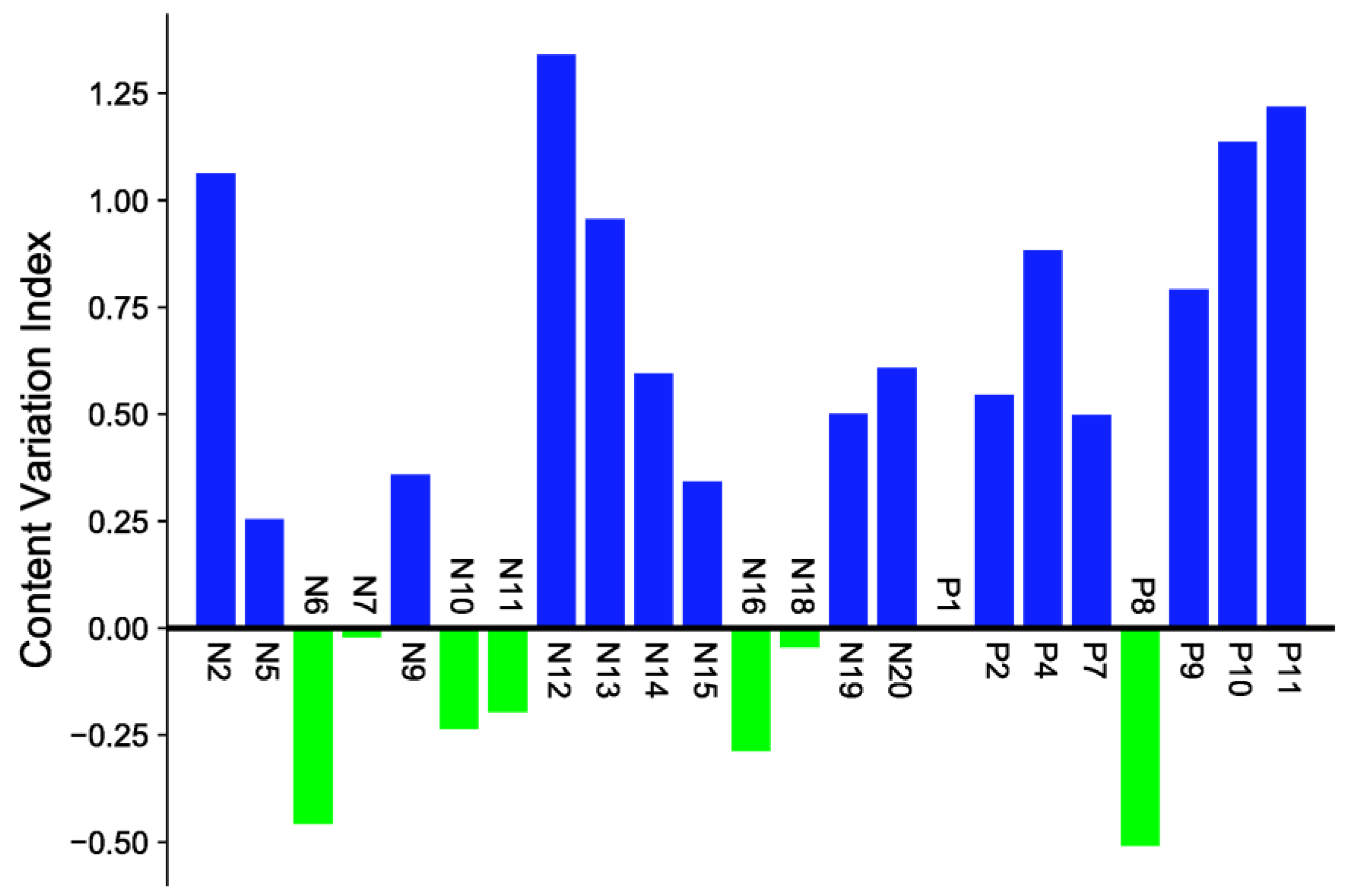
2.3. Quantitative Determination in Sraw and Sprocessed
2.4. Multivariate Statistical Analysis
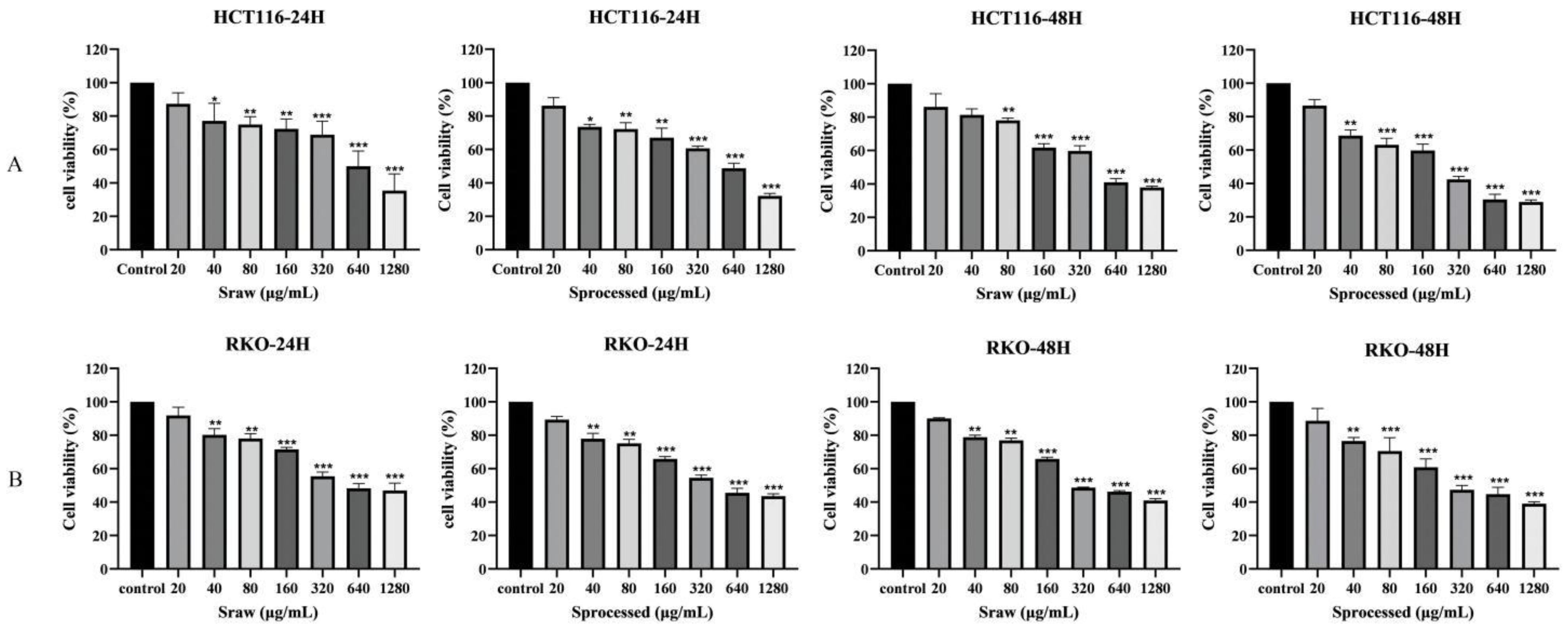
2.5. Effects of Sraw and Sprocessed on the Growth of Colon Cancer Cells In Vitro
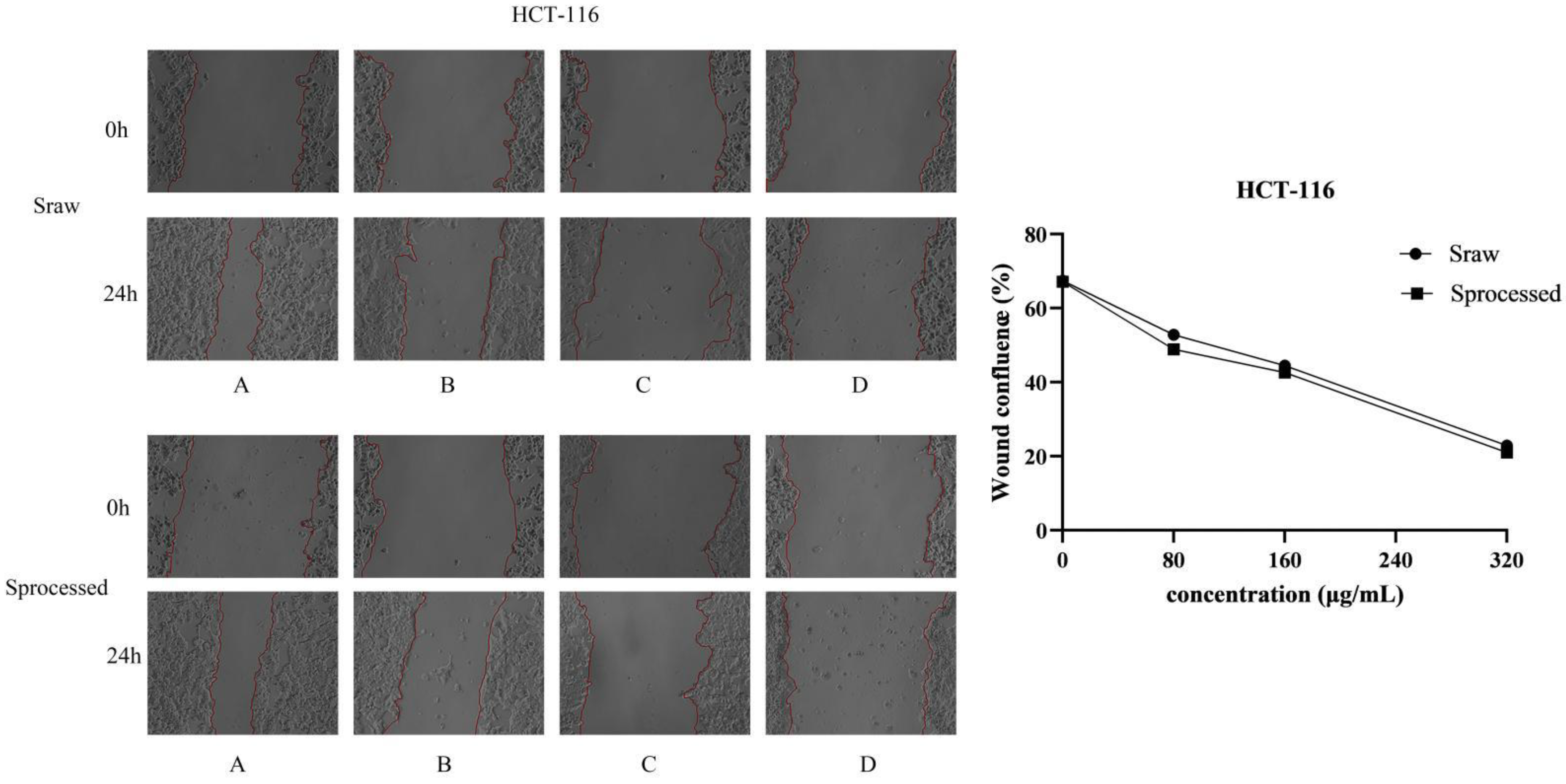
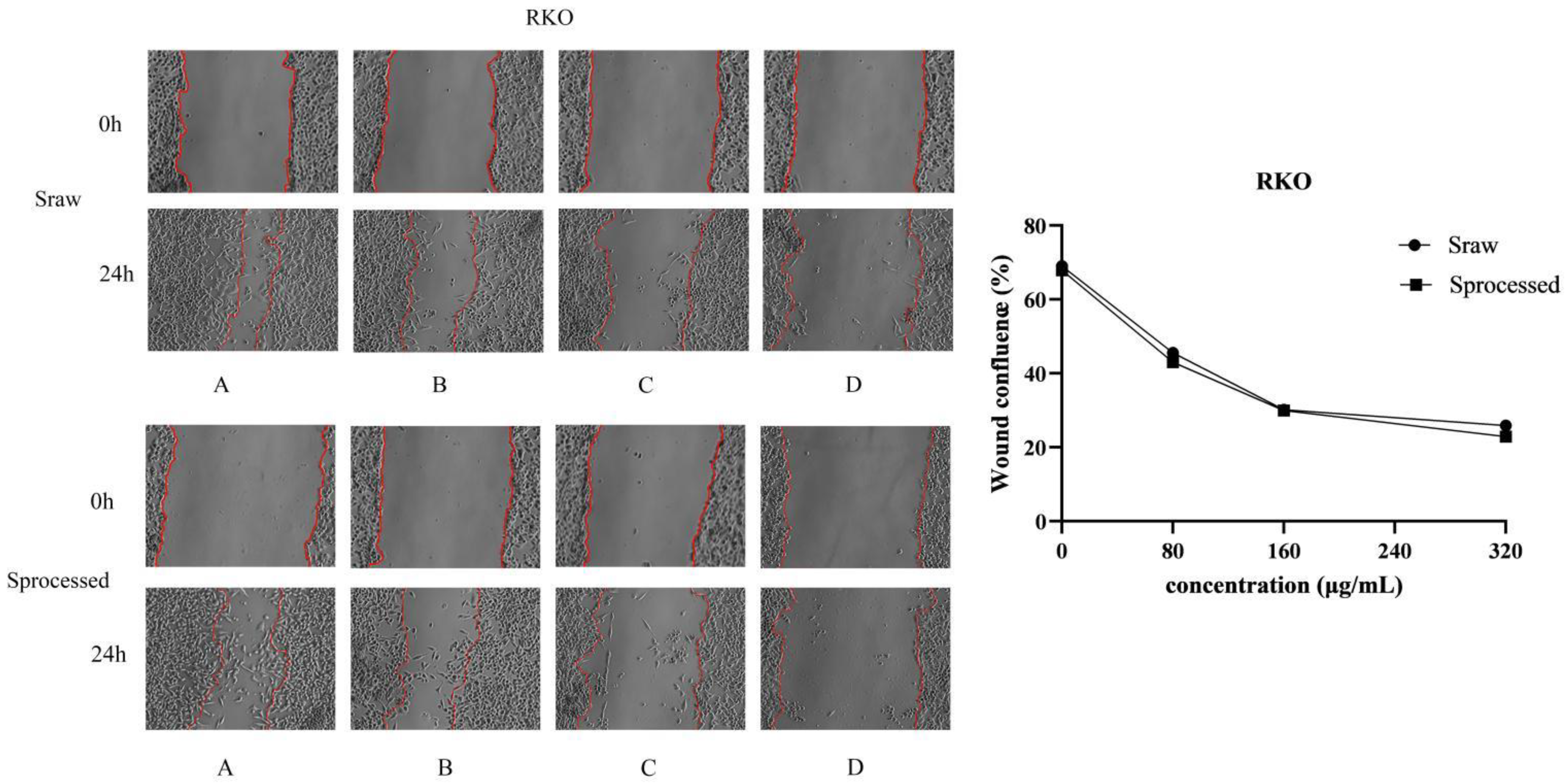
2.6. Sraw and Sprocessed Inhibited the Migration of HCT116 and RKO Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Sanguisorba officinalis L. before and after Samples
4.3. Preparation of Standard Solutions
4.4. Liquid Chromatographic Conditions
4.5. MS Spectrometry Conditions
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. Cell Migration Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| TCM | Traditional Chinese medicine; |
| S-Saponins | S. officinalis Saponins; |
| S. officinalis. | Sanguisorba officinalis L.; |
| Sraw | Sanguisorba officinalis L. saponins raw; |
| Sprocessed | Sanguisorba officinalis L. saponins processed; |
| UPLC-MS/MS | ultra-performance liquid chromatography–tandem mass spectrometry; |
| PCA | principal component analysis; |
| OPLS-DA | orthogonal partial least squares-discriminant analysis; |
| VIP | variable importance in projection |
References
- Fidelle, M.; Yonekura, S.; Picard, M.; Cogdill, A.; Hollebecque, A.; Roberti, M.P.; Zitvogel, L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front. Immunol. 2020, 11, 600886. [Google Scholar] [CrossRef]
- Otani, K.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Sasaki, K.; Kaneko, M.; Murono, K.; Emoto, S.; Nozawa, H. Colon cancer with perforation. Surg. Today 2019, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M. Colon Cancer. Surg. Oncol. Clin. N. Am. 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Pallag, A.; Roşca, E.; Tit, D.M.; MuŢiu, G.; Bungău, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2015, 56, 1103–1109. [Google Scholar]
- Gelibter, A.J.; Caponnetto, S.; Urbano, F.; Emiliani, A.; Scagnoli, S.; Sirgiovanni, G.; Napoli, V.M.; Cortesi, E. Adjuvant Chemotherapy in Resected Colon Cancer: When, How and How Long? Surg. Oncol. 2019, 30, 100–107. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liang, W.; Wang, T.; Sui, J.; Wang, J.; Deng, Z.; Chen, D. Saponins Regulate Intestinal Inflammation in Colon Cancer and IBD. Pharmacol. Res. 2019, 144, 66–72. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Geng, C.; Li, T.; Li, Y.; Guo, Y.; Wang, C. Ginsenoside Rg3 Suppresses Epithelial-Mesenchymal Transition via Downregulating Notch-Hes1 Signaling in Colon Cancer Cells. Am. J. Chin. Med. 2021, 49, 217–235. [Google Scholar] [CrossRef]
- Xia, X.; Lin, Q.; Zhao, N.; Zeng, J.; Yang, J.; Liu, Z.; Huang, R. Anti-Colon Cancer Activity of Dietary Phytochemical Soyasaponin I and the Induction of Metabolic Shifts in HCT116. Molecules 2022, 27, 4382. [Google Scholar] [CrossRef]
- Ma, J.; Gao, G.; Lu, H.; Fang, D.; Li, L.; Wei, G.; Chen, A.; Yang, Y.; Zhang, H.; Huo, J. Reversal Effect of Ginsenoside Rh2 on Oxaliplatin-resistant Colon Cancer Cells and Its Mechanism. Exp. Ther. Med. 2019, 18, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Sun, Y.; Cheng, Y.; Ye, W.; Jiang, C.; Liu, M.; Ji, Q.; Zhang, B.; Mei, Q.; Liu, D.; et al. MCP Mediated Active Targeting Calcium Phosphate Hybrid Nanoparticles for the Treatment of Orthotopic Drug-Resistant Colon Cancer. J. Nanobiotechnology 2021, 19, 367. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Sun, B.; Gong, T.; Pan, Z.; Meng, Q.; Ju, W. Ginsenoside Rb2 Inhibits Epithelial-Mesenchymal Transition of Colorectal Cancer Cells by Suppressing TGF-β/Smad Signaling. Phytomedicine 2019, 56, 126–135. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Wang, K.; Lu, J.; Bao, X.; Wang, R.; Qiu, Y.; Wang, T.; Yu, H. Cellular Senescence and Cancer: Focusing on Traditional Chinese Medicine and Natural Products. Cell Prolif. 2020, 53, e12894. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, Z.-L.; Liu, X.; Zhang, S.; He, L.; Wang, Z.; Wang, G.-S. Terpene Glycosides from the Roots of Sanguisorba Officinalis L. and Their Hemostatic Activities. Molecules 2012, 17, 7629–7636. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Guillén-Bejarano, R.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; López, S. In Vitro Toxicity of Asparagus Saponins in Distinct Multidrug-Resistant Colon Cancer Cells. Chem. Biodivers. 2018, 15, e1800282. [Google Scholar] [CrossRef]
- Lkhagvasuren, K.; Kim, J.-K. Ziyuglycoside II Induces Caspases-Dependent and Caspases-Independent Apoptosis in Human Colon Cancer Cells. Toxicol. Vitr. 2019, 59, 255–262. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.-Y.; Zhou, F.; Cai, Y.-J.; Sun, R.; Liu, R.-P. Ziyuglycoside II Inhibits the Growth of Digestive System Cancer Cells through Multiple Mechanisms. Chin. J. Nat. Med. 2021, 19, 351–363. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Xu, H.; Liu, Y.; He, H.; Zhou, M.; Jin, C.; Yang, M.; Ai, Z.; Su, D. Study on the Mechanism of Reducing Biofilm Toxicity and Increasing Antioxidant Activity in Vinegar Processing Phytomedicines Containing Pentacyclic Triterpenoid Saponins. J. Ethnopharmacol. 2022, 290, 115112. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.-Y.; Yang, Y.; Li, Z.-Y. A Review on Charred Traditional Chinese Herbs: Carbonization to Yield a Haemostatic Effect. Pharm. Biol. 2019, 57, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the Unseen of Chinese Herbal Medicine Processing (Paozhi): Advances in New Perspectives. Chin. Med. 2018, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Guo, M.-Q.; Li, X.-M.; Yang, X.-W. Simultaneous Qualitative and Quantitative Evaluation of the Coptidis Rhizoma and Euodiae Fructus Herbal Pair by Using UHPLC-ESI-QTOF-MS and UHPLC-DAD. Molecules 2020, 25, 4782. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, S.; Shi, Q.; Xiang, X.; Jin, Y.; Wei, S.; Zhang, L.; Yang, M.; Song, C.; Huang, R.; et al. Changes in Triterpenes in Alismatis Rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules 2021, 27, 185. [Google Scholar] [CrossRef]
- Yasueda, A.; Kayama, H.; Murohashi, M.; Nishimura, J.; Wakame, K.; Komatsu, K.; Ogino, T.; Miyoshi, N.; Takahashi, H.; Uemura, M.; et al. Sanguisorba Officinalis L. Derived from Herbal Medicine Prevents Intestinal Inflammation by Inducing Autophagy in Macrophages. Sci. Rep. 2020, 10, 9972. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Jin, M.; Jin, L.; Qi, Y.; Cao, L.; Zhou, W.; Li, G. A New Triterpenoid and Other Constituents with Cytotoxic Activity from the Roots of Sanguisorba officinalis L. Nat. Prod. Res. 2021, 35, 3341–3345. [Google Scholar] [CrossRef]
- Kayce, P.; Kirmizigul, S. Isolation and Identification of a New Saponin from Cephalaria Aytachii. Nat. Prod. Res. 2017, 31, 50–57. [Google Scholar] [CrossRef]
- Wu, C.; Yao, M.; Li, W.; Cui, B.; Dong, H.; Ren, Y.; Yang, C.; Gan, C. Simultaneous Determination and Pharmacokinetics Study of Six Triterpenes in Rat Plasma by UHPLC-MS/MS after Oral Administration of Sanguisorba officinalis L. Extract. Molecules 2018, 23, 2980. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. Determined with the LC-DAD-ESI-QTOF-MS/MS Analysis and Their In Vitro Antioxidant, Antidiabetic, Antiproliferative Potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- Cai, P.; Qiu, H.; Qi, F.; Zhang, X. The Toxicity and Safety of Traditional Chinese Medicines: Please Treat with Rationality. Biosci. Trends 2019, 13, 367–373. [Google Scholar] [CrossRef]
- Wang, B.; Ji, J.; Zhao, S.; Dong, J.; Tan, P.; Na, S.; Liu, Y. An Efficient High-Performance Liquid Chromatography Combined with Electrospray Ionization Tandem Mass Spectrometry Method to Elaborate the Changes of Components between the Raw and Processed Radix Aconitum Kusnezoffii. Pharmacogn. Mag. 2016, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Piao, J.-H.; Jiang, J.-G. Typical Toxic Components in Traditional Chinese Medicine. Expert Opin. Drug Saf. 2012, 11, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Chen, Y.; Zhu, Z.; Chen, J.; Gao, T.; Limsila, B.; Techadamrongsin, Y.; Wang, L.; Yu, J.; Fu, C.; et al. Vinegar-Processed Curcuma Phaeocaulis Promotes Anti-Angiogenic Activity and Reduces Toxicity in Zebrafish and Rat Models. Pharm. Biol. 2021, 59, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, Y.; Mao, Z.; Wu, J.; Ren, D.; Zhu, B.; Qin, L. Processing and Compatibility of Corydalis Yanhusuo: Phytochemistry, Pharmacology, Pharmacokinetics, and Safety. Evid. Based Complement. 2021, 2021, 1271953. [Google Scholar] [CrossRef]
- Shen, M.-R.; He, Y.; Shi, S.-M. Development of Chromatographic Technologies for the Quality Control of Traditional Chinese Medicine in the Chinese Pharmacopoeia. J. Pharm. Anal. 2021, 11, 155–162. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Chen, S.; Li, H.; Dong, L.; Fu, X. A New Discovery: Total Bupleurum Saponin Extracts Can Inhibit the Proliferation and Induce Apoptosis of Colon Cancer Cells by Regulating the PI3K/Akt/MTOR Pathway. J. Ethnopharmacol. 2022, 283, 114742. [Google Scholar] [CrossRef]
- Ellington, A.A. Induction of Macroautophagy in Human Colon Cancer Cells by Soybean B-Group Triterpenoid Saponins. Carcinogenesis 2004, 26, 159–167. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Koh, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. A Natural Degradant of Curcumin, Feruloylacetone Inhibits Cell Proliferation via Inducing Cell Cycle Arrest and a Mitochondrial Apoptotic Pathway in HCT116 Colon Cancer Cells. Molecules 2021, 26, 4884. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wang, S.-S.; Shen, K.-P.; Huang, X.-W.; Li, M.; Chen, L.; Peng, X.; An, H.-M.; Hu, B. Ursolic Acid Potentiated Oxaliplatin to Induce Apoptosis in Colorectal Cancer RKO Cells. Die Pharmazie. 2020, 75, 246–249. [Google Scholar]
- Yang, M.; Li, W.-Y.; Xie, J.; Wang, Z.-L.; Wen, Y.-L.; Zhao, C.-C.; Tao, L.; Li, L.-F.; Tian, Y.; Sheng, J. Astragalin Inhibits the Proliferation and Migration of Human Colon Cancer HCT116 Cells by Regulating the NF-ΚB Signaling Pathway. Front. Pharmacol. 2021, 12, 639256. [Google Scholar] [CrossRef]
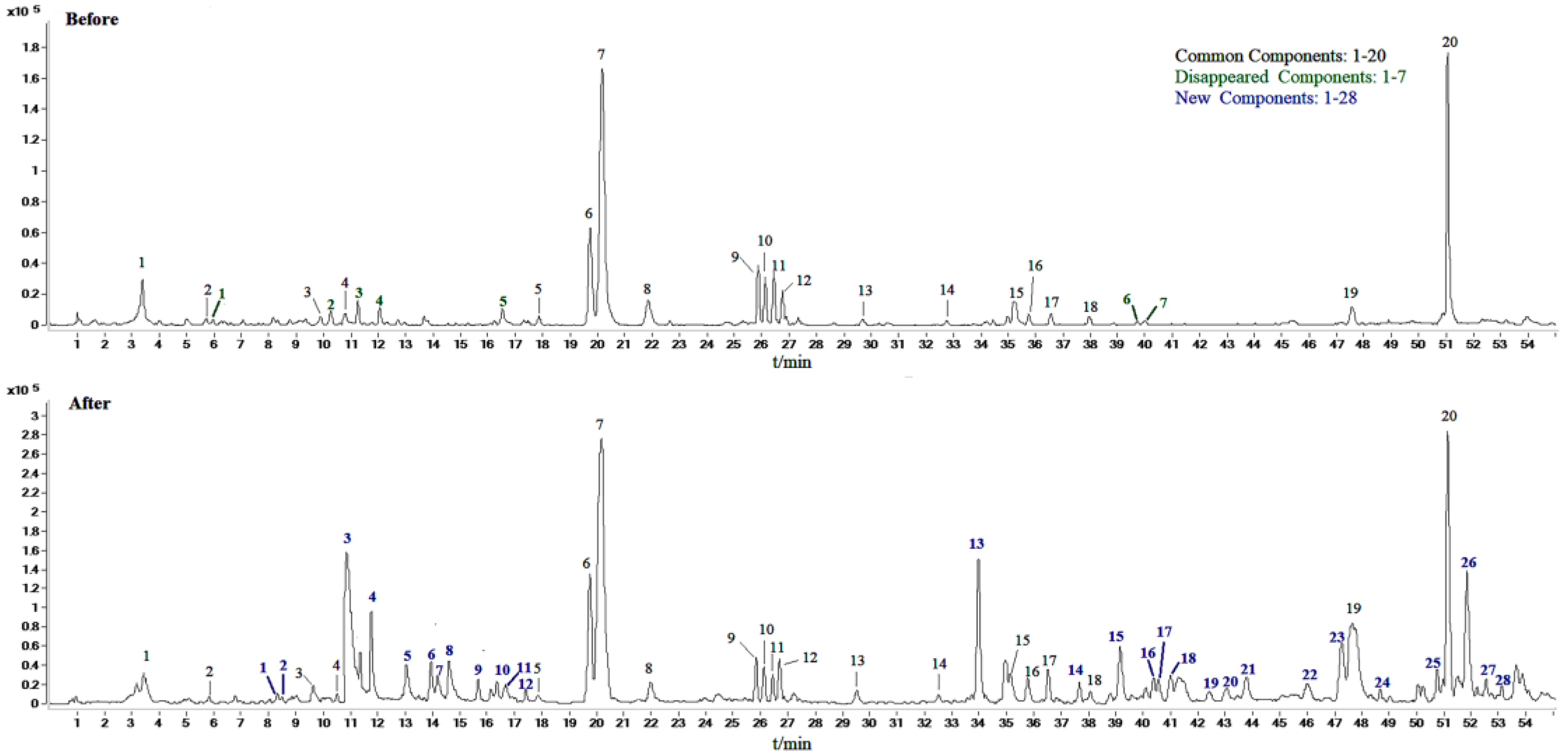
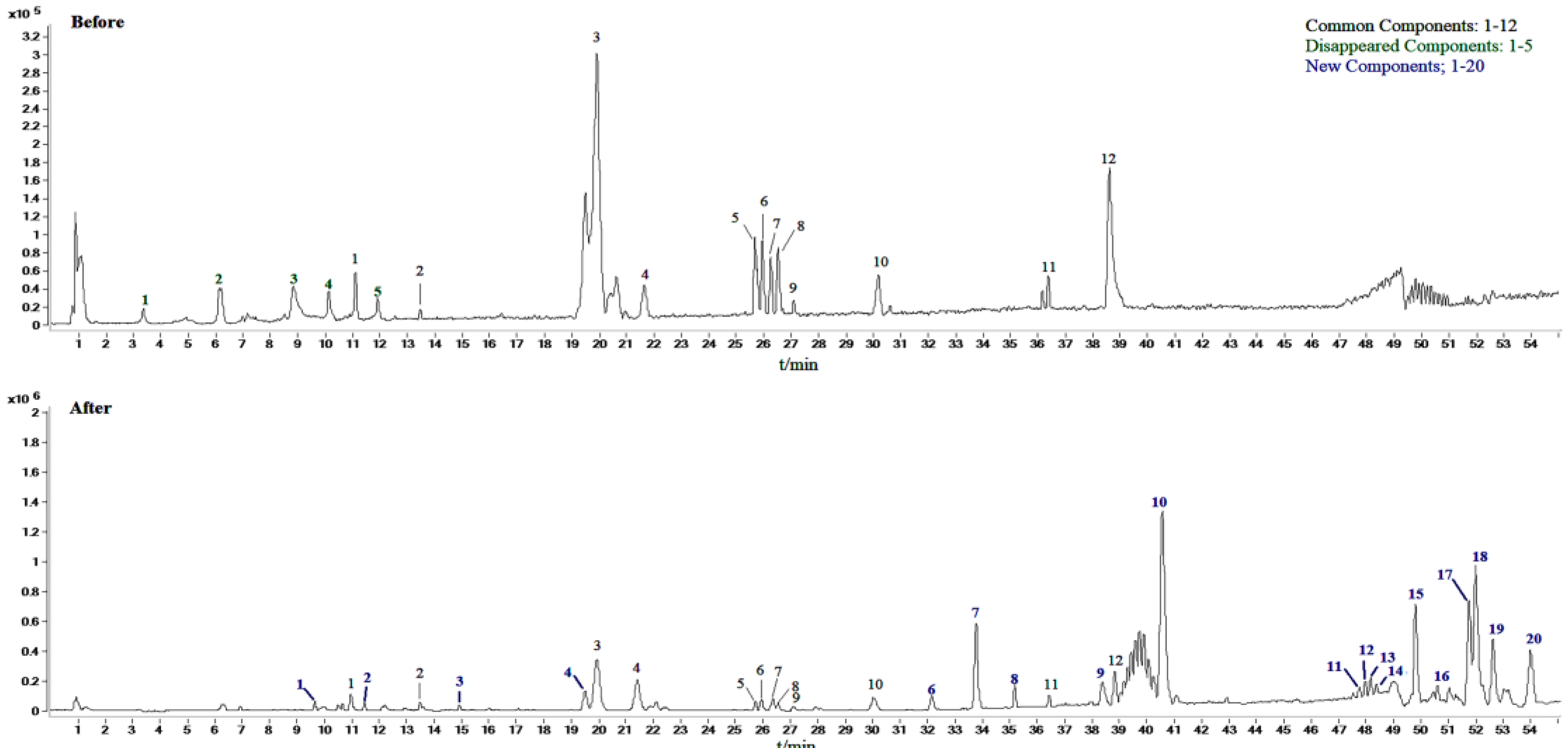
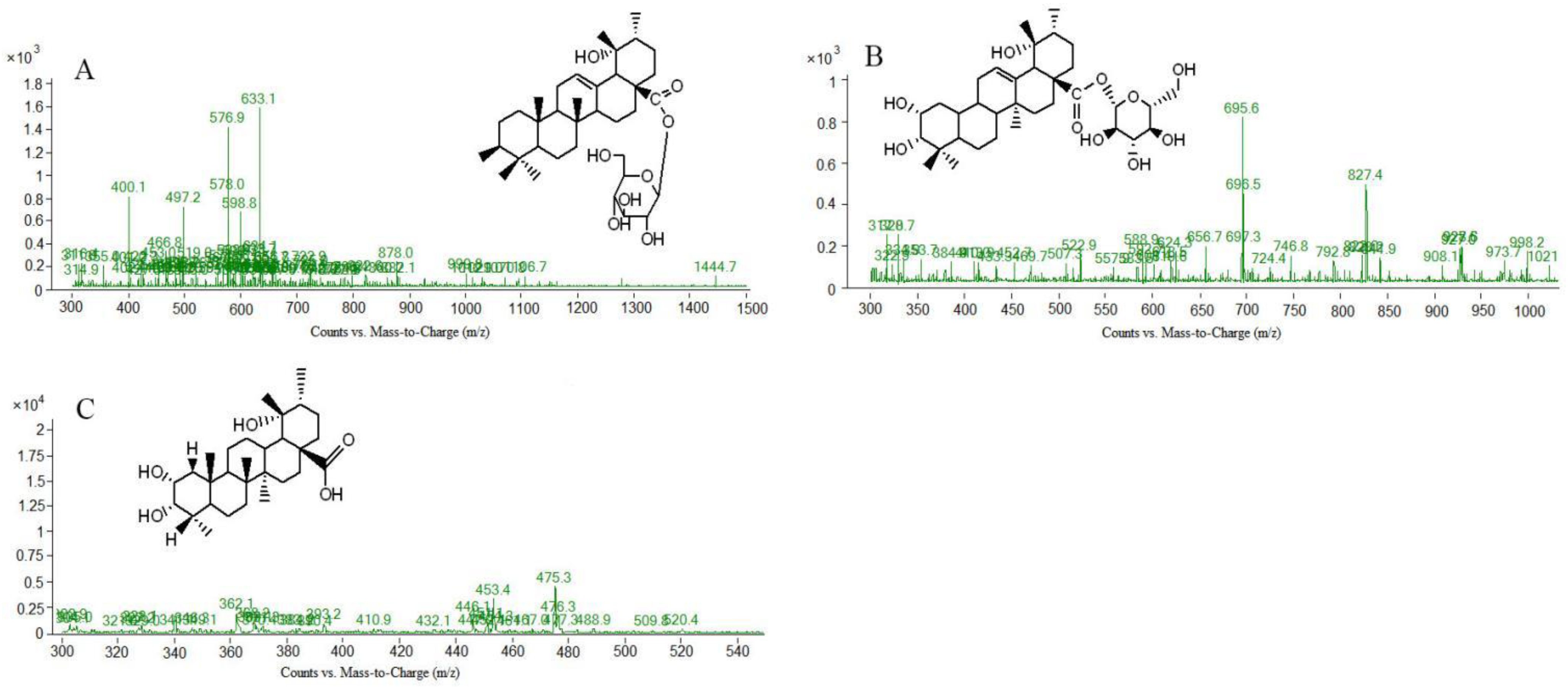
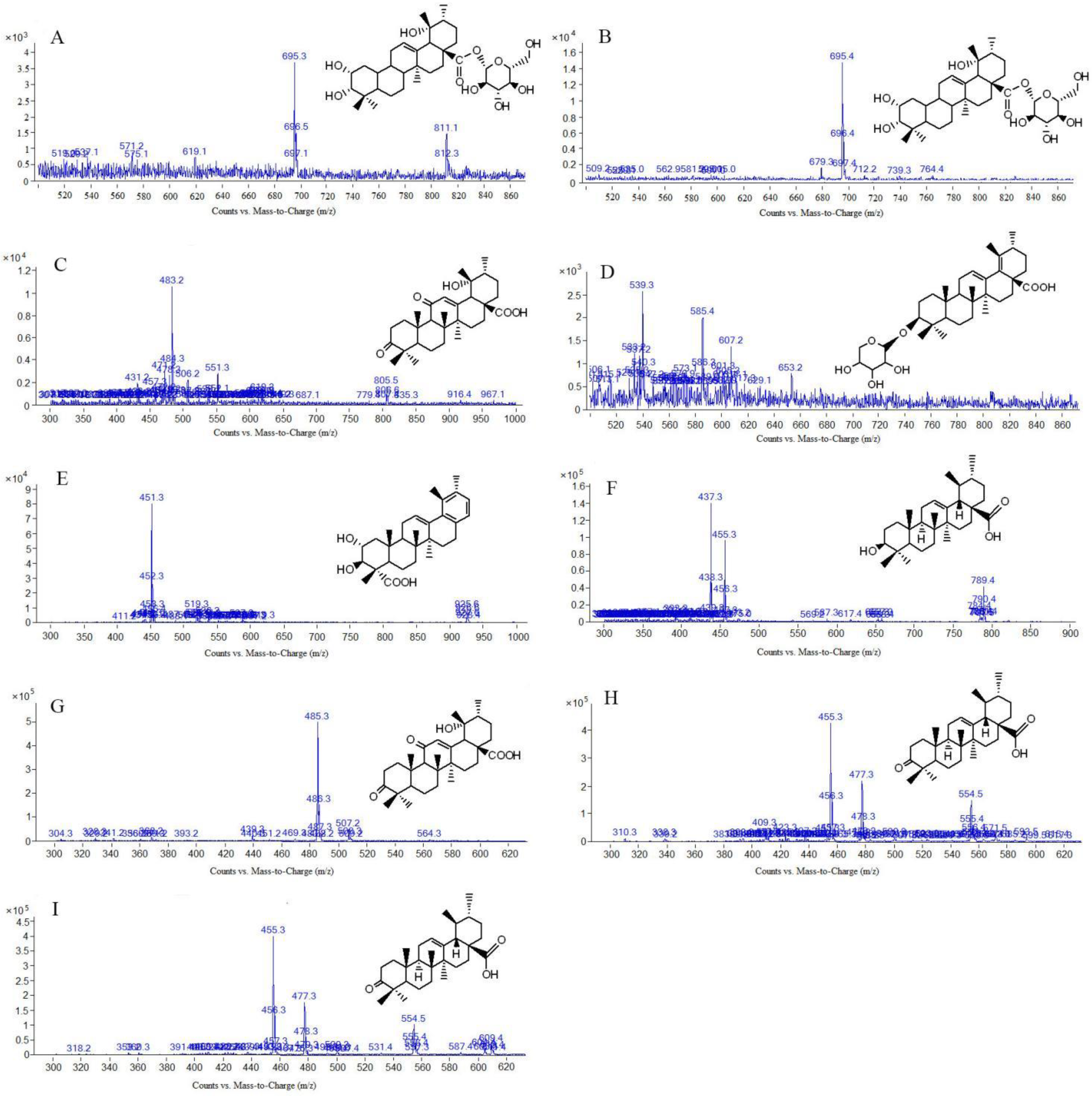



| Peak No | tR (min) | Molecular Weight | [M-H]− or [M+HCOOH-H]- | MS/MS m/z | Formula | Compound |
|---|---|---|---|---|---|---|
| 2 (N2) | 5.699 | 483.1 | 484.6 | 451.3; 338.1; 302.9; 264.1; 220.1 | C30H44O5 | 3,11-dioxo-19α-hydroxylurs-12-en-28-oic acid |
| 5 (N5) | 17.852 | 696.5 | 695.5 | 648.8; 487 | C36H58O10 | 2α,3β,19α-trihydroxyurs-12-en-28-acid-β-D-glucopyranosyl ester or isomer |
| 6 (N6) | 19.726 | 766.9 | 765.6 | 616.3; 603.5; 585.3; 471.3; 453.1 | C41H66O13 | 3-β[(α-L-arabinopyranosyl) oxy]-29-hydroxy olean-12-en-28-oic acid β-D-glucopyranosyl este |
| 7 * (N7) | 20.162 | 766.4 | 765.6; 811.3 | 764.9; 633.8; 616.3; 603.5; 585.3; 207.1; 471.3; 453.3 | C41H66O13 | Ziyuglycoside Ⅰ |
| 9 * (N9) | 25.852 | 748.4 | 793.4 | 657.1; 616.3; 584.6; 585.4; 453.1; 208.1; 190.1 | C41H64O12 | 3-β-O-α-L-arabinosylurs-12,18(19)-dien-28-acid-β-D-glucose ester |
| 10 * (N10) | 26.172 | 748.4 | 793.4 | 616.3; 585.3; 471.3; 453.1; 425.8; 377.3; 190.1 | C41H64O12 | 3-β-O-α-L-arabinosylurs-12,19(29)-dien-28-acid-β-D-glucose ester |
| 11 (N11) | 26.407 | 748.4 | 793.4 | 656.9; 616.3; 585.3; 541.3; 206.9 | C42H68O11 | 3β-O-α-L-arabinopyranosylurs-12,19-dien-28-β-D-glucopyranosyl ester |
| 12 * (N12) | 26.744 | 634.4 | 679.4 | 633.0; 541.3; 471.2; 453.1; 130.7 | C36H58O9 | 3β, l9α-dihydroxyursin-12-en-28-acid-β-D-glucose ester |
| 13 * (N13) | 29.599 | 504.3 | 503.4 | 485.5; 467.3; 443.2; 420.7; 313.0; 264.1; 220.1 | C30H48O6 | 1β-hydroxyrosic acid |
| 14 (N14) | 32.741 | 486.7 | 485.3 | 423.3; 407.4; 389.0; 373.3; 358.5; 264.1; 220.1; 137.8 | C30H46O5 | 2α,19α-dihydroxy-3-oxo-12-ursen-28-oic acid |
| 15 * (N15) | 35.229 | 488.3 | 487.3 | 469.5; 451.3; 406.9; 264.1; 220.1; 206.1 | C30H48O5 | Euscaphic acid |
| 16 (N16) | 35.745 | 488.7 | 487.4 | 469.2; 451.3; 425.3; 264.1 | C30H48O5 | 2α,3α,19α-trihydroxyurs-12-en-28-oic acid |
| 18 * (N18) | 37.955 | 604.5 | 603.5 | 585.3; 541.3; 471.3; 453.1; 130.7 | C35H56O8 | Ziyuglycoside II |
| 19 (N19) | 47.551 | 470.7 | 469.4 | 406,9; 264.1; 220.1; 206.1; 130.8; 108.2 | C31H50O3 | ursolic acid methyl ester |
| 20 (N20) | 51.040 | 484.5 | 483.3 | 443.0; 421.3; 355.5; 281.3; 253.4; 147.1; 133.5 | C30H44O5 | 2α,19α-dihydroxy-3-oxo-urs-11,13(18)-dien-28-oic acid |
| Peak No | tR (min) | Molecular Weight | [M+H] + | MS/MS m/z | Formula | Compound |
|---|---|---|---|---|---|---|
| 1 (P1) | 11.197 | 812.9 | 814.5 | 635.6; 604.6; 545.3; 472.4; 454.5; 428.2 | C42H68O15 | 3-O-β-D-glucopyranosyl-2α,19α-dihydroxyurs-12-en-28-oic acid β-D-glucopyranosyl ester |
| 2 (P2) | 13.452 | 470.3 | 471.3 | 453.2; 425.4; 410.9; 340.2; 379.3; 319.8; 238.1; 150.8; 136.6 | C30H46O4 | Pomeranic acid |
| 4 (P4) | 21.420 | 488.7 | 489.5 | 330.0; 314.9; 200.9; 145.1 | C30H48O5 | 1β,2α,3α,19α-tetrahydroxyurs-12-en-28-oic acid |
| 7 (P7) | 26.288 | 748.9 | 749.9 | 618.5; 560.5; 473.3; 454.5; 206.9; 130.1 | C42H68O11 | 3β-O-α-L-Arabinopyranosylusr-12,18-dien-28-β-D-glucopyranosyl ester |
| 8 (P8) | 26.435 | 748.9 | 749.9 | 618.6; 473.7; 454.6; 206.9; 189.1 | C42H68O11 | 3β-O-α-L-arabinopyranosylusr-12,19(29)-dien-28-β-D-glucopyranosyl ester |
| 9 (P9) | 27.032 | 454.5 | 455.3 | 437.4; 409.2; 201.8; 186.8; 179.1 | C30H46O3 | 3β-hydroxyurs-11,13(18)-dien-28-oic acid |
| 10 (P10) | 30.019 | 474.6 | 475.3 | 391.2; 286.9; 247.1; 230.7; 204.8; 191.2 | C29H46O5 | 2α,3α,19α-trihydroxyurs-12-en-28-oic-acid or isomer |
| 11 (P11) | 36.340 | 484.3 | 485.3 | 467.2; 439.5; 421.1; 403.2; 367.1; 331.2; 282.3; 251.0; 135.2 | C30H44O5 | 2α,19α-dihydroxy-3-oxo-urs-11,13(18)-dien-28-oic acid |
| Peak No | tR (min) | [M-H]− or [M+HCOOH-H]- | [M+H] + | MS/MS m/z | Formula | Compound |
|---|---|---|---|---|---|---|
| [1] (N1) | 6.235 | 633.1 | / | 603.5; 471.3; 453.1; 300.9; 274.7; 248.3 | C36H58O9 | 3β, l9α-dihydroxyursin-12-en-28-acid β-D-glucose ester |
| [3] (N3) | 11.231 | 695.4 | / | 649.4; 487.3; 471.3; 453.1; 425.3 | C36H58O10 | 2α,3α,19α-trihydroxyurs-12-en-28-acid-β-D-glucopyranosyl ester or isomer |
| [3] (P3) | 8.782 | / | 475.3 | 437.2; 409.3; 391.3; 201.8 | C29H46O5 | 2α,3α,19α-trihydroxyurs-12-en-28-oic-acid |
| Peak No | tR (min) | [M-H]− or [M+HCOOH-H]- | [M+H] + | MS/MS m/z | Formula | Compound |
|---|---|---|---|---|---|---|
| [11] (N11) | 16.670 | 695.3 | / | 649.4; 558.5; 487.3; 425.2; 303.0 | C36H58O10 | 2α,3,19-trihydroxyurs-12-en-28-acid-β-D-glucopyranosyl ester or isomer |
| [12] (N12) | 17.461 | 695.4 | / | 649.3; 648.9; 487.3; 475.1 | C36H58O10 | 2α,3,19-trihydroxyurs-12-en-28-acid-β-D-glucopyranosyl ester or isomer |
| [13] (N13) | 34.084 | 483.2 | / | 465.1; 421.1; 390.9; 224.9 | C30H44O5 | 3,11-dioxo-19α-hydroxy-urs-12-en-28-oic acid |
| [15] (N15) | 39.216 | 585.3 | / | 541.3; 471.3; 453.1; 348.7 | C35H54O7 | 3β-O-α-L-arabinopyranosylusr-12,18-dien-28-acid |
| [26] (N26) | 51.876 | 451.3 | / | 404.9; 363.6; 264.8; 252.3 | C29H40O4 | 2α,3β-dihydroxy-28-norurs-12,17,19(20),21-tetraen-23-oic acid |
| [4] (P4) | 19.403 | / | 458.3 | 436.8; 391.3; 373.3; 327.2; 276.5; 228.1; 214.9; 179.3; 153.0 | C30H48O3 | Ursolic acid |
| [7] (P7) | 33.765 | / | 485.3 | 439.5; 367.3; 340.9; 336.9; 329.1; 226.2; 201.2; 187.1; 174.6; 149.1; 109.0 | C30H44O5 | 3,11-dioxo-19α-hydroxy-urs-12-en-28-acid |
| [19] (P19) | 52.627 | / | 455.3 | 455.1; 440.2; 437.0; 305.0; 283.4; 267.4; 249.4; 201.3; 161.2; 147.2; 119.0; 107.1 | C30H46O3 | 3-oxo-12-en-28-ursolic acid |
| [20] (P20) | 53.923 | / | 455.3 | 455.1; 440.2; 391; 283.4; 267.4; 249.4; 201.3; 161.2; 147.2; 119.0; 107.1 | C30H46O3 | 3-oxo-12-en-28-ursolic acid or isomer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, C.; Wu, L.; Sun, J.; Wang, Z.; Wang, Z. Variation of Saponins in Sanguisorba officinalis L. before and after Processing (Paozhi) and Its Effects on Colon Cancer Cells In Vitro. Molecules 2022, 27, 9046. https://doi.org/10.3390/molecules27249046
Wang Z, Yang C, Wu L, Sun J, Wang Z, Wang Z. Variation of Saponins in Sanguisorba officinalis L. before and after Processing (Paozhi) and Its Effects on Colon Cancer Cells In Vitro. Molecules. 2022; 27(24):9046. https://doi.org/10.3390/molecules27249046
Chicago/Turabian StyleWang, Zhengyang, Chunjuan Yang, Lihong Wu, Jiahui Sun, Zhenyue Wang, and Zhibin Wang. 2022. "Variation of Saponins in Sanguisorba officinalis L. before and after Processing (Paozhi) and Its Effects on Colon Cancer Cells In Vitro" Molecules 27, no. 24: 9046. https://doi.org/10.3390/molecules27249046
APA StyleWang, Z., Yang, C., Wu, L., Sun, J., Wang, Z., & Wang, Z. (2022). Variation of Saponins in Sanguisorba officinalis L. before and after Processing (Paozhi) and Its Effects on Colon Cancer Cells In Vitro. Molecules, 27(24), 9046. https://doi.org/10.3390/molecules27249046






