Performance and Characterization of Bi-Metal Compound on Activated Carbon for Hydrogen Sulfide Removal in Biogas
Abstract
1. Introduction
2. Results and Discussion
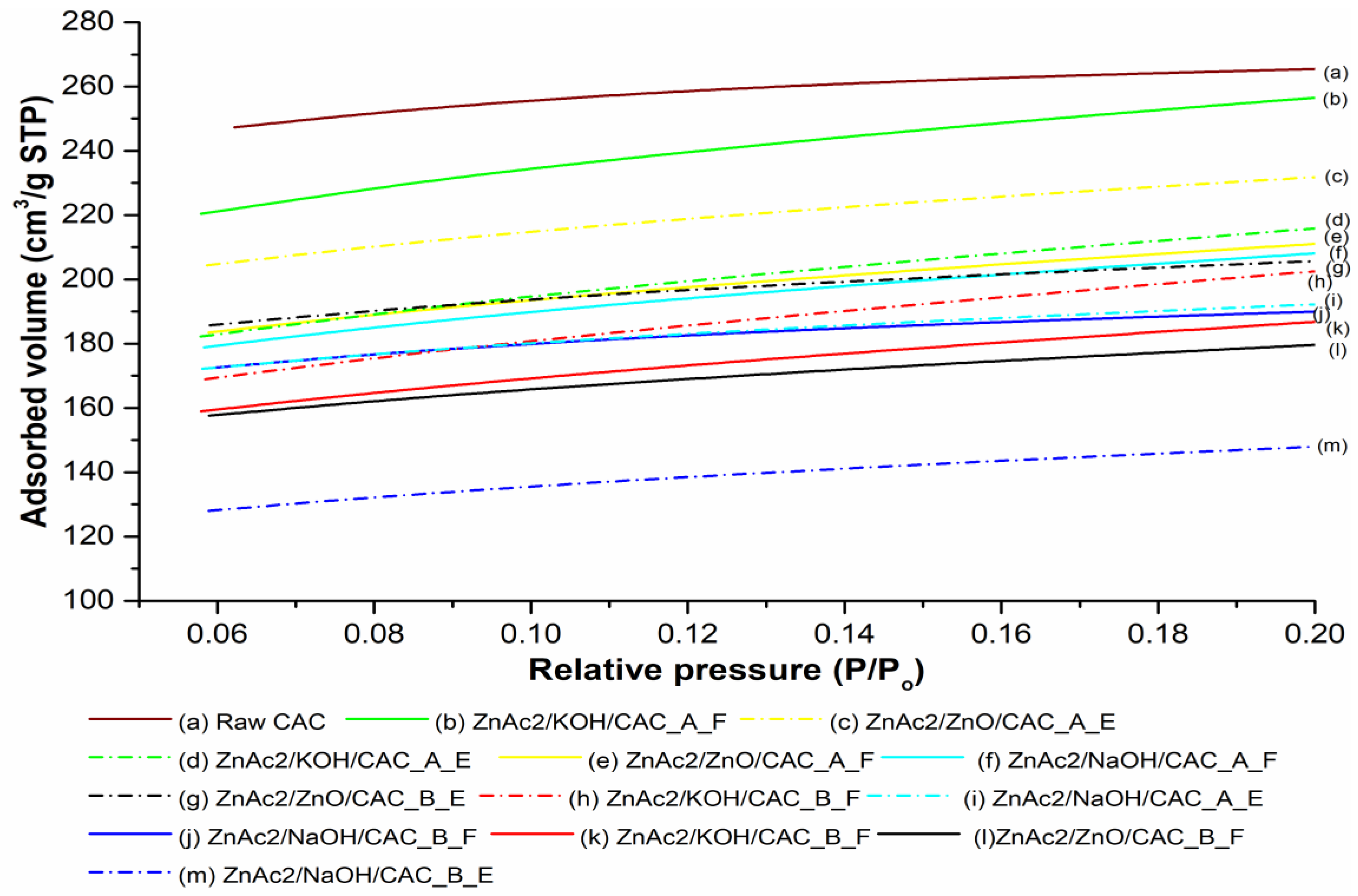
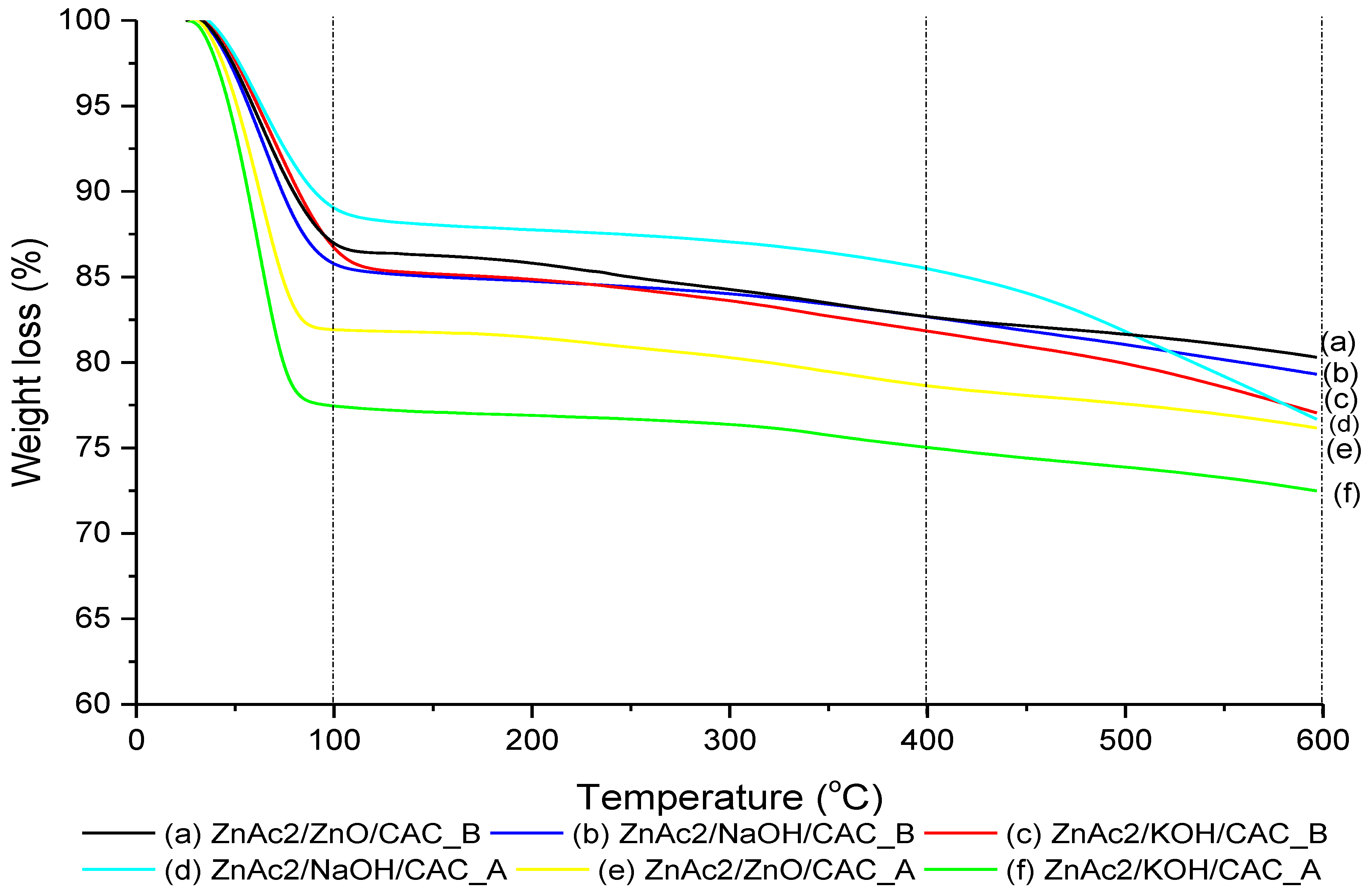
2.1. Surface Analysis of BMC Adsorbents
2.2. Performance of the H2S Adsorption-Desorption Test
2.3. Regeneration Adsorbent in H2S Adsorption–Desorption Test
3. Materials and Methods
3.1. Chemicals and Reagents
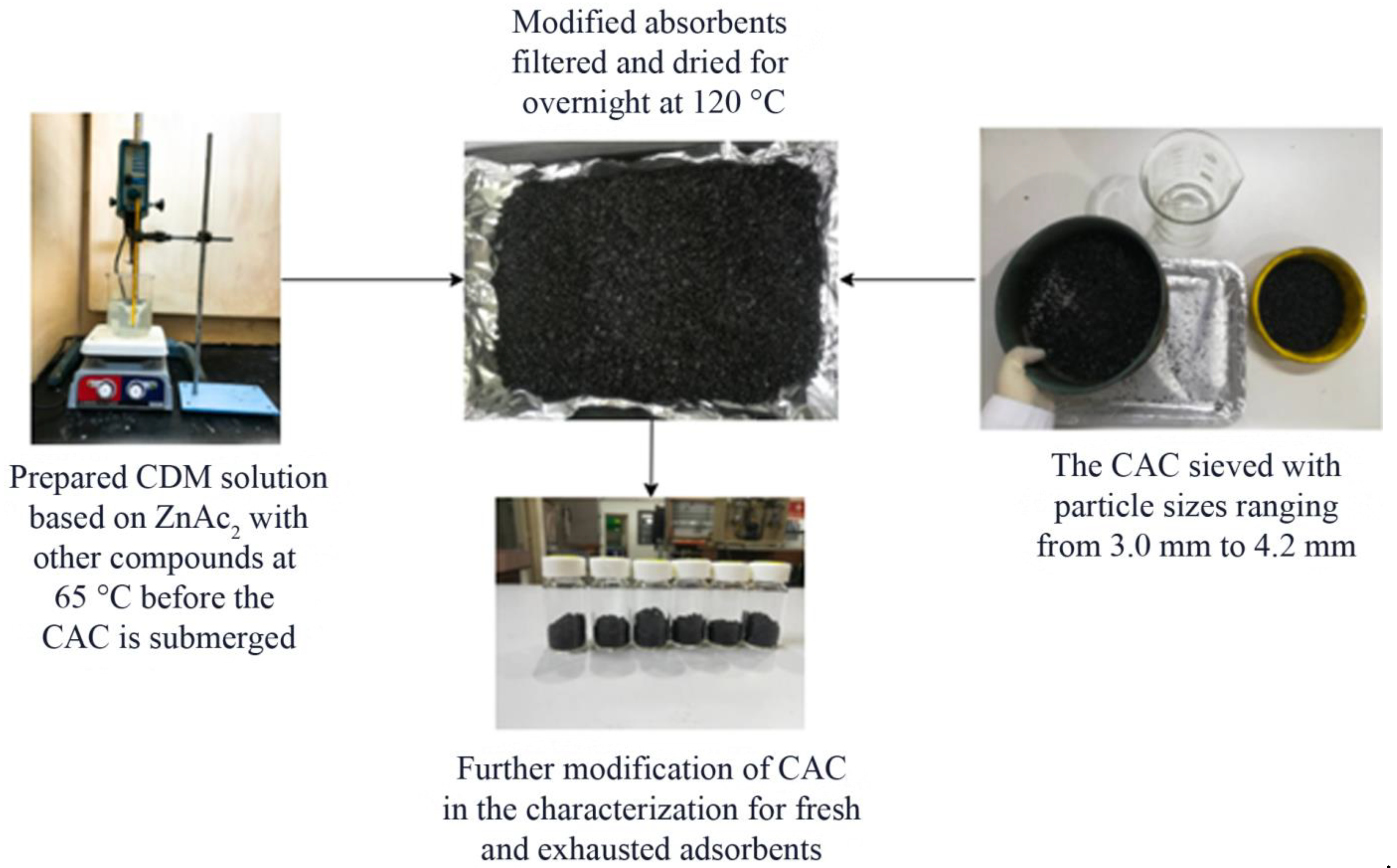
3.2. Preparation of Modified BMC/CAC
3.3. Characterization of Adsorbents
3.4. Real Test of H2S Adsorption–Desorption Process
3.5. Calculation of Adsorption Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Crisci, A.G.; Moniri, A.; Xu, Y. Hydrogen from hydrogen sulfide: Towards a more sustainable hydrogen economy. Int. J. Hydrog. Energy 2019, 44, 1299–1327. [Google Scholar] [CrossRef]
- Sublette, K.; Sylvester, N.D. Oxidation of hydrogen sulfide by Thiobacillus denitrificans: Desulfirization of natural gas. Biotechnol. Bioeng. 1987, 29, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/ chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Ramírez-Sáenz, D.; Zarate-Segura, P.B.; Guerrero-Barajas, C.; García-Peña, E.I. H2S and volatile fatty acids elimination by biofiltration: Clean-up process for biogas potential use. J. Hazard. Mater. 2009, 163, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. In Biomethanation II; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 82, pp. 1–33. [Google Scholar]
- Kegl, T.; Kralj, A.K. Multi-objective optimization of anaerobic digestion process using a gradient-based algorithm. Energy Convers. Manag. 2020, 226, 113560. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Anaerobic Biodegradation of Wheat Straw Lignin: The Influence of Wet Explosion Pretreatment. Energies 2021, 14, 5940. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, J.; Li, P.; Shen, P.; Wei, Y. Enhancement of methane production and antibiotic resistance genes reduction by ferrous chloride during anaerobic digestion of swine manure. Bioresour. Technol. 2020, 298, 122519. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Pradhan, A.K.; Pental, D. A highly contiguous genome assembly of Brassica nigra (BB) and revised nomenclature for the pseudochromosomes. BMC Genom. 2020, 21, 887. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Zhang, Y.; Banks, C.J. Antifoam, dilution and trace element addition as foaming control strategies in mesophilic anaerobic digestion of sugar beet pulp. Int. Biodeterior. Biodegrad. 2019, 145, 104812. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced anaerobic digestion of primary sludge with additives: Performance and mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef]
- Kegl, T. Consideration of biological and inorganic additives in upgraded anaerobic digestion BioModel. Bioresour. Technol. 2022, 355, 127252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; McDowell, R.G.; Martin, L.R.; Qiang, Y. Qiang. Selective extraction of heavy and light lanthanides from aqueous solution by advanced magnetic nanosorbents. Appl. Mater. Interfaces 2016, 8, 9523–9531. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Long, F.; Zhao, H.; Zhang, Y.; Liang, D.; Wang, L.; Lesnik, K.L.; Cao, H.; Zhang, Y.; Liu, H. Performance prediction of ZVI-based anaerobic digestion reactor using machine learning algorithms. Waste Manag. 2021, 121, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Khanal, S.K.; Li, Y. Bioenergy Principles and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2017; pp. 338–360. [Google Scholar]
- Zhang, Y.; Oshita, K.; Kusakabe, T.; Takaoka, M.; Kawasaki, Y.; Minami, D.; Tanaka, T. Simultaneous removal of siloxanes and H2S from biogas using an aerobic biotrickling filter. J. Hazard. Mater. 2020, 391, 122187. [Google Scholar] [CrossRef] [PubMed]
- Kiragosyan, K.; Picard, M.; Sorokin, D.Y.; Dijkstra, J.; Klok, J.; Roman, P.; Janssen, A.J.H. Effect of dimethyl disulfide on the sulfur formation and microbial community composition during the biological H2S removal from sour gas streams. J. Hazard. Mater. 2020, 386, 121916. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Wan Isahak, W.N.R.; Jahim, J.M.; Rejab, S.A.M.; Lye, C.C. Removal of hydrogen sulfide from a biogas mimic by using impregnated activated carbon adsorbent. PLoS ONE 2019, 14, e0211713. [Google Scholar] [CrossRef]
- Ajhar, M.; Travesset, M.; Yuce, S.; Melin, T. Siloxane removal from landfill and digester gas—A technology overview. Bioresour. Technol. 2010, 101, 2913–2923. [Google Scholar] [CrossRef]
- Yang, H.; Tatarchuk, B. Novel-doped zinc oxide sorbents for low temperature regenerable desulfurization applications. AIChE J. 2010, 56, 2898–2904. [Google Scholar] [CrossRef]
- Dhage, P.; Samokhvalov, A.; Repala, D.; Duin, C.E.; Bowman, M.; Tatarchuk, B.J. Copper-promoted ZnO/SiO2 regenerable sorbents for the room temperature removal of H2S from reformate gas streams. Ind. Eng. Chem. Res. 2010, 49, 8388–8396. [Google Scholar] [CrossRef]
- Yan, R.; Chin, T.; Ng, Y.L.; Duan, H.; Liang, D.T.; Tay, J.H. Influence of surface properties on the mechanism of H2S removal by alkaline activated carbons. Environ. Sci. Technol. 2004, 38, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thanh, D.; Bandosz, T.J. Activated carbons with metal containing bentonite binders as adsorbents of hydrogen sulphide. Carbon 2005, 43, 359–367. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J. On the mechanism of hydrogen sulfide removal from moist air on catalytic carbonaceous adsorbents. Ind. Eng. Chem. Res. 2005, 44, 530–538. [Google Scholar] [CrossRef]
- Feng, W.; Kwon, S.; Borguet, E.; Vidic, R. Adsorption of hydrogen sulfide onto activated carbon fibers: Effect of pore structure and surface chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749. [Google Scholar] [CrossRef] [PubMed]
- Cal, M.P.; Strickler, B.W.; Lizzio, A.A. High temperature hydrogen sulfide adsorption on activated carbon: I. Effects of gas composition and metal addition. Carbon 2000, 38, 1757–1765. [Google Scholar] [CrossRef]
- Siriwardane, I.W.; Udangawa, R.; de Silva, R.M.; Kumarasinghe, A.R.; Acres, R.G.; Hettiarachchi, A.; de Silva, K.M.N. Synthesis and characterization of nano magnesium oxide impregnated granular activated carbon composite for H2S removal applications. Mater. Des. 2017, 136, 127–136. [Google Scholar] [CrossRef]
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effect of KI and KOH impregnations over activated carbon on H2S adsorption performance at low and high temperatures. Sep. Sci. Technol. 2014, 49, 354–366. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Mathuray Veeran, L.S.; Noor Azam, A.M.I.; Masdar, M.S.; Wan Isahak, W.N.R. Effect of Bimetallic-Activated Carbon Impregnation on Adsorption– Desorption Performance for Hydrogen Sulfide (H2S) Capture. Materials 2022, 15, 5409. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Seladorai, R.; Masdar, M.S.; Mohd Sofian, N.; Wan Isahak, W.N.R. Core Shell Nanostructure: Impregnated Activated Carbon as Adsorbent for Hydrogen Sulfide Adsorption. Molecules 2022, 27, 1145. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Wan Isahak, W.N.R.; Abu Bakar, S.N.H.; Abu Hasan, H.; Mohd Sofian, N. Application of Response Surface Methodology for Preparation of ZnAC2/CAC Adsorbents for Hydrogen Sulfide (H2S) Capture. Catalysts 2021, 11, 545. [Google Scholar] [CrossRef]
- Rosso, I.; Galletti, C.; Bizzi, M.; Saracco, G.; Specchia, V. Zinc oxide sorbents for the removal of hydrogen sulfide from syngas. Ind. Eng. Chem. Res. 2003, 42, 1688–1697. [Google Scholar] [CrossRef]
- Garces, H.F.; Galindo, H.M.; Garces, L.J.; Hunt, J.; Morey, A.; Suib, S.L. Low temperature H2S dry-desulfurization with zinc-oxide. Microporous Mesoporous Mater. 2010, 127, 190–197. [Google Scholar] [CrossRef]
- Montes, D.; Tocuyo, E.; González, E.; Rodríguez, D.; Solano, R.; Atencio, R.; Moronta, A. Reactive H2S chemisorption on mesoporous silica molecular sieve supported CuO or ZnO. Microporous Mesoporous Mater 2013, 168, 111–120. [Google Scholar] [CrossRef]
- Elyassi, B.; Al Wahedi, Y.; Rajabbeigi, N.; Kumar, P.; Jeong, J.S.; Zhang, X.; Kumar, P.; Balasubramanian, V.V.; Katsiotis, M.S.; Mkhoyan, K.A.; et al. High-performance adsorbent for hydrogen sulfide removal. Microporous Mesoporous Mater 2014, 190, 152–155. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tayakout-Fayolle, M.; Geantet, C. Gas-phase removal of hydrogen sulfide using iron oxyhydroxide at low temperature: Measurement of breakthrough curve and modeling of sulfidation mechanism. Chem. Eng. J. 2015, 262, 702–709. [Google Scholar] [CrossRef]
- Girard, V.; Baudot, A.; Chiche, D.; Bazer-Bachi, D.; Bounie, C.; Geantet, C. Rational selection of single oxide sorbents for syngas desulfurization regenerable at reduced temperature: Thermochemical calculations and experimental study. Fuel 2014, 128, 220–230. [Google Scholar] [CrossRef]
- Kalidindi, S.; Vecha, M.; Kar, A.; Raychoudhury, T. Aluminium-cerium Double Metal Impregnated Activated Carbon: A Novel Composite for Flouride Removal from Aqueous Solution. Water Sci. Technol. Water Supply 2017, 17, 115–124. [Google Scholar] [CrossRef]
- Lau, L.C.; Nor, N.M.; Lee, K.T.; Mohamed, A.R. Hydrogen Sulfide Removal Using CeO2/NaOH/PSAC: Effect of Preparation Parameters. J. Environ. Chem. Eng. 2017, 6, 386–394. [Google Scholar] [CrossRef]
- Jiang, D.; Su, L.; Ma, L.; Yao, N.; Xu, X.; Tang, H.; Li, X. Cu–Zn–Al mixed metal oxides derived from hydroxycarbonate precursors for H2S removal at low temperature. Appl. Surf. Sci. 2010, 256, 3216–3223. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.; Chu, S. Effect of moisture on H2S adsorption by copper impregnated activated carbon. J. Hazard. Mater. 2006, 136, 866–873. [Google Scholar] [CrossRef]
- Hernández, S.P.; Chiappero, M.; Russo, N.; Fino, D. A novel ZnO-based adsorbent for biogas purification in H2 production systems. Chem. Eng. J. 2011, 176–177, 272–279. [Google Scholar]
- Sisani, E.; Cinti, G.; Discepoli, G.; Penchini, D.; Desideri, U.; Marmottini, F. Adsorptive removal of H2S in biogas conditions for high temperature fuel cell systems. Int. J. Hydrog. Energy 2014, 39, 21753–21766. [Google Scholar] [CrossRef]
- Phooratsamee, W.; Hussaro, K.; Teekasap, S.; Hirunlabh, J. Increasing adsorption of activated carbon from Palm Oil Shell for adsorb H2S from biogas production by impregnation. Am. J. Environ. Sci. 2014, 10, 431–445. [Google Scholar] [CrossRef]
- Isik-Gulsac, I. Investigation of impregnated activated carbon properties used in hydrogen sulfide fine removal. Braz. J. Chem. Eng. 2016, 33, 1021–1030. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Kenneth Sing, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Roge, B. Water vapour sorption isotherms and the caking of food powders. Food Chem. 2003, 82, 61–71. [Google Scholar] [CrossRef]
- Roque-Malherbe, R.M.A. Adsorption and Diffusion in Nanoporous Materials; CRC Press, Taylor & Francis Group: Boca Raton, Florida, USA, 2007. [Google Scholar]
- Ghazali, Z.; Hassan, N.H.; Yarmo, M.A.; Peng, T.L.; Othaman, R. Immobilization of Choline Chloride: Urea onto Mesoporous Silica for Carbon Dioxide Capture. Sains Malays. 2019, 48, 1025–1033. [Google Scholar] [CrossRef]
- Yusuf, N.Y.M.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Wu, S.Y.; Rejab, S.A.M.; Lye, C.C. Impregnated carboneionic liquid as innovative adsorbent for H2/CO2 separation from biohydrogen. Int. J. Hydrog. Energy 2019, 44, 3414–3424. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.R.; Zabaniotou, A.; Stavropoulos, G.; Sakellaropoulos, G.P. Enhanced mercury adsorption in activated carbons from biomass materials and waste tires. Fuel Process. Technol. 2007, 88, 749–758. [Google Scholar] [CrossRef]
- Bai, B.C.; Lee, C.W.; Lee, Y.S.; Im, J.S. Metal impregnate on activated carbon fiber for SO2 gas removal: Assessment of pore structure, Cu supporter, breakthrough, and bed utilization. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 73–79. [Google Scholar] [CrossRef]
- Fan, X.; Li, C.; Zeng, G.; Gao, Z.; Chen, L.; Zhang, W.; Gao, H. Removal of gas-phase element mercury by activated carbon fiber impregnated with CeO2. Energy Fuels 2010, 24, 4250–4254. [Google Scholar] [CrossRef]
- Sumathi, S.; Bhatia, K.; Lee, T.; Mohamed, A.R. Selection of best impregnated palm shell activated carbon (PSAC) for simultaneous removal of SO2 and NOx. J. Hazard. Mater. 2010, 176, 1093–1096. [Google Scholar] [CrossRef]
- Hidayu, A.R.; Muda, N. Preparation and Characterization of Impregnated Activated Carbon from Palm Kernel Shell and Coconut Shell for CO2 Capture. Procedia Eng. 2016, 148, 106–113. [Google Scholar] [CrossRef]
- Balsamo, M.; Cimino, S.; de Falco, G.; Erto, A.; Lisi, L. ZnO-CuO supported on activated carbon for H2S removal at room temperature. Chem. Eng. J. 2016, 304, 399–407. [Google Scholar] [CrossRef]
- Farzad, S.; Taghikhan, V.; Ghotbi, C.; Aminshahidi, B.; Nemati, N.L. Experimental and theoretical study of the effect of moisture on methane adsorption and desorption by activated carbon at 273.3K. J. Nat. Gas Chem. 2008, 16, 22–30. [Google Scholar] [CrossRef]
- Sidek, M.Z.; Masdar, M.S.; Nik Dir, N.M.H.; Amran, N.F.A.; Ajit Sing, S.K.D.; Wong, W.L. Integrasi Sistem Penulenan Biohidrogen dan Aplikasi Sel Fuel. J. Kejuruter. 2018, 1, 41–48. [Google Scholar]
- Papurello, D.; Lanzini, A. SOFC single cells fed by biogas: Experimental tests with trace contaminants. Waste Manag. 2018, 72, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sidek, M.Z.; Jun, C.Y.; Zulkefli, N.N.; Yusuf, N.Y.M.; Isahak, W.N.R.W.; Sitanggang, R.; Masdar, M.S. Effect of Impregnated Activated Carbon on Carbon Dioxide Adsorption Performance for Biohydrogen Purification. Mater. Res. Express 2019, 6, 015510. [Google Scholar] [CrossRef]




| Adsorbents | C | Ca | Zn | O | K | Na | S | |
|---|---|---|---|---|---|---|---|---|
| ZnAc2/ZnO/CAC_A | Fresh | 56.1 | 0.3 | 29.1 | 24.5 | 0.0 | 0.0 | 0.0 |
| Exhausted | 68.3 | 0.3 | 11.2 | 17.0 | 0.0 | 0.0 | 3.23 | |
| ZnAc2/ZnO/CAC_B | Fresh | 28.1 | 0.4 | 45.5 | 26.1 | 0.00 | 0.0 | 0.0 |
| Exhausted | 64.3 | 0.3 | 10.9 | 21.8 | 0.0 | 0.0 | 2.7 | |
| ZnAc2/KOH/CAC_A | Fresh | 45.3 | 0.3 | 22.7 | 30.7 | 1.0 | 0.0 | 0.0 |
| Exhausted | 49.8 | 1.8 | 11.1 | 26.9 | 7.3 | 0.0 | 3.2 | |
| ZnAc2/KOH/CAC_B | Fresh | 29.0 | 0.5 | 36.6 | 31.0 | 2.9 | 0.0 | 0.0 |
| Exhausted | 53.2 | 0.8 | 19.6 | 21.0 | 3.8 | 0.0 | 0.8 | |
| ZnAc2/NaOH/CAC_A | Fresh | 35.4 | 0.6 | 11.6 | 40.2 | 0.0 | 12.1 | 0.0 |
| Exhausted | 63.8 | 0.5 | 5.2 | 27.2 | 0.0 | 2.0 | 1.3 | |
| ZnAc2/NaOH/CAC_B | Fresh | 10.4 | 0.5 | 27.7 | 44.5 | 0.0 | 17.0 | 0.0 |
| Exhausted | 42.4 | 0.3 | 15.6 | 38.8 | 0.0 | 2.7 | 0.3 |
| Adsorbents | BET Surface Area (m2/g) | Average Pore Size (Å) | Pore Volume (cm3/g) | |
|---|---|---|---|---|
| ZnAc2/ZnO/CAC_A | Fresh | 732 | 26.6 | 0.3 |
| Exhausted | 797 | 25.4 | 0.5 | |
| ZnAc2/ZnO/CAC_B | Fresh | 624 | 25.6 | 0.2 |
| Exhausted | 702 | 23.0 | 0.4 | |
| ZnAc2/KOH/CAC_A | Fresh | 890 | 26.5 | 0.3 |
| Exhausted | 753 | 28.4 | 0.5 | |
| ZnAc2/KOH/CAC_B | Fresh | 649 | 27.7 | 0.3 |
| Exhausted | 710 | 30.0 | 0.5 | |
| ZnAc2/NaOH/CAC_A | Fresh | 722 | 26.7 | 0.3 |
| Exhausted | 657 | 23.8 | 0.4 | |
| ZnAc2/NaOH/CAC_B | Fresh | 659 | 21.8 | 0.1 |
| Exhausted | 512 | 26.0 | 0.3 |
| Adsorbents | Breakthrough Time, Tb (min) | Adsorption Capacity, Q (mg H2S/g) |
|---|---|---|
| ZnAc2/ZnO/CAC_A | 182 | 1.33 |
| ZnAc2/ZnO/CAC_B | 276 | 2.01 |
| ZnAc2/KOH/CAC_A | 237 | 1.73 |
| ZnAc2/KOH/CAC_B | 155 | 1.13 |
| ZnAc2/NaOH/CAC_A | 113 | 0.82 |
| ZnAc2/NaOH/CAC_B | 139 | 1.01 |
| ZnAc2/CAC | 68 | 0.37 |
| Raw CAC | 28 | 0.15 |
| Number of Cycles | Breakthrough Time, Tb (min) | Adsorption Capacity, Q (mg H2S/g) | Degradation, % |
|---|---|---|---|
| 1 | 276 | 1.49 | 0 |
| 2 | 276 | 1.49 | 0 |
| 3 | 276 | 1.49 | 0 |
| 4 | 274 | 1.48 | 0.67 |
| 5 | 273 | 1.47 | 1.34 |
| Adsorbents | Ratio |
|---|---|
| ZnAc2/ZnO/CAC_A | 1:1 |
| ZnAc2/ZnO/CAC_B | 2:1 |
| ZnAc2/KOH/CAC_A | 1:1 |
| ZnAc2/KOH/CAC_B | 2:1 |
| ZnAc2/NaOH/CAC_A | 1:1 |
| ZnAc2/NaOH/CAC_B | 2:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkefli, N.N.; Noor Azam, A.M.I.; Masdar, M.S.; Baharuddin, N.A.; Wan Isahak, W.N.R.; Mohd Sofian, N. Performance and Characterization of Bi-Metal Compound on Activated Carbon for Hydrogen Sulfide Removal in Biogas. Molecules 2022, 27, 9024. https://doi.org/10.3390/molecules27249024
Zulkefli NN, Noor Azam AMI, Masdar MS, Baharuddin NA, Wan Isahak WNR, Mohd Sofian N. Performance and Characterization of Bi-Metal Compound on Activated Carbon for Hydrogen Sulfide Removal in Biogas. Molecules. 2022; 27(24):9024. https://doi.org/10.3390/molecules27249024
Chicago/Turabian StyleZulkefli, Nurul Noramelya, Adam Mohd Izhan Noor Azam, Mohd Shahbudin Masdar, Nurul Akidah Baharuddin, Wan Nor Roslam Wan Isahak, and Nabilah Mohd Sofian. 2022. "Performance and Characterization of Bi-Metal Compound on Activated Carbon for Hydrogen Sulfide Removal in Biogas" Molecules 27, no. 24: 9024. https://doi.org/10.3390/molecules27249024
APA StyleZulkefli, N. N., Noor Azam, A. M. I., Masdar, M. S., Baharuddin, N. A., Wan Isahak, W. N. R., & Mohd Sofian, N. (2022). Performance and Characterization of Bi-Metal Compound on Activated Carbon for Hydrogen Sulfide Removal in Biogas. Molecules, 27(24), 9024. https://doi.org/10.3390/molecules27249024









