Computational Quantification of the Zwitterionic/Quinoid Ratio of Phenolate Dyes for Their Solvatochromic Prediction
Abstract
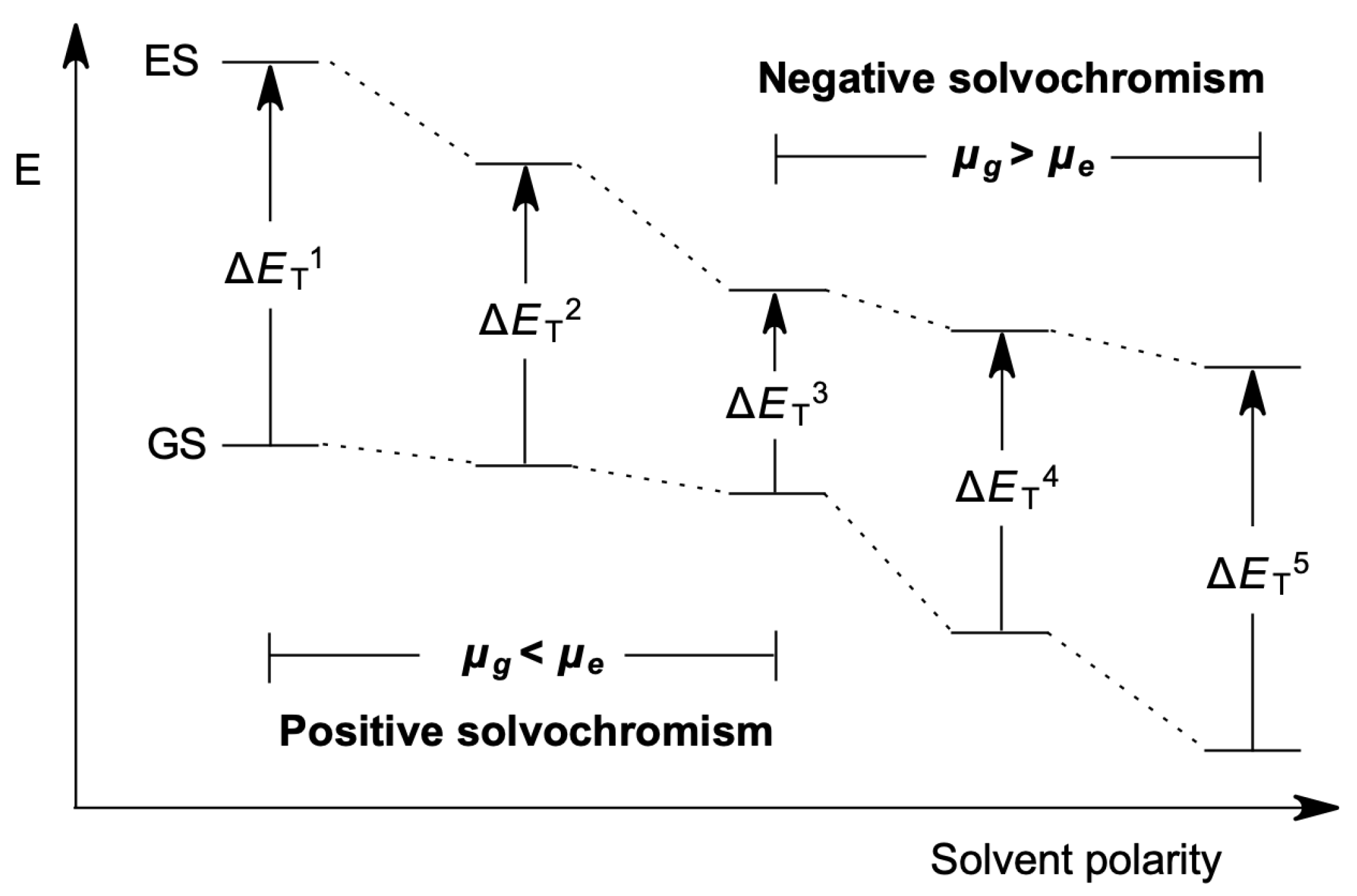
1. Introduction
2. Results and Discussions
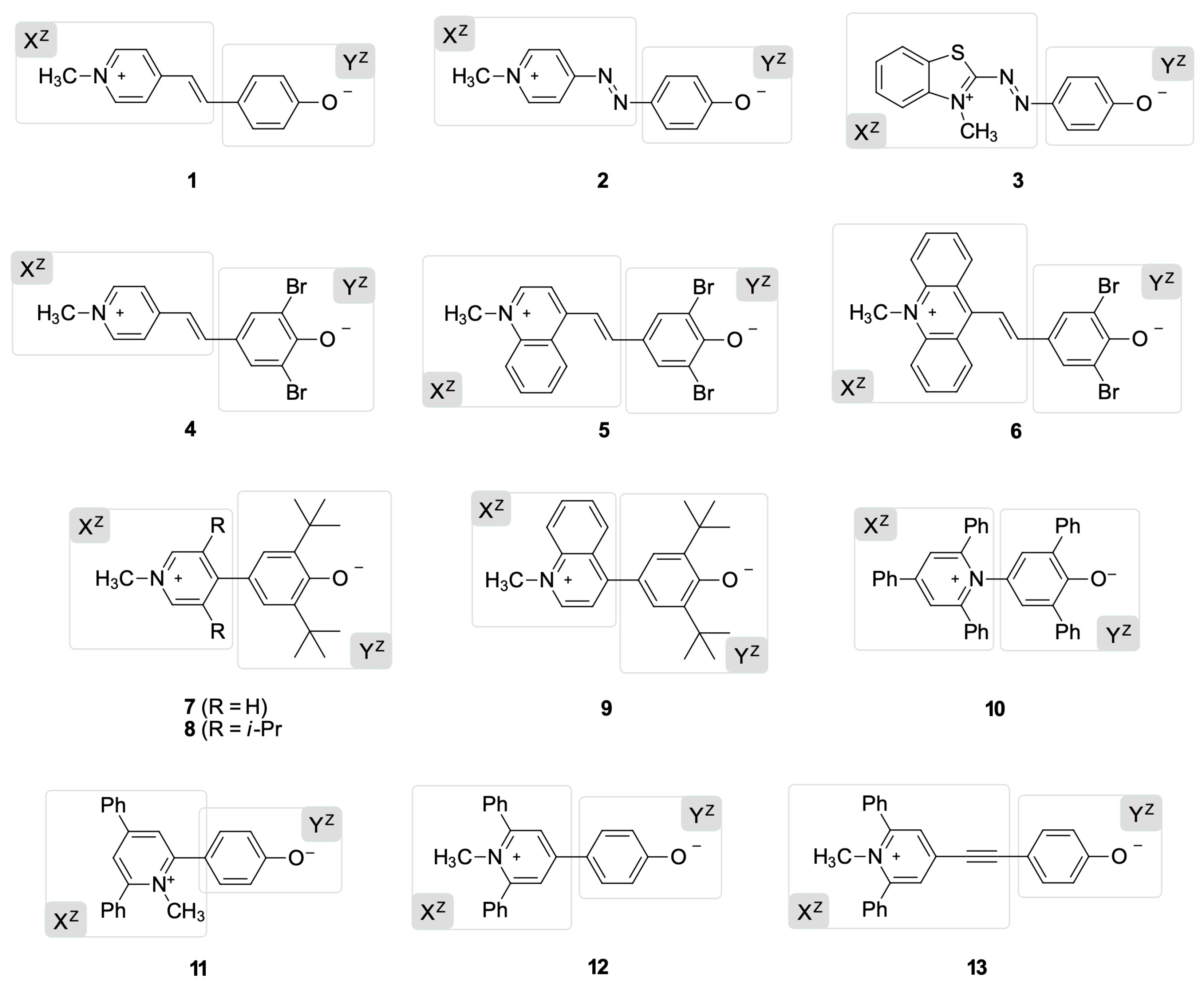
2.1. The Library Employed in This Work
2.2. The BLA and BOA Indices for Solvatochromic Tendency Predictions
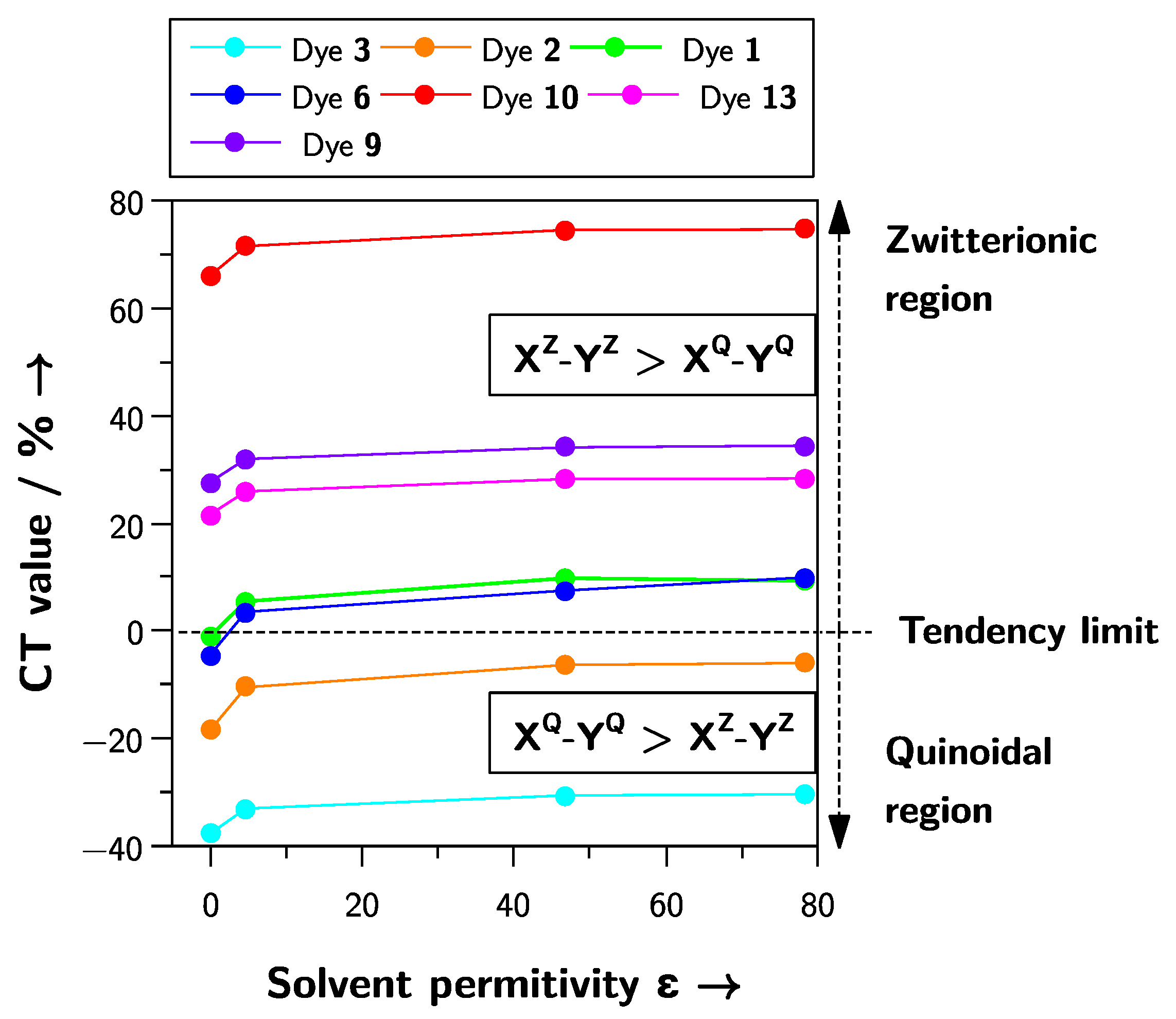
2.3. Prediction of the Solvatochromic Tendencies by the Natural Population Analysis (NPA) of the Natural Bond Orbitals (NBO)
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nandi, L.G.; Facin, F.; Marini, V.G.; Zimmermann, L.M.; Giusti, L.A.; da Silva, R.; Caramori, G.F.; Machado, V.G. Nitro-Substituted 4-[(Phenylmethylene)Imino]Phenolates: Solvatochromism and Their Use as Solvatochromic Switches and as Probes for the Investigation of Preferential Solvation in Solvent Mixtures. J. Org. Chem. 2012, 77, 10668–10679. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Vologdin, N.; Achelle, S.; Barsella, A.; Caro, B.; Robin-le Guen, F. Methylenepyran Based Dipolar and Quadrupolar Dyes: Synthesis, Electrochemical and Photochemical Properties. Tetrahedron 2013, 69, 8392–8399. [Google Scholar] [CrossRef]
- Sato, B.M.; Martins, C.T.; El Seoud, O.A. Solvation in Aqueous Binary Mixtures: Consequences of the Hydrophobic Character of the Ionic Liquids and the Solvatochromic Probes. New J. Chem. 2012, 36, 2353. [Google Scholar] [CrossRef]
- Mati, S.S.; Sarkar, S.; Sarkar, P.; Bhattacharya, S.C. Explicit Spectral Response of the Geometrical Isomers of a Bio-Active Pyrazoline Derivative Encapsulated in β-Cyclodextrin Nanocavity: A Photophysical and Quantum Chemical Analysis. J. Phys. Chem. A 2012, 116, 10371–10382. [Google Scholar] [CrossRef] [PubMed]
- Kanski, R.; Murray, C.J. Enzymichromism: Determination of the Dielectric Properties of an Enzyme Active Site. Tetrahedron Lett. 1993, 34, 2263–2266. [Google Scholar] [CrossRef]
- Farafonov, V.S.; Lebed, A.V.; Mchedlov-Petrossyan, N.O. Character of Localization and Microenvironment of Solvatochromic Reichardt’s Betaine Dye in Sodium n-Dodecyl Sulfate and Cetyltrimethylammonium Bromide Micelles: Molecular Dynamics Simulation Study. Langmuir 2017, 33, 8342–8352. [Google Scholar] [CrossRef]
- Khristenko, I.V.; Panteleimonov, A.V.; Iliashenko, R.Y.; Doroshenko, A.O.; Ivanov, V.V.; Tkachenko, O.S.; Benvenutti, E.V.; Kholin, Y.V. Heterogeneous Polarity and Surface Acidity of Silica-Organic Materials with Fixed 1-n-Propyl-3-Methylimidazolium Chloride as Probed by Solvatochromic and Fluorescent Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 280–286. [Google Scholar] [CrossRef]
- Le Bahers, T.; Pauporté, T.; Lainé, P.P.; Labat, F.; Adamo, C.; Ciofini, I. Modeling Dye-Sensitized Solar Cells: From Theory to Experiment. J. Phys. Chem. Lett. 2013, 4, 1044–1050. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Machado, V.G.; Stock, R.I.; Reichardt, C. Pyridinium N-Phenolate Betaine Dyes. Chem. Rev. 2014, 114, 10429–10475. [Google Scholar] [CrossRef]
- Lantzke, I.R.; Irish, D.E.; Gough, T.E. Spectroscopic Measurements. In Physical Chemistry of Organic Solvent Systems; Springer: Boston, MA, USA, 1973; pp. 405–523. ISBN 9781468419610. [Google Scholar]
- Bayliss, N.S.; McRae, E.G. Solvent Effects in Organic Spectra: Dipole Forces and the Franck–Condon Principle. J. Phys. Chem. 1954, 58, 1002–1006. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvent Effects on the Absorption Spectra of Organic Compounds. In Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 359–424. ISBN 9783527632220. [Google Scholar]
- Botrel, A.; le Beuze, A.; Jacques, P.; Strub, H. Solvatochromism of a Typical Merocyanine Dye. A Theoretical Investigation through the CNDO/SCI Method Including Solvation. J. Chem. Soc. 1984, 80, 1235. [Google Scholar] [CrossRef]
- Morley, J.O.; Morley, R.M.; Docherty, R.; Charlton, M.H. Fundamental Studies on Brooker’s Merocyanine. J. Am. Chem. Soc. 1997, 119, 10192–10202. [Google Scholar] [CrossRef]
- Baraldi, I.; Brancolini, G.; Momicchioli, F.; Ponterini, G.; Vanossi, D. Solvent Influence on Absorption and Fluorescence Spectra of Merocyanine Dyes: A Theoretical and Experimental Study. Chem. Phys. 2003, 288, 309–325. [Google Scholar] [CrossRef]
- Han, W.-G.; Liu, T.; Himo, F.; Toutchkine, A.; Bashford, D.; Hahn, K.M.; Noodleman, L. A Theoretical Study of the UV/Visible Absorption and Emission Solvatochromic Properties of Solvent-Sensitive Dyes. Chemphyschem 2003, 4, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Archetti, G.; Schmidt, R.; Kuball, H.-G. Solvent Effect on Color, Band Shape, and Charge-Density Distribution for Merocyanine Dyes Close to the Cyanine Limit. Angew. Chem. Int. Ed. Engl. 2008, 47, 4529–4532. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Ishchenko, A.A. Merocyanine Dyes: Synthesis, Structure, Properties and Applications. Russ. Chem. Rev. 2009, 78, 141–164. [Google Scholar] [CrossRef]
- Benson, H.G.; Murrell, J.N. Some Studies of Benzenoid-Quinonoid Resonance. Part 2—The Effect of Solvent Polarity on the Structure and Properties of Merocyanine Dyes. J. Chem. Soc. 1972, 68, 137–143. [Google Scholar] [CrossRef]
- Jacques, P. On the Relative Contributions of Nonspecific and Specific Interactions to the Unusual Solvtochromism of a Typical Merocyanine Dye. J. Phys. Chem. 1986, 90, 5535–5539. [Google Scholar] [CrossRef]
- Domínguez, M.; Rezende, M.C. Towards a Unified View of the Solvatochromism of Phenolate Betaine Dyes. J. Phys. Org. Chem. 2010, 23, 156–170. [Google Scholar] [CrossRef]
- Rezende, M.C.; Dominguez, M.; Aracena, A.; Millán, D. Solvatochromism and Electrophilicity. Chem. Phys. Lett. 2011, 514, 267–273. [Google Scholar] [CrossRef]
- Rezende, M.C.; Aracena, A. Electrophilicity and Solvatochromic Reversal of Pyridinium Phenolate Betaine Dyes. Chem. Phys. Lett. 2012, 542, 147–152. [Google Scholar] [CrossRef]
- Rezende, M.C.; Aracena, A. A General Framework for the Solvatochromism of Pyridinium Phenolate Betaine Dyes. Chem. Phys. Lett. 2013, 558, 77–81. [Google Scholar] [CrossRef]
- Manzoni, V.; Coutinho, K.; Canuto, S. An Insightful Approach for Understanding Solvatochromic Reversal. Chem. Phys. Lett. 2016, 655–656, 30–34. [Google Scholar] [CrossRef]
- Franco, L.R.; Brandão, I.; Fonseca, T.L.; Georg, H.C. Elucidating the Structure of Merocyanine Dyes with the ASEC-FEG Method. Phenol Blue in Solution. J. Chem. Phys. 2016, 145, 194301. [Google Scholar] [CrossRef] [PubMed]
- Gieseking, R.L.; Risko, C.; Brédas, J.-L. Distinguishing the Effects of Bond-Length Alternation versus Bond-Order Alternation on the Nonlinear Optical Properties of π-Conjugated Chromophores. J. Phys. Chem. Lett. 2015, 6, 2158–2162. [Google Scholar] [CrossRef]
- Rajagopal, S.; Buncel, E. Synthesis and Electronic Spectral Characteristics of Some New Azo Merocyanine Dyes. Dyes Pigment. 1991, 17, 303–321. [Google Scholar] [CrossRef]
- Martins, C.T.; El Seoud, O.A. Thermo-Solvatochromism of Merocyanine Polarity Probes—What Are the Consequences of Increasing Probe Lipophilicity through Annelation? Eur. J. Org. Chem. 2008, 2008, 1165–1180. [Google Scholar] [CrossRef]
- Chaumeil, H.; Neuburger, M.; Jacques, P.; Tschamber, T.; Diemer, V.; Carré, C. Conformational Analysis of Some Pyridinium Phenolates and Synthetic Precursors Based on X-Ray and IR Characterisations. Tetrahedron 2014, 70, 3116–3122. [Google Scholar] [CrossRef][Green Version]
- Runser, C.; Fort, A.; Barzoukas, M.; Combellas, C.; Suba, C.; Thiébault, A.; Graff, R.; Kintzinger, J.P. Solvent Effect on the Intramolecular Charge Transfer of Zwitterions. Structures and Quadratic Hyperpolarizabilities. Chem. Phys. 1995, 193, 309–319. [Google Scholar] [CrossRef]
- Aliaga, C.; Galdames, J.S.; Rezende, M.C. On the Solvatochromic Reversal of Merocyanine Dyes. Part 2.1 An Experimental and Semi-Empirical Study of the Solvatochromism of α- and γ-Vinylogous Pyridones. J. Chem. Soc. Perkin Trans. 2 1997, 1055–1058. [Google Scholar] [CrossRef]
- Reissig, H.-U.; Domínguez, M. N -Methylpyridinium-4-Phenolates: Generation of a Betaine Dye Library Bearing Different Spacer Units and Their Solvatochromism. ChemistrySelect 2016, 1, 5270–5275. [Google Scholar] [CrossRef]
- Jung, C.; Ristau, O.; Jung, C. An INDO-CI Method in π-Approximation for the Calculation of Transition Metal Complexes with Organic Ligands?Application to Iron(II)-Trisdiimine Complexes. Theoret. Chim. Acta 1983, 63, 143–159. [Google Scholar] [CrossRef]
- Wolcan, E. On the Origins of the Absorption Spectroscopy of Pterin and Re(CO)3(Pterin)(H2O) Aqueous Solutions. A Combined Theoretical and Experimental Study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.C. A Generalized Reversal Model for the Solvatochromism of Merocyanines. J. Phys. Org. Chem. 2016, 29, 460–467. [Google Scholar] [CrossRef]
- Mera-Adasme, R.; Moraga, D.; Medina, R.; Domínguez, M. Mapping the Solute-Solvent Interactions for the Interpretation of the Three Types of Solvatochromism Exhibited by Phenolate-Based Dyes. J. Mol. Liq. 2022, 359, 119302. [Google Scholar] [CrossRef]
- Jacques, P.; Graff, B.; Diemer, V.; Ay, E.; Chaumeil, H.; Carré, C.; Malval, J.-P. Negative Solvatochromism of a Series of Pyridinium Phenolate Betaine Dyes with Increasing Steric Hindrance. Chem. Phys. Lett. 2012, 531, 242–246. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian∼09 Revision E.01; Gaussian Inc.: Wallingford, UK.
- Gorelsky, S.I.; Lever, A.B.P. Electronic Structure and Spectra of Ruthenium Diimine Complexes by Density Functional Theory and INDO/S. Comparison of the Two Methods. J. Organomet. Chem. 2001, 635, 187–196. [Google Scholar] [CrossRef]
- Mera-Adasme, R.; Rezende, M.C.; Domínguez, M. On the Physical-Chemical Nature of Solvent Polarizability and Dipolarity. Spectrochim. Acta. Part A 2020, 229, 118008. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kawajiri, I.; Ueki, M.; Morimoto, T.; Nishida, J.-I.; Ikeda, H.; Tanaka, M.; Kawase, T. A New Fluorophore Displaying Remarkable Solvatofluorochromism and Solid-State Light Emission, and Serving as a Turn-on Fluorescent Sensor for Cyanide Ions. Org. Chem. Front. 2017, 4, 743–749. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, J.; Vidal, M.; Pastenes, C.; Aliaga, C.; Rezende, M.C.; Domínguez, M. The Solvatofluorochromism of 2,4,6-Triarylpyrimidine Derivatives. Photochem. Photobiol. 2018, 94, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Vidal, M.; Pastenes, C.; Rezende, M.C.; Domínguez, M. Solvatofluorochromism of Conjugated 4-Methoxyphenyl-Pyridinium Electron Donor-Acceptor Pairs. Dyes Pigment. 2019, 166, 395–402. [Google Scholar] [CrossRef]
- Rathod, P.V.; Puguan, J.M.C.; Kim, H. Multi-stimuli Responsive Thiazolothiazole Viologen-containing Poly(2-isopropyl-2-oxazoline) and Its Multi-modal Thermochromism, Photochromism, Electrochromism, and Solvatofluorochromism Applications. Adv. Mater. Interfaces 2022, 2201227. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, X.; Zhang, L.; Eubank, J.F.; Liu, X.; Liu, Y. Unique Fluorescence Turn-on and Turn-off-on Responses to Acids by a Carbazole-Based Metal-Organic Framework and Theoretical Studies. J. Am. Chem. Soc. 2022, 144, 17054–17063. [Google Scholar] [CrossRef]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines Using a Thiadiazole-Functionalized Zr(IV)-Based Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef]

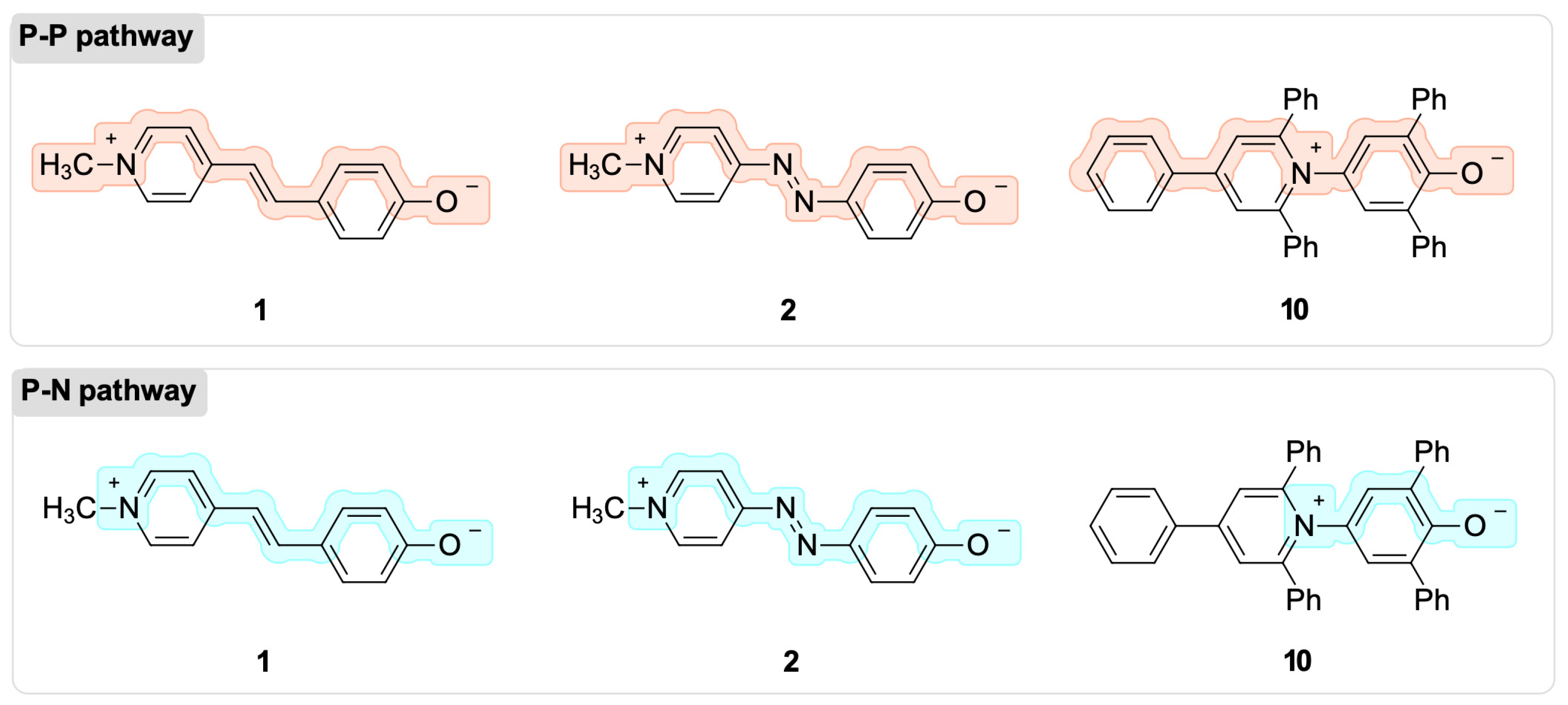

| BLA Index | ||||||
| Dye | Solvatochromism | Pathway | Gas-Phase | CHCl3 | Me2SO | H2O |
| 1 | Inverted | P–P | 0.064 | 0.044 | 0.032 | 0.034 |
| P–N | 0.083 | −0.062 | −0.049 | −0.051 | ||
| 2 | Positive | P–P | 0.074 | 0.058 | 0.049 | 0.049 |
| P–N | −0.098 | −0.082 | −0.072 | −0.072 | ||
| 10 | Negative | P–P | −0.027 | −0.017 | −0.013 | −0.013 |
| P–N | 0.088 | 0.069 | 0.062 | 0.062 | ||
| BOA Index | ||||||
| Dye | Solvatochromism | Pathway | Gas-Phase | CHCl3 | Me2SO | H2O |
| 1 | Inverted | P–P | −0.302 | −0.266 | −0.240 | −0.244 |
| P–N | −0.408 | 0.369 | 0.341 | 0.345 | ||
| 2 | Positive | P–P | −0.317 | −0.290 | −0.273 | −0.272 |
| P–N | 0.421 | 0.391 | 0.373 | 0.372 | ||
| 10 | Negative | P–P | 0.051 | 0.033 | 0.025 | 0.025 |
| P–N | −0.144 | −0.112 | −0.097 | −0.096 | ||
| Dye | Solvatochromism | Gas-Phase | CHCl3 | Me2SO | H2O |
|---|---|---|---|---|---|
| 1 | Inverted | −1.0 | 5.5 | 9.8 | 9.3 |
| 2 | Positive | −18.5 | −10.5 | −6.3 | −6.0 |
| 3 | Positive | −37.7 | −33.0 | −30.6 | −30.4 |
| 4 | Negative | 4.9 | 13.2 | 17.4 | 17.7 |
| 5 | Negative 1 | 6.3 | 15.5 | 21.0 | 21.3 |
| 6 | Inverted | −4.5 | 3.5 | 7.5 | 9.9 |
| 7 | Negative 1 | 38.6 | 44.2 | 47.3 | 47.5 |
| 8 | Negative | 41.7 | 48.2 | 52.5 | 52.8 |
| 9 | Inverted | 27.6 | 31.9 | 34.1 | 34.4 |
| 10 | Negative | 66.0 | 71.5 | 74.5 | 74.6 |
| 11 | Negative 1 | 51.4 | 57.0 | 59.7 | 59.8 |
| 12 | Negative 1 | 33.4 | 37.1 | 38.7 | 38.8 |
| 13 | Negative 1 | 21.6 | 25.9 | 28.2 | 28.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aracena, A.; Domínguez, M. Computational Quantification of the Zwitterionic/Quinoid Ratio of Phenolate Dyes for Their Solvatochromic Prediction. Molecules 2022, 27, 9023. https://doi.org/10.3390/molecules27249023
Aracena A, Domínguez M. Computational Quantification of the Zwitterionic/Quinoid Ratio of Phenolate Dyes for Their Solvatochromic Prediction. Molecules. 2022; 27(24):9023. https://doi.org/10.3390/molecules27249023
Chicago/Turabian StyleAracena, Andrés, and Moisés Domínguez. 2022. "Computational Quantification of the Zwitterionic/Quinoid Ratio of Phenolate Dyes for Their Solvatochromic Prediction" Molecules 27, no. 24: 9023. https://doi.org/10.3390/molecules27249023
APA StyleAracena, A., & Domínguez, M. (2022). Computational Quantification of the Zwitterionic/Quinoid Ratio of Phenolate Dyes for Their Solvatochromic Prediction. Molecules, 27(24), 9023. https://doi.org/10.3390/molecules27249023




