Real-Time Monitoring of the Atrazine Degradation by Liquid Chromatography and High-Resolution Mass Spectrometry: Effect of Fenton Process and Ultrasound Treatment
Abstract
1. Introduction
2. Results and Discussion
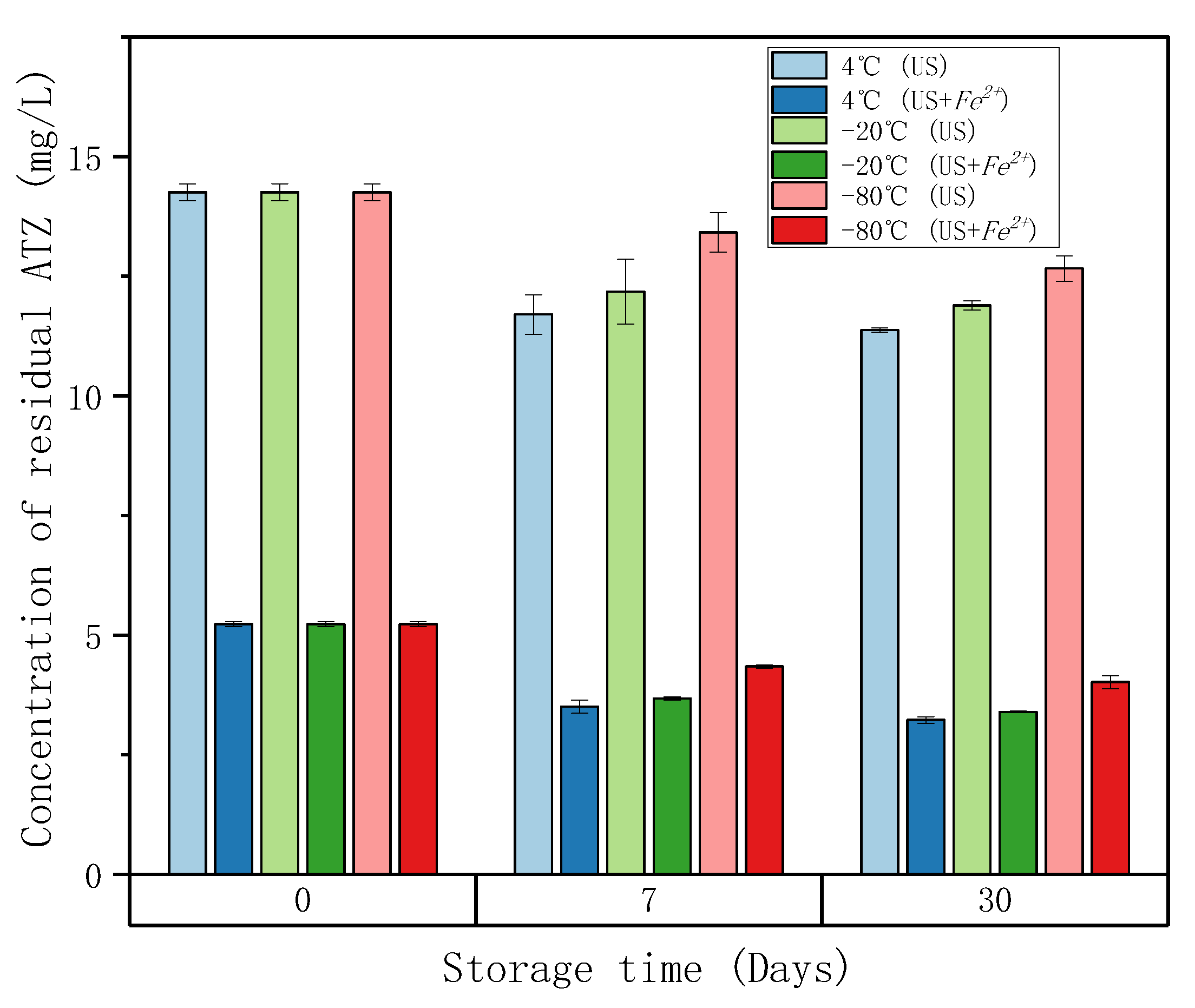
2.1. Influence of Sample Storage after High Frequency Ultrasound (HFUS) Treatment
2.2. Sonolysis and Fenton Reaction Reactor Coupled to LC-HRMS
2.3. Related Compounds
2.4. Degradation Rate of Atrazine
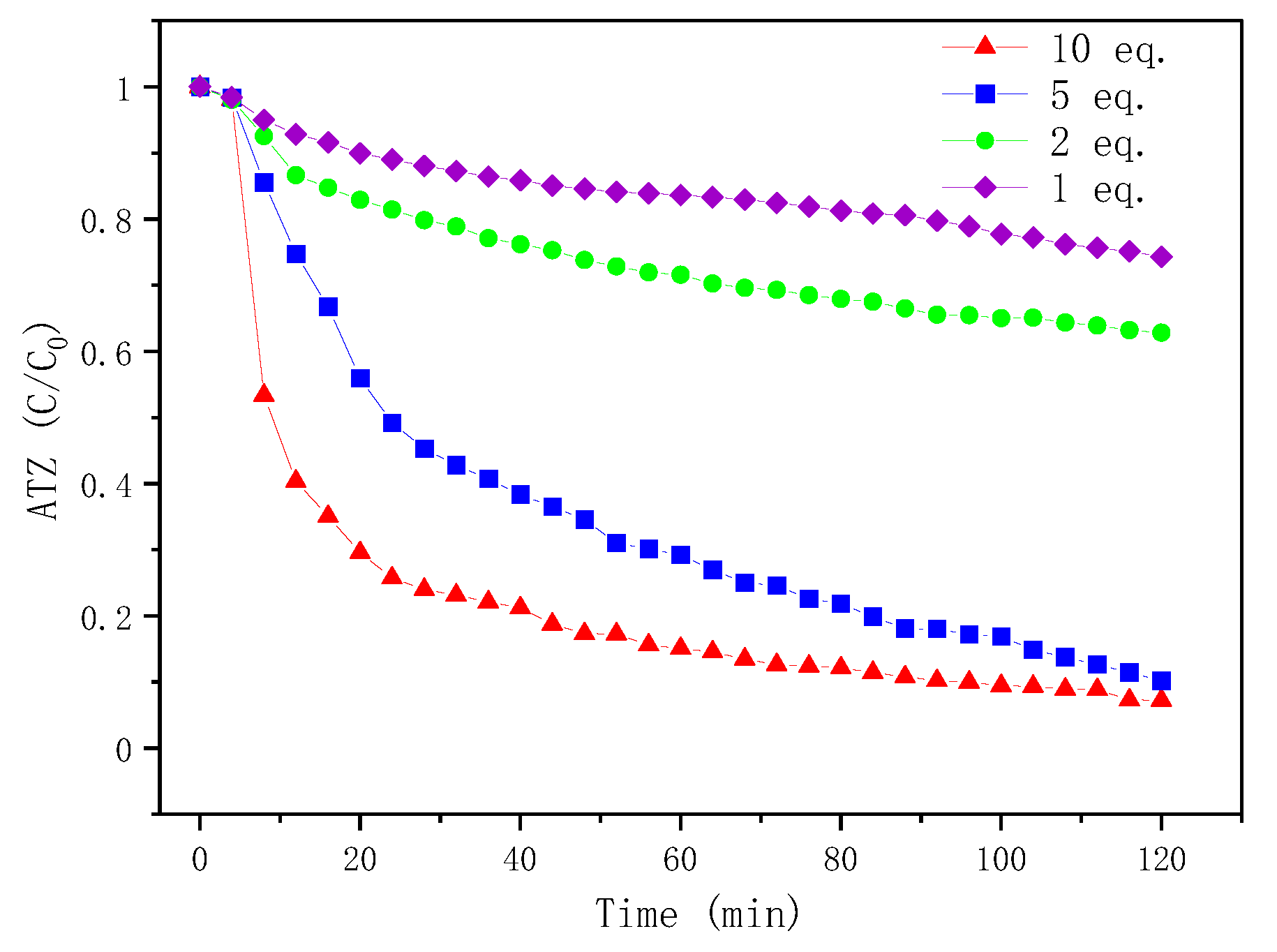
2.4.1. Effect of Fenton Reagents Equivalents
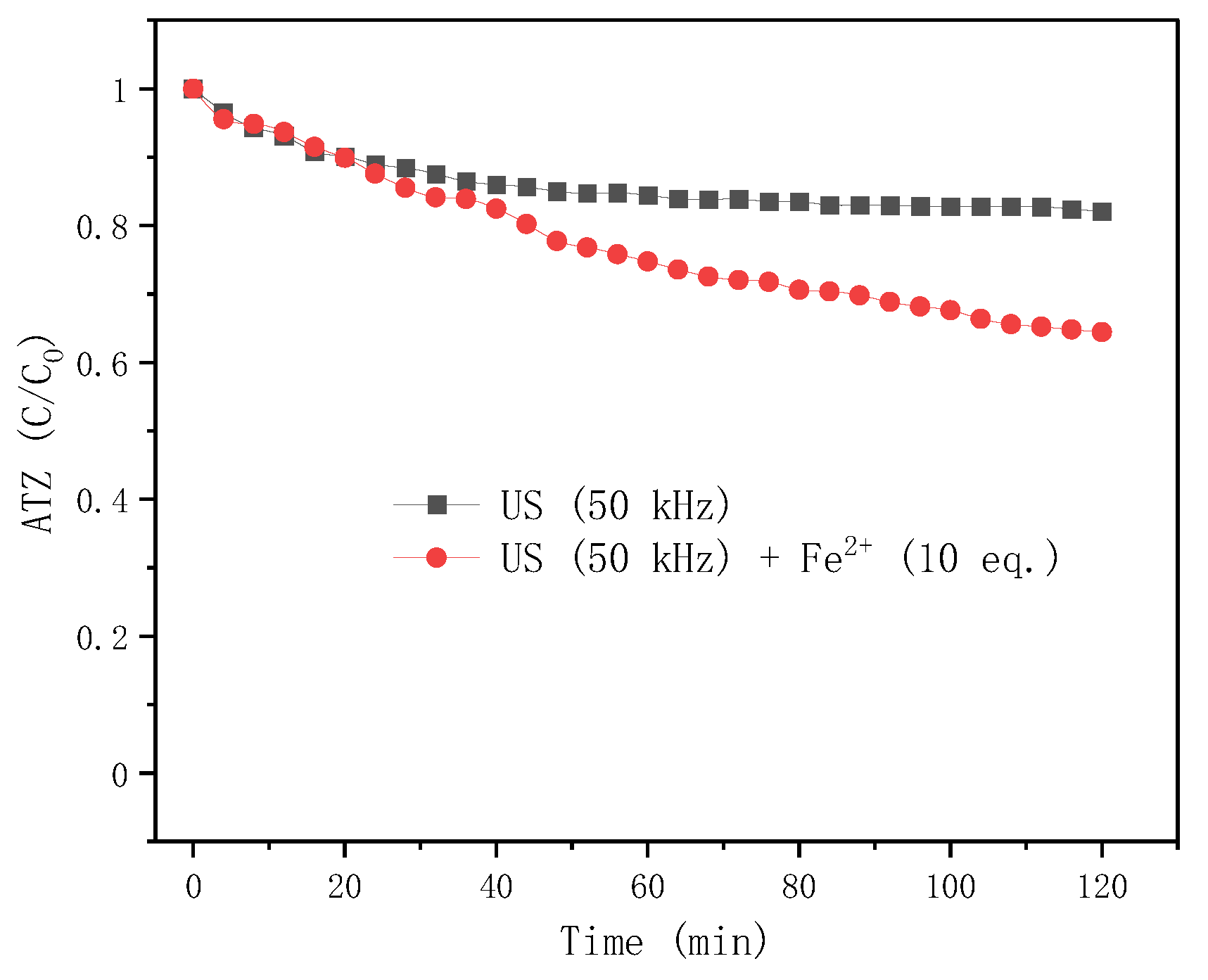
2.4.2. Effect of LFUS Treatment with/without Fe2+
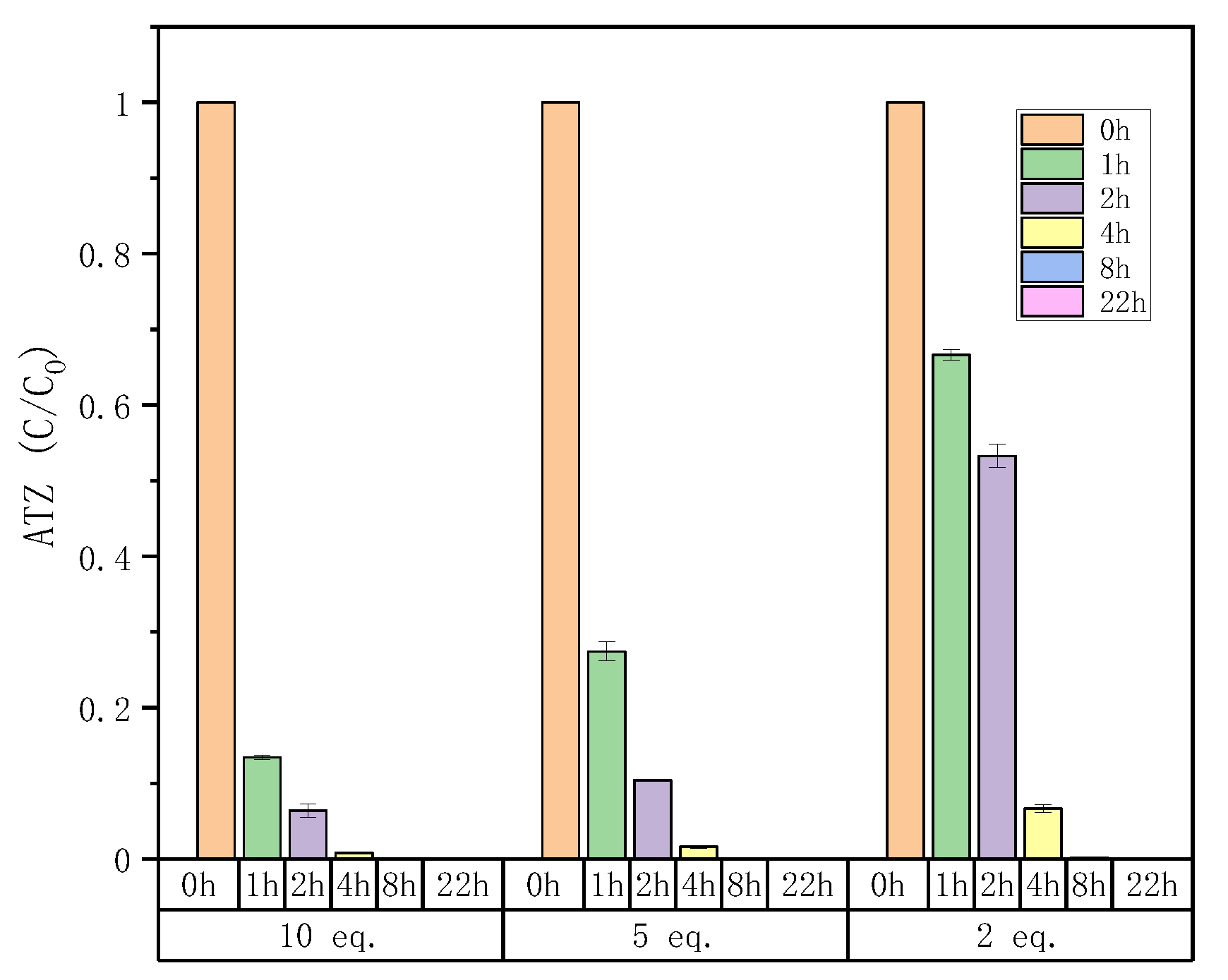
2.4.3. Atrazine Degradation of Offline Fenton Experiments
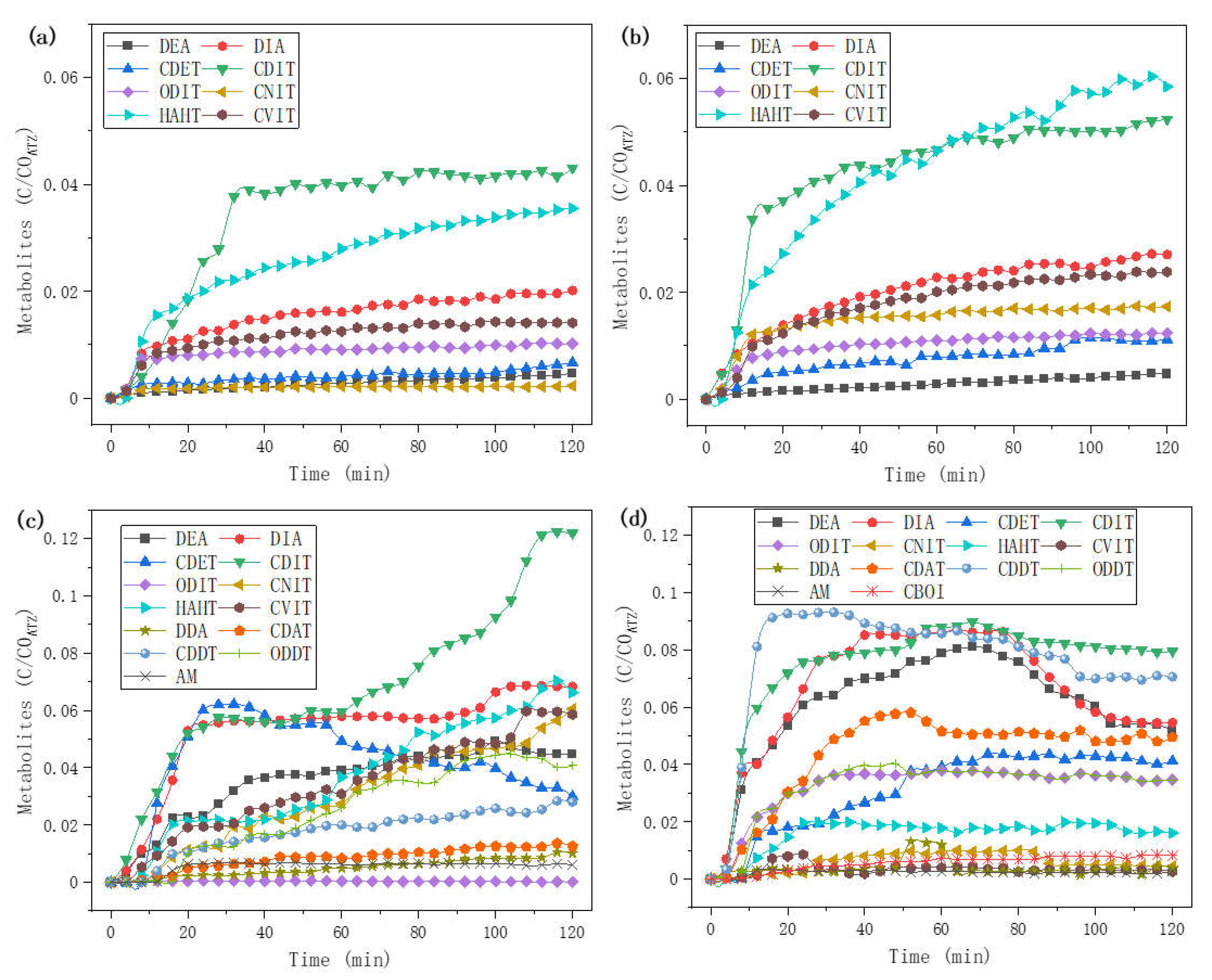
2.5. Kinetics of Metabolites
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Internal Standard (IS) Preparation
3.3. Preparation of Calibration Standard and Quality Control (QC) Samples
3.4. Sample Preparation
3.5. Instrumentation and Analytical Conditions
3.5.1. Offline HFUS Experiment with/without Fe2+
3.5.2. Online Fenton Experiment
3.5.3. Online LFUS Experiment with/without Fe2+
3.5.4. Offline Fenton Experiment
3.5.5. HPLC-HRMS
3.5.6. HPLC-HRMS Online Setup
3.6. Data Analysis
3.7. Compound Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munz, N.; Leu, C.; Wittmer, I. Pesticides Dans Les Cours d’eau Suisses. Aqua Gas. 2013, 93, 78–87. [Google Scholar]
- Udiković-Kolić, N.; Scott, C.; Martin-Laurent, F. Evolution of Atrazine-Degrading Capabilities in the Environment. Appl. Microbiol. Biotechnol. 2012, 96, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Song, F. Bioremediation of Atrazine: Recent Advances and Promises. J. Soils Sediments 2014, 14, 1727–1737. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed]
- Komang Ralebitso, T.; Senior, E.; Van Verseveld, H.W. Microbial Aspects of Atrazine Degradation in Natural Environments. Biodegradation 2002, 13, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Boussetta, N.; Enderlin, G.; Merlier, F.; Grimi, N. Degradation of Residual Herbicide Atrazine in Agri-Food and Washing Water. Foods 2022, 11, 2416. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.B.; Khoury, V.; Narayan, A.; Nazir, M.; Parka, A.; Brown, T.; Adame, L.; Chan, E.; Buchholz, D.; Stueve, T.; et al. Atrazine Induces Complete Feminization and Chemical Castration in Male African Clawed Frogs (Xenopus Laevis). Proc. Natl. Acad. Sci. USA 2010, 107, 4612–4617. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Zhu, J.; Wang, Z.; Li, J. Study on the Efficacy and Mechanism of Fe-TiO2 Visible Heterogeneous Fenton Catalytic Degradation of Atrazine. Chemosphere 2020, 252, 126333. [Google Scholar] [CrossRef]
- Sass, J.; MacLennan, P.A.; Delzell, E.; Sathiakumar, N.; Myers, S.L.; Cheng, H.; Grizzle, W.; Chen, V.W.; Wu, X.C. Cancer Incidence among Triazine Herbicide Manufacturing Workers [1] (Multiple Letters). J. Occup. Environ. Med. 2003, 45, 343–344. [Google Scholar] [CrossRef]
- Xiong, G.; Liang, J.; Zou, S.; Zhang, Z. Microwave-Assisted Extraction of Atrazine from Soil Followed by Rapid Detection Using Commercial ELISA Kit. Anal. Chim. Acta 1998, 371, 97–103. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tataraki, D.; Rassias, G. Degradation of Atrazine in Soil by Dielectric Barrier Discharge Plasma—Potential Singlet Oxygen Mediation. Chem. Eng. J. 2018, 347, 682–694. [Google Scholar] [CrossRef]
- Xu, L.J.; Chu, W.; Graham, N. Atrazine Degradation Using Chemical-Free Process of USUV: Analysis of the Micro-Heterogeneous Environments and the Degradation Mechanisms. J. Hazard. Mater. 2014, 275, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Petrier, C.; David, B.; Laguian, S. Ultrasonic Degradation at 20 KHz and 500 KHz of Atrazine and Pentachlorophenol in Aqueous Solution : Preliminary Results. Chemosphere 1996, 32, 1709–1718. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; von Gunten, U. Degradation Kinetics of Atrazine and Its Degradation Products with Ozone and OH Radicals: A Predictive Tool for Drinking Water Treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Ventura, A.; Jacquet, G.; Bermond, A.; Camel, V. Electrochemical Generation of the Fenton’s Reagent: Application to Atrazine Degradation. Water Res. 2002, 36, 3517–3522. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Cao, B.; Hu, M.; Wang, Z.; Dong, X. Metabolic Ability and Gene Characteristics of Arthrobacter Sp. Strain DNS10, the Sole Atrazine-Degrading Strain in a Consortium Isolated from Black Soil. Int. Biodeterior. Biodegrad. 2011, 65, 1140–1144. [Google Scholar] [CrossRef]
- Mortureux, M. Avis de l’Agence Nationale de Sécurité Sanitaire de l’ Alimentation, de l’ Environnement et Du Travail; Agence Nationale Sécurité Sanitaire Alimentaire Nationale (Anses): Cedex, France, 2012; Volume 33, ISBN 9782110958433. [Google Scholar]
- Chen, H.; Bramanti, E.; Longo, I.; Onor, M.; Ferrari, C. Oxidative Decomposition of Atrazine in Water in the Presence of Hydrogen Peroxide Using an Innovative Microwave Photochemical Reactor. J. Hazard. Mater. 2011, 186, 1808–1815. [Google Scholar] [CrossRef]
- Kuklenyik, Z.; Panuwet, P.; Jayatilaka, N.K.; Pirkle, J.L.; Calafat, A.M. Two-Dimensional High Performance Liquid Chromatography Separation and Tandem Mass Spectrometry Detection of Atrazine and Its Metabolic and Hydrolysis Products in Urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 901, 1–8. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Martín-Torres, S.; Jimenez-Carvelo, A.M.; Cuadros-Rodríguez, L.; Frenich, A.G. Applying an Instrument-Agnostizing Methodology for the Standardization of Pesticide Quantitation Using Different Liquid Chromatography-Mass Spectrometry Platforms: A Case Study; Elsevier B.V.: Amsterdam, The Netherlands, 2021; ISBN 0000000308069. [Google Scholar]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of Peroxymonosulfate by Cobalt-Impregnated Biochar for Atrazine Degradation: The Pivotal Roles of Persistent Free Radicals and Ecotoxicity Assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Merlier, F.; Jellali, R.; Leclerc, E. Online Monitoring of Hepatic Rat Metabolism by Coupling a Liver Biochip and a Mass Spectrometer. Analyst 2017, 142, 3747–3757. [Google Scholar] [CrossRef] [PubMed]
- Merayo, N.; Hermosilla, D.; Negro, C.; Blanco, Á. On-Line FTIR as a Novel Tool to Monitor Fenton Process Behavior. Chem. Eng. J. 2013, 232, 519–526. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, D.; Zhang, H.; Yu, K.; Li, N.; Zheng, G. Degradation Mechanism Study of Fluoroquinolones in UV/Fe2+/Peroxydisulfate by on-Line Mass Spectrometry. Chemosphere 2020, 239, 124737. [Google Scholar] [CrossRef]
- Fu, Y.; Li, W.; Li, H.; Huang, M. High-Precision and on-Line Measurement of Dissolved Organic Matter in Electro-Fenton Process Based on Dual Wavelength Analysis with Combination of Fluorescence Emission and Ultraviolet Absorption Spectroscopy. Anal. Chem. Acta 2021, 1181, 338904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Vieira, C.L.Z.; Wolfson, J.M.; Pingtian, G.; Huang, S. Review on the Treatment of Organic Pollutants in Water by Ultrasonic Technology. Ultrason. Sonochem. 2019, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guan, C.; Luo, L.; Wang, Z.; Pan, H.; Jiang, J. New Insights into Atrazine Degradation by the Novel Manganese Dioxide/Bisulfite System: Product Formation and Mn Reuse. J. Clean. Prod. 2022, 368, 133106. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of Iron-Free Fenton-like Systems for Activating H2O2 in Advanced Oxidation Processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Model Applications and Mechanism Study on the Degradation of Atrazine by Fenton’s System. J. Hazard. Mater. 2005, 118, 227–237. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on Sonochemical Methods in the Presence of Catalysts and Chemical Additives for Treatment of Organic Pollutants in Wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Tang, C.; Wang, W.; Liu, M.; Zhao, G. Mechanisim Investigation on the Enhanced and Selective Photoelectrochemical Oxidation of Atrazine on Molecular Imprinted Mesoporous TiO2. Appl. Catal. B 2019, 246, 50–60. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Balci, B.; Oturan, N.; Cherrier, R.; Oturan, M.A. Degradation of atrazine in aqueous medium by electrocatalytically generated hydroxyl radicals. A kinetic and mechanistic study. Water Res. 2009, 43, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Basfar, A.A.; Mohamed, K.A.; Al-Abduly, A.J.; Al-Shahrani, A.A. Radiolytic degradation of atrazine aqueous solution containing humic substances. Ecotoxicol. Environ. Saf. 2009, 72, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.L.; Pirola, C.; Ragaini, V.; Selli, E. Mechanism and efficiency of atrazine degradation under combined oxidation processes. Appl. Catal. B. 2006, 64, 131–138. [Google Scholar] [CrossRef]
- Chen, S.; He, P.; Wang, X.; Xiao, F.; Zhou, P.; He, Q.; Jia, L.; Dong, F.; Zhang, H.; Jia, B.; et al. Co/Sm-modified Ti/PbO2 anode for atrazine degradation: Effective electrocatalytic performance and degradation mechanism. Chemosphere 2021, 268, 128799. [Google Scholar] [CrossRef]
- Deng, S.; Liu, L.; Cagnetta, G.; Huang, J.; Yu, G. Mechanochemically synthesized S-ZVIbm composites for the activation of persulfate in the pH-independent degradation of atrazine: Effects of sulfur dose and ball-milling conditions. Chem. Eng. J. 2021, 423, 129789. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.J.; Garcia-Anton, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Granados-Oliveros, G.; Páez-Mozo, E.A.; Ortega, F.M.; Ferronato, C.; Chovelon, J.M. Degradation of atrazine using metalloporphyrins supported on TiO2 under visible light irradiation. Appl. Catal. B. 2009, 89, 448–454. [Google Scholar] [CrossRef]
- Hu, E.; Cheng, H. Catalytic effect of transition metals on microwave-induced degradation of atrazine in mineral micropores. Water Res. 2014, 57, 8–19. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4−. Appl. Catal. B 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Y.; Jiang, S.; Wang, Y.; Li, H.; Han, W.; Qu, J.; Wang, L.; Hu, Y. Graphene-like carbon sheet-supported nZVI for efficient atrazine oxidation degradation by persulfate activation. Chem. Eng. J. 2021, 403, 126309. [Google Scholar] [CrossRef]
- Khan, J.A.; Shah, N.S.; Khan, H.M. Decomposition of atrazine by ionizing radiation: Kinetics, degradation pathways and influence of radical scavengers. Sep. Purif. Technol. 2015, 156, 140–147. [Google Scholar] [CrossRef]
- Khan, J.A.; Shah, N.S.; Nawaz, S.; Ismail, M.; Rehman, F.; Khan, H.M. Role of eaq−, OH and H in radiolytic degradation of atrazine: A kinetic and mechanistic approach. J. Hazard. Mater. 2015, 288, 147–157. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Dong, X.; Sun, Z.; Duan, X.; Ren, B.; Zheng, S.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Li, G.; Guo, Y.; Jin, Y.; Tan, W.; Liu, F.; Yin, H. Intrinsic mechanisms of calcium sulfite activation by siderite for atrazine degradation. Chem. Eng. J. 2021, 426, 131917. [Google Scholar] [CrossRef]
- Lu, Y.C.; Feng, S.J.; Zhang, J.J.; Luo, F.; Zhang, S.; Yang, H. Genome-wide identification of DNA methylation provides insights into the association of gene expression in rice exposed to pesticide atrazine. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Mahlalela, L.C.; Casado, C.; Marugan, J.; Septien, S.; Ndlovu, T.; Dlamini, L.N. Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused with Bi2O3. J. Environ. Chem. Eng. 2020, 8, 104215. [Google Scholar] [CrossRef]
- McBeath, S.T.; Graham, N.J. In-situ electrochemical generation of permanganate for the treatment of atrazine. Sep. Purif. Rev. 2021, 260, 118252. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Jiang, X.; Zhang, Y.; Chen, Q.; Lai, B.; Yao, G. Degradation of atrazine by persulfate activation with copper sulfide (CuS): Kinetics study, degradation pathways and mechanism. Chem. Eng. J. 2018, 354, 740–752. [Google Scholar] [CrossRef]
- Qu, M.; Li, N.; Li, H.; Yang, T.; Liu, W.; Yan, Y.; Feng, X.; Zhu, D. Phytoextraction and biodegradation of atrazine by Myriophyllum spicatum and evaluation of bacterial communities involved in atrazine degradation in lake sediment. Chemosphere 2018, 209, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Saltmiras, D.A.; Lemley, A.T. Atrazine degradation by anodic Fenton treatment. Water Res. 2002, 36, 5113–5119. [Google Scholar] [CrossRef]
- Saylor, G.L.; Zhao, C.; Kupferle, M.J. Synergistic enhancement of oxidative degradation of atrazine using combined electrolysis and ozonation. J. Water Process. Eng. 2018, 21, 154–162. [Google Scholar] [CrossRef]
- Sun, X.; Qi, H.; Sun, Z. Bifunctional nickel foam composite cathode co-modified with CoFe@ NC and CNTs for electrocatalytic degradation of atrazine over wide pH range. Chemosphere 2022, 286, 131972. [Google Scholar] [CrossRef]
- Ta, N.; Hong, J.; Liu, T.; Sun, C. Degradation of atrazine by microwave-assisted electrodeless discharge mercury lamp in aqueous solution. J. Hazard. Mater. 2006, 138, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Li, J.; Wang, J.; Liu, J.; Ge, X.; Gu, T. Effective degradation of atrazine in wastewater by three-dimensional electrochemical system using fly ash-red mud particle electrode: Mechanism and pathway. Sep. Purif. Technol. 2021, 267, 118661. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, C.; Zhu, J.; Wang, L.; Gao, S.; Xia, X. Enhanced degradation of atrazine by nanoscale LaFe1−xCuxO3−δ perovskite activated peroxymonosulfate: Performance and mechanism. Sci. Total Environ. 2019, 673, 565–575. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, A.; Li, P.; Héroux, P.; Zhang, H.; Yu, X.; Liu, Y. Degradation of aqueous atrazine using persulfate activated by electrochemical plasma coupling with microbubbles: Removal mechanisms and potential applications. J. Hazard. Mater. 2021, 403, 124087. [Google Scholar] [CrossRef]
- Wang, T.; Huang, T.; Jiang, H.; Ma, R. Electrochemical degradation of atrazine by BDD anode: Evidence from compound-specific stable isotope analysis and DFT simulations. Chemosphere 2021, 273, 129754. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, J.J.; Gao, M.; Huang, Y.X.; Zhang, X.; Yu, H.Q. Photocatalytic degradation of atrazine by boron-doped TiO2 with a tunable rutile/anatase ratio. Appl. Catal. B. 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, N.; Shi, Y.; Zhang, L. Pyrite enables persulfate activation for efficient atrazine degradation. Chemosphere 2020, 244, 125568. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, H.; Li, X.; Yang, C.; Zeng, G.; Wu, B.; He, S.; Lu, L. Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: Performances and mechanisms. Chem. Eng. J. 2018, 341, 126–136. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Li, X.; He, H.; Yang, C. Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem. Eng. J. 2018, 353, 533–541. [Google Scholar] [CrossRef]
- Xie, S.; Tang, C.; Shi, H.; Zhao, G. Highly efficient photoelectrochemical removal of atrazine and the mechanism investigation: Bias potential effect and reactive species. J. Hazard. Mater. 2021, 415, 125681. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yao, J.Z.; Tang, L.; Yang, X.Y.; Zheng, M.; Wang, H.; Wu, M.H. Electron beam induced degradation of atrazine in aqueous solution. Chem. Eng. J. 2015, 275, 374–380. [Google Scholar] [CrossRef]
- Xu, X.; Chen, W.; Zong, S.; Ren, X.; Liu, D. Atrazine degradation using Fe3O4-sepiolite catalyzed persulfate: Reactivity, mechanism and stability. J. Hazard. Mater. 2019, 377, 62–69. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Dong, W.; Ma, J.; Cao, J.; Li, T.; Li, J.; Gu, J.; Liu, P. Study on enhanced degradation of atrazine by ozonation in the presence of hydroxylamine. J. Hazard. Mater. 2016, 316, 110–121. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, H.; Peng, P.; Bo, H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J. Hazard. Mater. 2014, 279, 444–451. [Google Scholar] [CrossRef]
- Ye, G.; Luo, P.; Zhao, Y.; Qiu, G.; Hu, Y.; Preis, S.; Wei, C. Three-dimensional Co/Ni bimetallic organic frameworks for high-efficient catalytic ozonation of atrazine: Mechanism, effect parameters, and degradation pathways analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef]
- Yola, M.L.; Eren, T.; Atar, N. A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem. Eng. J. 2014, 250, 288–294. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yan, X.; Xu, H.; Li, D.; Sun, L.; Cao, G.; Xia, D. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. J. Environ. Sci. (China). 2017, 61, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Lu, Y.C.; Yang, H. Chemical modification and degradation of atrazine in Medicago sativa through multiple pathways. J. Agric. Food Chem. 2014, 62, 9657–9668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3/Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Y.; Liu, D.; Bian, W. The role of dissolved oxygen in the Ta (O) N-driven visible Fenton-like degradation of atrazine. J. Environ. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Qu, J.; Zhang, S.; Han, W. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chem. Eng. J. 2021, 420, 129868. [Google Scholar] [CrossRef]
- Zhanqi, G.; Shaogui, Y.; Na, T.; Cheng, S. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J. Hazard. Mater. 2007, 145, 424–430. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, B.; Yu, Y.; Bu, L.; Deng, J.; Zhou, S. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]


| Entries | Chemical Structure | Molecular Formula | Abbreviation | Name | m/z | Retention Time (min) | Metabolite Level | |
|---|---|---|---|---|---|---|---|---|
| Online | Offline | |||||||
| 1 | 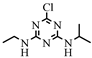 | C8H14ClN5 | ATZ | Atrazine | 216.1010 | 2.558 | 2.178 | 0 |
| 2 | 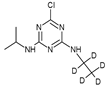 | C8H9D5ClN5 | ATZ-D5 | Atrazine-D5 | 221.1324 | 2.579 | 2.168 | \ |
| 3 | 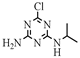 | C6H10ClN5 | DEA | Deethylatrazine | 188.0698 | 1.910 | 1.751 | 1 |
| 4 |  | C5H8ClN5 | DIA | Deisopropylatrazine | 174.0541 | 1.620 | 1.463 | 1 |
| 5 |  | C3H4ClN5 | DDA | Didealkylatrazine | 146.0228 | 1.023 | 0.905 | 2 |
| 6 |  | C7H10ClN5O | CDET | Simazine amide | 216.0647 | 1.820 | 1.691 | 1 |
| 7 | 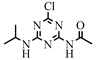 | C8H12ClN5O | CDIT | Atrazine amide | 230.0804 | 2.091 | 1.875 | 1 |
| 8 |  | C5H6ClN5O | CDAT | Deisopropylatrazine amide | 188.0334 | 1.264 | 1.257 | 2 |
| 9 |  | C7H8ClN5 O2 | CDDT | N,N’-(6-Chloro-1,3,5-triazine-2,4-diyl)diacetamide | 230.0440 | 1.451 | 1.407 | 3 |
| 10 | 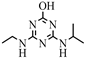 | C8H15N5O | HA | Hydroxyatrazine | 198.1350 | 1.233 | 1 | |
| 11 | 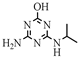 | C6H11N5O | DEHA | Deethylhydroxyatrazine | 170.1037 | 0.810 | 2 | |
| 12 | 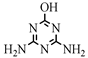 | C3H5N5O | AM | Ammeline | 128.0567 | 0.490 | 0.475 | 3 |
| 13 | 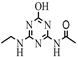 | C7H11N5O2 | ODET | N-[6-(Ethylamino)-4-oxo-1,4-dihydro-1,3,5-triazin-2-yl]acetamide | 198.0986 | 1.082 | 1.048 | 2 |
| 14 | 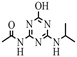 | C8H13N5O2 | ODIT | Hydroxyatrazine amide | 212.1142 | 1.213 | 1.067 | 2 |
| 15 |  | C7H9N5O3 | ODDT | N,N’-(6-hydroxy-1,3,5-triazine-2,4-diyl)diacetamide | 212.0778 | 1.141 | 1.013 | 3 |
| 16 | 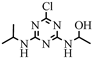 | C8H14ClN5O | CNIT | 1-({4-chloro-6-[(propan-2-yl)amino]-1,3,5-triazin-2-yl}amino)ethan-1-ol | 232.0960 | 1.868 | 1.732 | 1 |
| 17 | 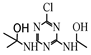 | C8H14ClN5O2 | HAHT | 2-({4-chloro-6-[(1-hydroxyethyl)amino]-1,3,5-triazin-2-yl}amino)propan-2-ol | 248.0909 | 2.212 | 1.933 | 2 |
| 18 |  | C8H12ClN5 | CVIT | 6-chloro-N2-ethenyl-N4-(propan-2-yl)-1,3,5-triazine-2,4-diamine | 214.0854 | 2.070 | 2 | |
| 19 | 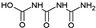 | C3H5N3O4 | CBOI | 1-carboxybiuret | 148.0353 | 0.416 | 4 | |
| Treatment | 50 min | 100 min | ||||||
|---|---|---|---|---|---|---|---|---|
| Cl | OH | Ring Opening | C/C0(ATZ) | Cl | OH | Ring Opening | C/C0(ATZ) | |
| a Fenton_1 eq. | 92.56% | 7.44% | 84.08% | 93.32% | 6.68% | 77.75% | ||
| a Fenton_2 eq. | 94.20% | 5.80% | 72.84% | 94.45% | 5.55% | 65.00% | ||
| a Fenton_5 eq. | 91.23% | 8.77% | 31.02% | 89.30% | 10.70% | 16.90% | ||
| a Fenton_10 eq. | 85.70% | 13.19% | 1.10% | 17.27% | 83.37% | 14.92% | 1.71% | 9.46% |
| US_50 kHz | 89.86% | 10.14% | 84.73% | 86.44% | 13.56% | 82.81% | ||
| 81.01% | 18.99% | 76.80% | 64.54% | 35.46% | 67.62% | |||
| Treatment | Amidation | Dealkylation | Dehydrogenation | Hydroxylation | Substitution (Conversion of ATZ to HA) |
|---|---|---|---|---|---|
| 50 min | |||||
| Fenton_1 eq. | 47.65% | 16.58% | 10.81% | 24.96% | |
| Fenton_2 eq. | 38.33% | 14.15% | 11.37% | 36.15% | |
| Fenton_5 eq. | 47.46% | 29.32% | 8.45% | 14.77% | |
| Fenton_10 eq. | 61.77% | 32.39% | 0.70% | 5.14% | |
| US_50 kHz | 41.94% | 57.51% | 0.55% | ||
| 15.82% | 80.14% | 4.04% | |||
| 100 min | |||||
| Fenton_1 eq. | 43.96% | 17.23% | 11.01% | 27.80% | |
| Fenton_2 eq. | 37.40% | 14.21% | 11.56% | 36.83% | |
| Fenton_5 eq. | 43.58% | 26.04% | 9.64% | 20.75% | |
| Fenton_10 eq. | 67.20% | 26.82% | 0.71% | 5.27% | |
| US_50 kHz | 47.09% | 52.04% | 0.88% | ||
| 28.44% | 63.32% | 8.25% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Boussetta, N.; Enderlin, G.; Grimi, N.; Merlier, F. Real-Time Monitoring of the Atrazine Degradation by Liquid Chromatography and High-Resolution Mass Spectrometry: Effect of Fenton Process and Ultrasound Treatment. Molecules 2022, 27, 9021. https://doi.org/10.3390/molecules27249021
Hong J, Boussetta N, Enderlin G, Grimi N, Merlier F. Real-Time Monitoring of the Atrazine Degradation by Liquid Chromatography and High-Resolution Mass Spectrometry: Effect of Fenton Process and Ultrasound Treatment. Molecules. 2022; 27(24):9021. https://doi.org/10.3390/molecules27249021
Chicago/Turabian StyleHong, Junting, Nadia Boussetta, Gérald Enderlin, Nabil Grimi, and Franck Merlier. 2022. "Real-Time Monitoring of the Atrazine Degradation by Liquid Chromatography and High-Resolution Mass Spectrometry: Effect of Fenton Process and Ultrasound Treatment" Molecules 27, no. 24: 9021. https://doi.org/10.3390/molecules27249021
APA StyleHong, J., Boussetta, N., Enderlin, G., Grimi, N., & Merlier, F. (2022). Real-Time Monitoring of the Atrazine Degradation by Liquid Chromatography and High-Resolution Mass Spectrometry: Effect of Fenton Process and Ultrasound Treatment. Molecules, 27(24), 9021. https://doi.org/10.3390/molecules27249021









