Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon
Abstract
1. Introduction
2. Results and Discussion
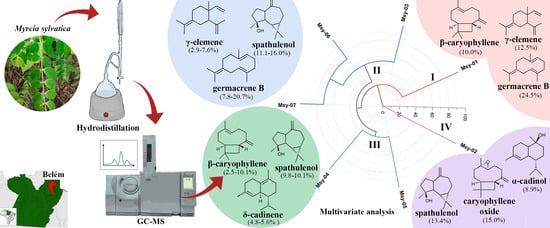
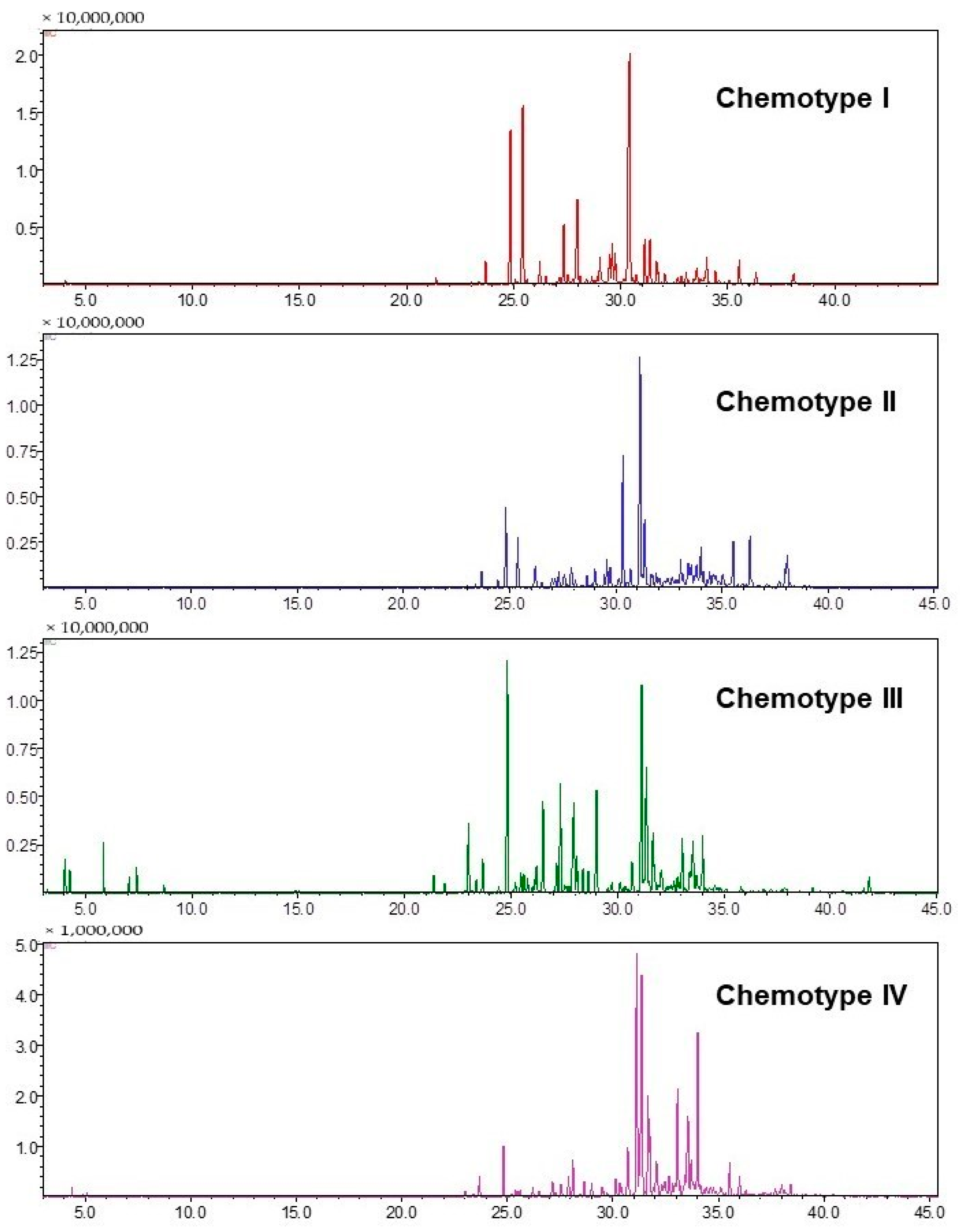
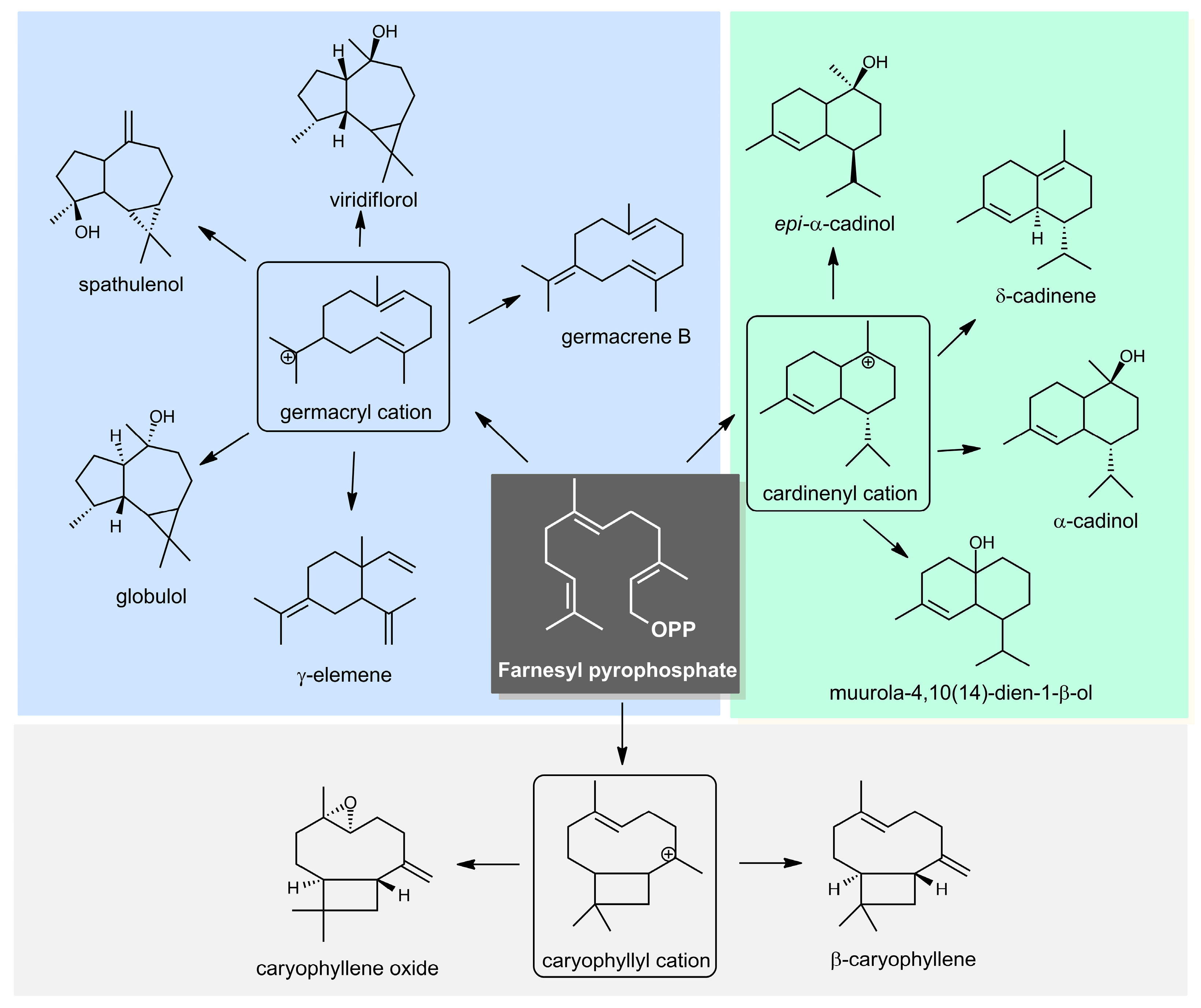
2.1. Yield and Chemical Composition of the Essential Oils
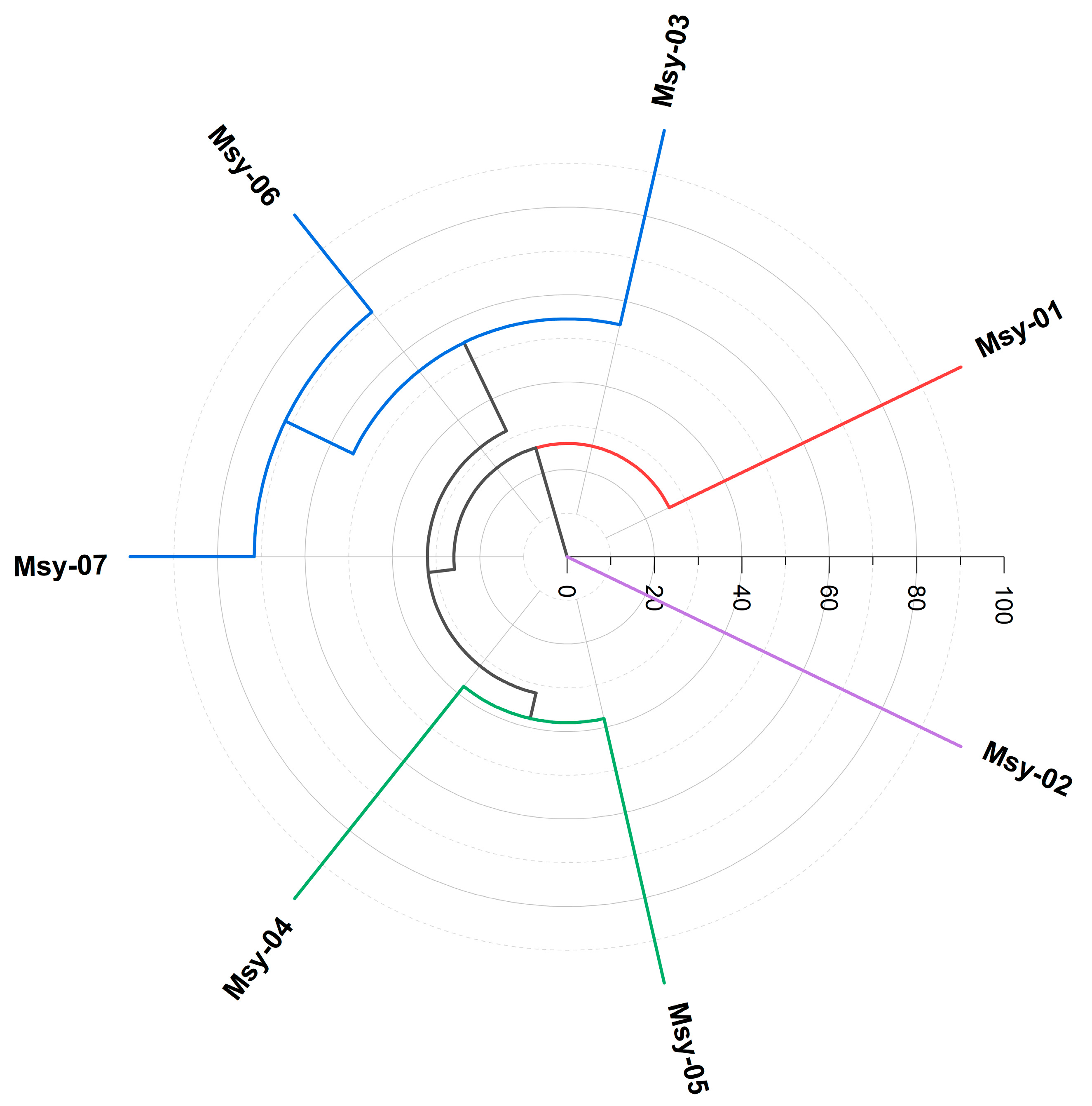
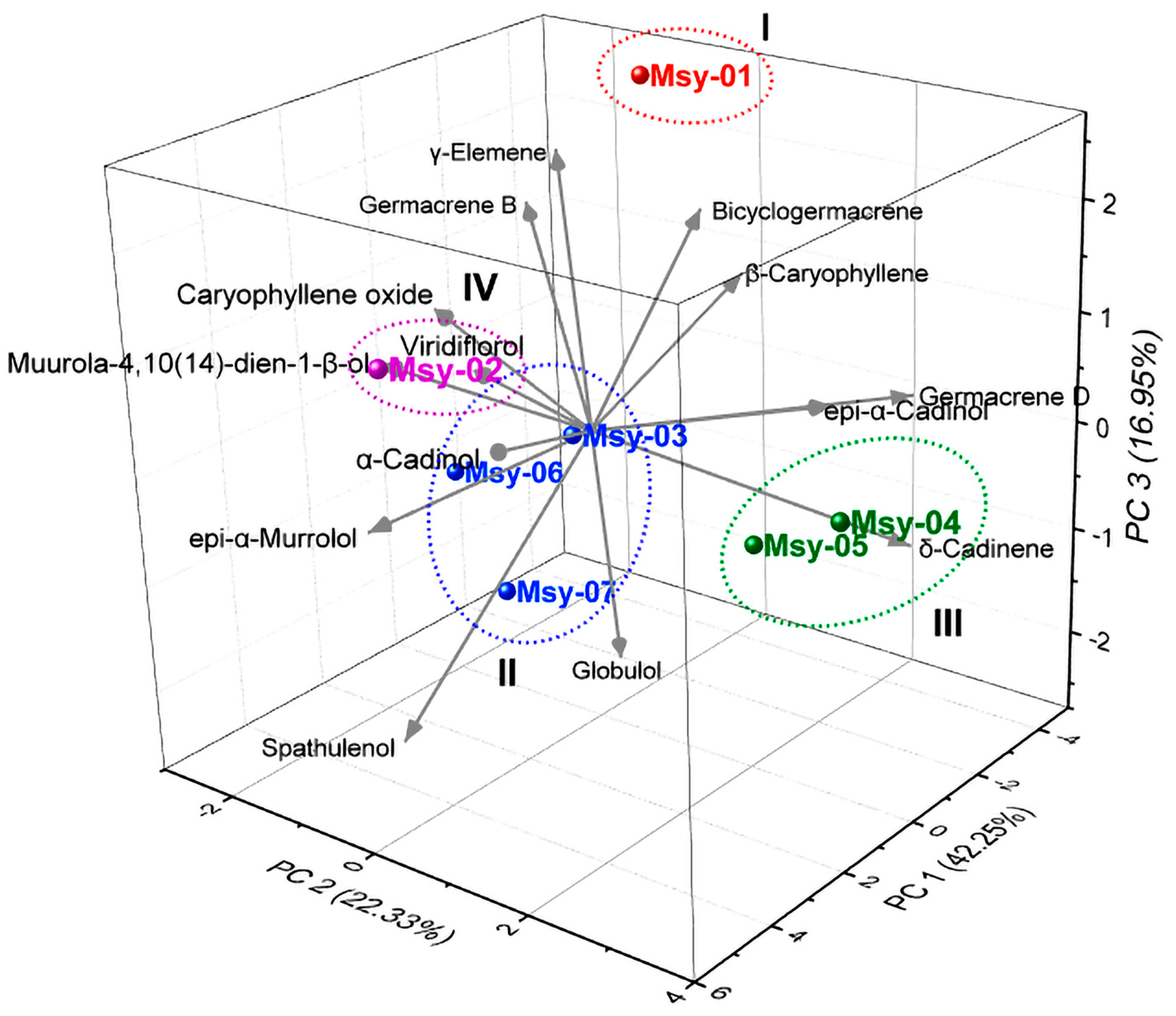
2.2. Chemical Variability in the Specimens
3. Materials and Methods
3.1. Plant Material
3.2. Analysis of Essential Oil Composition
3.3. Multivariate Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lucas, E.J.; Amorim, B.S.; Lima, D.F.; Lima-Lourenço, A.R.; Nic Lughadha, E.M.; Proença, C.E.B.; Rosa, P.O.; Rosário, A.S.; Santos, L.L.; Santos, M.F.; et al. A New Infra-Generic Classification of the Species-Rich Neotropical Genus Myrcia s.L. Kew Bull. 2018, 73, 9. [Google Scholar] [CrossRef]
- Amorim, B.S.; Vasconcelos, T.N.C.; Souza, G.; Alves, M.; Antonelli, A.; Lucas, E. Advanced Understanding of Phylogenetic Relationships, Morphological Evolution and Biogeographic History of the Mega-Diverse Plant Genus Myrcia and Its Relatives (Myrtaceae: Myrteae). Mol. Phylogenet. Evol. 2019, 138, 65–88. [Google Scholar] [CrossRef] [PubMed]
- APG IV An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [CrossRef]
- Santos, M.F.; Lucas, E.; Sano, P.T.; Buerki, S.; Staggemeier, V.G.; Forest, F. Biogeographical Patterns of Myrcia s.l. (Myrtaceae) and Their Correlation with Geological and Climatic History in the Neotropics. Mol. Phylogenet. Evol. 2017, 108, 34–48. [Google Scholar] [CrossRef]
- Gressler, E.; Pizo, M.A.; Morellato, L.P.C. Polinização e Dispersão de Sementes Em Myrtaceae Do Brasil. Rev. Bras. Botânica 2006, 29, 509–530. [Google Scholar] [CrossRef]
- Brack, P.; Köhler, M.; Corrêa, C.A.; Ardissone, R.E.; Sobral, M.E.G.; Kinupp, V.F. Frutas Nativas Do Rio Grande Do Sul, Brasil: Riqueza e Potencial Alimentício. Rodriguésia 2020, 71, 1–12. [Google Scholar] [CrossRef]
- Cruz, A.V.d.M.; Kaplan, M.A.C. Uso Medicinal de Espécies Das Famílias Myrtaceae e Melastomataceae No Brasil. Floresta e Ambient. 2004, 11, 47–52. [Google Scholar]
- Silva, F.K.S.d.; Rosário, A.S.d.; Secco, R.d.S.; Zoghbi, M.d.G.B. Levantamento Das Espécies Conhecidas Como Pedra-Ume-Caá (Myrtaceae), Com Ênfase Nas Comercializadas Na Cidade de Belém, Pará, Brasil. Biota Amaz. 2015, 5, 7–15. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; de Aguiar Andrade, E.H.; das Graças Bichara Zoghbi, M.; da Silva Santos, L. Constituents and Pharmacological Activities of Myrcia (Myrtaceae): A Review of an Aromatic and Medicinal Group of Plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef]
- Zoghbi, M.d.G.B.; Andrade, E.H.A.; da Silva, M.H.L.; Carreira, L.M.M.; Maia, J.G.S. Essential Oils from Three Myrcia Species. Flavour Fragr. J. 2003, 18, 421–424. [Google Scholar] [CrossRef]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Myrtaceae in Lista de Espécies Da Flora Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB19882 (accessed on 14 December 2022).
- Raposo, J.D.A.; Figueiredo, P.L.B.; Santana, R.L.; da Silva Junior, A.Q.; Suemitsu, C.; da Silva, R.; Mourão, R.H.V.; Maia, J.G.S. Seasonal and Circadian Study of the Essential Oil of Myrcia Sylvatica (G. Mey) DC., a Valuable Aromatic Species Occurring in the Lower Amazon River Region. Biochem. Syst. Ecol. 2018, 79, 21–29. [Google Scholar] [CrossRef]
- Saccol, E.M.; Londero, P.; Bressan, C.A.; Salbego, J.; Gressler, L.T.; Silva, L.V.; Mourão, R.H.; Oliveira, R.B.; Llesuy, S.F.; Baldisserotto, B.; et al. Oxidative and Biochemical Responses in Brycon Amazonicus Anesthetized and Sedated with Myrcia Sylvatica (G. Mey.) DC. and Curcuma Longa L. Essential Oils. Vet. Anaesth. Analg. 2017, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.A.; Sarrazin, S.L.F.; Oliveira, R.B.; Suemitsu, C.; Maia, J.G.S.; Mourão, R.H.V. Composition and Antimicrobial Activity of Leaf Essential Oils of Myrcia Sylvatica (G. Mey.) DC. European J. Med. Plants 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Rosa, C.S.; Veras, K.S.; Silva, P.R.; Lopes Neto, J.J.; Cardoso, H.L.M.; Alves, L.P.L.; Brito, M.C.A.; Amaral, F.M.M.; Maia, J.G.S.; Monteiro, O.S.; et al. Composição Química e Toxicidade Frente Aedes Aegypti L. e Artemia Salina Leach Do Óleo Essencial Das Folhas de Myrcia Sylvatica (G. Mey.) DC. Rev. Bras. Plantas Med. 2016, 18, 19–26. [Google Scholar] [CrossRef]
- Costa, J.S.d.; da Cruz, E.d.N.; Setzer, W.N.; da Silva, J.K.d.R.; Maia, J.G.S.; Figueiredo, P.L.B. Essentials Oils from Brazilian Eugenia and Syzygium Species and Their Biological Activities. Biomolecules 2020, 10, 1155. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Pereira, R.A.; Zoghbi, M.d.G.B.; Bastos, M.d.N.d.C. Essential Oils of Twelve Species of Myrtaceae Growing Wild in the Sandbank of the Resex Maracanã, State of Pará, Brazil. J. Essent. Oil Bear. Plants 2013, 13, 440–450. [Google Scholar] [CrossRef]
- de Moraes, A.B.; Ferreira, O.O.; da Costa, L.S.; Almeida, L.Q.; Varela, E.L.P.; Cascaes, M.M.; Franco, C.D.J.P.; Percário, S.; Nascimento, L.D.D.; de Oliveira, M.S.; et al. Phytochemical Profile, Preliminary Toxicity, and Antioxidant Capacity of the Essential Oils of Myrciaria Floribunda (H. West Ex Willd.) O. Berg. and Myrcia Sylvatica (G. Mey) DC. (Myrtaceae). Antioxidants 2022, 11, 2076. [Google Scholar] [CrossRef]
- Silva, R.C.e.; Costa, J.S.d.; Figueiredo, R.O.d.; Setzer, W.N.; Silva, J.K.R.d.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef]
- da Costa, J.S.; Andrade, W.M.S.; de Figueiredo, R.O.; Santos, P.V.L.; Freitas, J.J.d.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Chemical Composition and Variability of the Volatile Components of Myrciaria Species Growing in the Amazon Region. Molecules 2022, 27, 2234. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, Antioxidant Capacity and Cytotoxic Activity of Eugenia Uniflora L. Chemotype-Oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, O.; Magalhães, M. Modified Distillation Trap. Chem. Anal. 1960, 49, 114. [Google Scholar]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids From Brazil. Nat. Prod. Commun. 2021, 16, 1934578X2199615. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
| RI(C) | RI(L) | Constituents (%) * | Msy-01 | Msy-02 | Msy-03 | Msy-04 | Msy-05 | Msy-06 | Msy-07 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 847 | 846 a | Hex-(2E)-enal | 0.1 | tr | 0.1 | 0.5 | tr | tr | |
| 2 | 850 | 850 a | Hex-(3Z)-enol | 0.1 | tr | 0.1 | 0.4 | 0.7 | 0.1 | tr |
| 3 | 858 | 859 a | Hex-(2Z)-enol | 0.1 | 0.2 | |||||
| 4 | 862 | 863 a | n-Hexanol | 0.1 | 0.1 | 0.2 | 0.5 | tr | tr | |
| 5 | 904 | 907 a | Butyl propanoate | 0.1 | ||||||
| 6 | 934 | 932 a | α-Pinene | 0.1 | 1.2 | 0.1 | ||||
| 7 | 977 | 974 a | β-Pinene | 0.1 | 0.4 | tr | ||||
| 8 | 991 | 988 a | Myrcene | 0.1 | 0.7 | tr | ||||
| 9 | 1028 | 1024 a | Limonene | tr | 0.2 | 0.1 | tr | |||
| 10 | 1190 | 1186 a | α-Terpineol | 0.1 | 0.1 | |||||
| 11 | 1194 | 1190 a | Methyl salicylate | 0.1 | ||||||
| 12 | 1195 | 1195 a | Myrtenal | 0.1 | ||||||
| 13 | 1338 | 1335 a | δ-Elemene | 0.4 | 0.1 | 0.3 | 0.8 | 0.1 | 0.1 | |
| 14 | 1349 | 1345 a | α-Cubebene | 0.1 | 0.2 | 0.4 | tr | |||
| 15 | 1352 | 1350 a | Citronellyl acetate | 0.1 | ||||||
| 16 | 1368 | 1367 b | Cyclosativene | 0.1 | 0.2 | 0.1 | ||||
| 17 | 1371 | 1373 a | α-Ylangene | 0.1 | 0.3 | 0.1 | tr | 0.1 | ||
| 18 | 1374 | 1374 a | Isoledene | tr | 0.1 | |||||
| 19 | 1377 | 1374 a | α-Copaene | 0.1 | 0.3 | 0.1 | 1.8 | 2.8 | 0.3 | 0.2 |
| 20 | 1381 | 1378 a | Hex-(3Z)-enyl hexenoate | 0.1 | ||||||
| 21 | 1386 | 1387 a | β-Bourbonene | 0.2 | 0.1 | 0.3 | 0.8 | 0.6 | 0.2 | 0.2 |
| 22 | 1390 | 1387 a | β-Cubebene | 0.1 | ||||||
| 23 | 1392 | 1389 a | β-Elemene | 1.3 | 0.9 | 1.1 | 1.8 | 1.4 | 1.6 | 0.9 |
| 24 | 1410 | 1409 a | α-Gurjunene | 0.1 | tr | 0.1 | 0.3 | 0.1 | ||
| 25 | 1422 | 1417 a | β-Caryophyllene | 10.0 | 2.4 | 4.3 | 2.5 | 10.1 | 1.8 | 4.7 |
| 26 | 1428 | 1428 a | (E)-α-Ionone | 0.2 | 0.2 | 0.1 | ||||
| 27 | 1429 | 1430 a | β-Copaene | 0.2 | 0.1 | 0.3 | 0.5 | 0.1 | 0.1 | |
| 28 | 1429 | 1431 a | β-Gurjunene | 0.9 | ||||||
| 29 | 1434 | 1434 a | γ-Elemene | 12.5 | 0.3 | 2.9 | 0.7 | 7.6 | 2.9 | |
| 30 | 1437 | 1432 a | α-trans-Bergamotene | 0.6 | 0.3 | 0.3 | 0.3 | 1.0 | ||
| 31 | 1440 | 1439 a | Aromadendrene | 0.4 | 0.4 | 0.2 | 0.2 | 0.9 | 0.4 | 0.2 |
| 32 | 1440 | 1437 a | α-Guaiene | 0.5 | ||||||
| 33 | 1443 | 1442 a | Guaia-6,9-diene | 0.6 | ||||||
| 34 | 1444 | 1445 b | Selina-5,11-diene | 0.1 | ||||||
| 35 | 1447 | 1448 a | cis-Muurola-3,5-diene | tr | 0.5 | |||||
| 36 | 1451 | 1447 a | Isogermacrene D | 0.2 | 0.2 | |||||
| 37 | 1451 | 1451 a | trans-Muurola-3,5-diene | 0.1 | 0.5 | |||||
| 38 | 1454 | 1452 a | α-Humulene | 1.4 | 0.4 | 1.1 | 1.6 | 1.2 | 1.9 | 1.4 |
| 39 | 1462 | 1460 a | allo-Aromadendrene | 0.6 | 0.2 | |||||
| 40 | 1461 | 1464 a | 9-epi-(E)-Caryophyllene | 0.3 | 0.9 | 3.9 | 0.6 | 0.3 | ||
| 41 | 1463 | 1472 b | cis-Cadina-1(6),4-diene | 0.2 | ||||||
| 42 | 1463 | 1465 a | cis-Muurola-4(14),5-diene | 0.1 | ||||||
| 43 | 1466 | 1471 a | Dauca-5,8-diene | 0.1 | ||||||
| 44 | 1474 | 1475 a | trans-Cadina-1(6),4-diene | 0.2 | 0.2 | |||||
| 45 | 1477 | 1478 a | γ-Muurolene | 0.6 | 0.8 | 0.9 | 3.0 | 1.4 | 0.7 | |
| 46 | 1481 | 1483 a | α-Amorphene | 0.2 | ||||||
| 47 | 1482 | 1484 a | Germacrene D | 3.8 | 3.5 | 5.0 | 4.9 | 0.5 | 1.1 | |
| 48 | 1485 | 1476 b | Selina-4,11-diene | 0.7 | ||||||
| 49 | 1487 | 1492 a | β-Selinene | 0.6 | 0.8 | 0.8 | 2.1 | 0.4 | 1.1 | 0.7 |
| 50 | 1491 | 1491 a | 10,11-epoxy-Calamenene | 0.2 | 0.2 | |||||
| 51 | 1491 | 1493 a | trans-Muurola-4(14),5-diene | 0.5 | ||||||
| 52 | 1492 | 1489 a | δ-Selinene | 0.3 | ||||||
| 53 | 1496 | 1496 a | Viridiflorene | 2.0 | 1.1 | 1.2 | 3.1 | 2.3 | 2.2 | 1.2 |
| 54 | 1497 | 1500 a | Bicyclogermacrene | 5.0 | 0.8 | 3.8 | 1.4 | 0.8 | ||
| 55 | 1501 | 1500 a | α-Muurolene | 0.5 | 1.8 | 0.8 | 2.7 | 1.5 | 0.9 | 0.5 |
| 56 | 1508 | 1502 a | trans-β-Guaiene | 1.0 | ||||||
| 57 | 1508 | 1509 a | α-Bulnesene | 0.4 | 0.1 | |||||
| 58 | 1508 | 1511 a | δ-Amorphene | 0.4 | 0.2 | |||||
| 59 | 1509 | 1505 a | β-Bisabolene | 0.2 | 0.9 | |||||
| 60 | 1515 | 1513 a | γ-Cadinene | 0.7 | 0.7 | 1.0 | 2.6 | 0.9 | 0.4 | 0.7 |
| 61 | 1519 | 1514 a | Cubebol | 0.3 | 0.2 | |||||
| 62 | 1523 | 1521 a | trans-Calamenene | 0.6 | ||||||
| 63 | 1524 | 1522 a | δ-Cadinene | 1.8 | 2.7 | 5.6 | 4.8 | 1.1 | 1.3 | |
| 64 | 1531 | 1532 a | γ-Cuprenene | 0.1 | ||||||
| 65 | 1533 | 1533 a | trans-Cadina-1,4-diene | 0.1 | tr | 0.1 | tr | |||
| 66 | 1536 | 1528 a | Zonarene | 0.3 | 0.2 | 0.2 | ||||
| 67 | 1539 | 1537 a | α-Cadinene | 0.2 | 0.7 | 0.3 | ||||
| 68 | 1539 | 1540 b | Selina-4(15),7(11)-diene | 1.6 | 0.6 | 1.5 | 0.7 | |||
| 69 | 1543 | 1545 a | Selina-3,7(11)-diene | 1.9 | 0.2 | 1.5 | 0.6 | |||
| 70 | 1544 | 1544 a | α-Calacorene | 1.4 | 1.9 | 0.6 | 0.5 | 0.9 | ||
| 71 | 1558 | 1559 a | Germacrene B | 24.5 | 0.7 | 7.8 | 1.3 | 0.3 | 20.7 | 7.9 |
| 72 | 1558 | 1562 a | epi-Longipinanol | 0.3 | ||||||
| 73 | 1563 | 1564 a | β-Calacorene | 0.3 | 0.2 | 0.1 | ||||
| 74 | 1568 | 1567 a | Palustrol | 0.8 | 3.3 | 1.8 | ||||
| 75 | 1578 | 1577 a | Spathulenol | 2.9 | 13.4 | 11.1 | 9.8 | 10.1 | 15.7 | 16.0 |
| 76 | 1584 | 1582 a | Caryophyllene oxide | 3.3 | 15.0 | 9.6 | 3.7 | 0.4 | 0.1 | 0.6 |
| 77 | 1592 | 1592 a | Viridiflorol | 1.5 | 5.3 | 1.0 | 1.2 | 2.9 | 0.9 | 0.8 |
| 78 | 1583 | 1590 a | Globulol | 7.4 | 3.4 | 5.5 | ||||
| 79 | 1595 | 1595 a | Cubeban-11-ol | 0.7 | 2.9 | 0.9 | 1.1 | 0.6 | 0.7 | |
| 80 | 1602 | 1600 a | Rosifoliol | 0.9 | 3.0 | 0.7 | 0.9 | 1.5 | 0.8 | 0.9 |
| 81 | 1609 | 1608 a | Humulene epoxide | 0.9 | 1.0 | 0.5 | 0.4 | 0.2 | 0.3 | |
| 82 | 1615 | 1618 a | 1,10-di-epi-Cubenol | 0.3 | 0.4 | 0.4 | 1.2 | 1.0 | ||
| 83 | 1618 | 1618 a | Junenol | 1.3 | 0.9 | 1.1 | 0.6 | |||
| 84 | 1629 | 1627 a | 1-epi-Cubenol | 1.7 | 2.6 | 2.6 | 1.8 | |||
| 85 | 1629 | 1630 a | Muurola-4,10(14)-dien-1β-ol | 5.8 | ||||||
| 86 | 1629 | 1632 a | α-Acorenol | 0.8 | ||||||
| 87 | 1633 | 1630 a | γ-Eudesmol | 0.3 | 0.6 | 1.0 | ||||
| 88 | 1637 | 1639 a | Caryophylla-4(12),8(13)-dien-5β-ol | 0.7 | 0.6 | |||||
| 89 | 1643 | 1645 a | Cubenol | 0.3 | 0.7 | |||||
| 90 | 1643 | 1640 a | epi-α-Murrolol | 4.8 | 3.2 | 2.2 | 1.2 | 2.3 | ||
| 91 | 1643 | 1640 b | epi-α-Cadinol | 1.6 | 1.4 | 5.1 | 1.2 | |||
| 92 | 1647 | 1644 a | α-Muurolol | 0.4 | 2.3 | 1.1 | 2.0 | 1.2 | ||
| 93 | 1649 | 1648 a | cis-Guaia-3,9-dien-11-ol | 0.9 | ||||||
| 94 | 1655 | 1652 a | α-Cadinol | 1.9 | 8.9 | 3.3 | 5.5 | 2.5 | 1.7 | 3.1 |
| 95 | 1666 | 1668 b | Intermedeol | 0.9 | 0.7 | 0.8 | 0.8 | 1.0 | ||
| 96 | 1668 | 1668 a | trans-Calamenen-10-ol | 0.1 | 0.1 | |||||
| 97 | 1671 | 1668 a | 14-hydroxy-9-epi-(E)-Caryophyllene | 1.9 | ||||||
| 98 | 1675 | 1675 a | Cadalene | 0.5 | 0.6 | 0.8 | ||||
| 99 | 1677 | 1676 a | Mustakone | 0.7 | ||||||
| 100 | 1685 | 1679 a | Kusinol | 0.7 | ||||||
| 101 | 1686 | 1664 a | Longiborneol acetate | 0.1 | ||||||
| 102 | 1690 | 1685 a | Germacra-4(15),5,10(14)-trien-1α-ol | 0.2 | 0.1 | 0.2 | ||||
| 103 | 1696 | 1696 b | Juniper camphor | 1.5 | 1.6 | 2.0 | 0.2 | 2.1 | 3.3 | |
| 104 | 1701 | 1702 a | 10-nor-Calamenen-10-one | 0.2 | ||||||
| 105 | 1739 | 1733 a | Isobicyclogermacrenal | 0.2 | 0.1 | |||||
| 106 | 1762 | 1766 a | Drimenol | 0.2 | ||||||
| 107 | 1771 | 1767 a | 14-oxy-α-Muurolene | 0.2 | ||||||
| 108 | 1780 | 1779 a | 14-hydroxy-α-Muurolene | 0.1 | 0.1 | tr | 0.2 | |||
| 109 | 1798 | 1792 a | β-Eudesmol acetate | 0.2 | ||||||
| 110 | 1801 | 1803 a | 14-hydroxy-δ-Cadinene | tr | 0.1 | 0.1 | ||||
| 111 | 1836 | 1845 a | (2E,6E)-Farnesyl acetate | 0.1 | ||||||
| 112 | 2113 | 2106 b | Phytol | 0.1 | 0.1 | 0.3 | ||||
| Monoterpene hydrocarbons | - | - | - | 0.3 | 2.5 | 0.2 | tr | |||
| Oxygenated monoterpenoids | - | - | tr | 0.3 | 0.1 | 0.1 | - | |||
| Sesquiterpene hydrocarbons | 71.8 | 12.5 | 33.1 | 43.5 | 47.7 | 48.0 | 29.0 | |||
| Oxygenated sesquiterpenoids | 17.4 | 71.5 | 39.7 | 36.0 | 36.7 | 29.9 | 40.4 | |||
| Others | 0.4 | 0.7 | 1.3 | 2.5 | 2.1 | 0.2 | 0.2 | |||
| Total | 89.6 | 84.7 | 74.1 | 82.5 | 89.2 | 78.4 | 69.6 | |||
| Oil yield (%) * | 0.7 | 0.9 | 0.6 | 0.3 | 0.3 | 0.3 | 0.5 | |||
| Code | Voucher Number | Coordinates Latitude/Longitude |
|---|---|---|
| Msyl-1 | MG-228738 | 1°15′52.65″S, 48°28′12.85″W |
| Msyl-2 | MG-229217 | 1°14′52.69″S, 48°26′30.20″W |
| Msyl-3 | MG-229955 | 1°15′52.42″S, 48°28′12.58″W |
| Msyl-4 | MG-229956 | 1°15′52.41″S, 48°28′12.69″W |
| Msyl-5 | MG-229954 | 1°15′42.54″S, 48°28′1.78″W |
| Msyl-6 | MG-233283 | 1°14′51.71″S, 48°26′29.66″W |
| Msyl-7 | MG-233284 | 1°14′20.79″S, 48°26′9.94″W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.S.d.; Freitas, J.J.d.S.; Setzer, W.N.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon. Molecules 2022, 27, 8975. https://doi.org/10.3390/molecules27248975
Costa JSd, Freitas JJdS, Setzer WN, da Silva JKR, Maia JGS, Figueiredo PLB. Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon. Molecules. 2022; 27(24):8975. https://doi.org/10.3390/molecules27248975
Chicago/Turabian StyleCosta, Jamile Silva da, Jofre Jacob da Silva Freitas, William N. Setzer, Joyce Kelly R. da Silva, José Guilherme S. Maia, and Pablo Luis B. Figueiredo. 2022. "Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon" Molecules 27, no. 24: 8975. https://doi.org/10.3390/molecules27248975
APA StyleCosta, J. S. d., Freitas, J. J. d. S., Setzer, W. N., da Silva, J. K. R., Maia, J. G. S., & Figueiredo, P. L. B. (2022). Variability in the Chemical Composition of Myrcia sylvatica (G. Mey) DC. Essential Oils Growing in the Brazilian Amazon. Molecules, 27(24), 8975. https://doi.org/10.3390/molecules27248975