Abstract
The preparation of anodic TiO2 nanotube layers has been performed using electrochemical anodization of Ti foil for 4 h at different voltages (from 0 V to 80 V). In addition, a TiO2 thin layer has been also prepared using the sol–gel method. All the photocatalysts have been characterized by XRD, SEM, and DRS to investigate the crystalline phase composition, the surface morphology, and the optical properties, respectively. The performance of the photocatalyst has been assessed in versatile photocatalytic reactions including the reduction of N2O gas and the oxidation of aqueous sulfamethoxazole. Due to their high specific surface area and excellent charge carriers transport, anodic TiO2 nanotube layers have exhibited the highest N2O conversion rate (up to 10% after 22 h) and the highest degradation extent of sulfamethoxazole (about 65% after 4 h) under UVA light. The degradation mechanism of sulfamethoxazole has been investigated by analyzing its transformation products by LC-MS and the predominant role of hydroxyl radicals has been confirmed. Finally, the efficiency of the anodic TiO2 nanotube layer has been tested in real wastewater reaching up to 45% of sulfamethoxazole degradation after 4 h.
1. Introduction
With regard to a number of publications, titania (TiO2) is the most investigated photocatalyst in a multitude of applications such as, for example, antibacterial coatings and water and air treatments [1,2,3,4,5,6]. Efficient TiO2 photocatalysts are prepared in the form of nanomaterials ranging from 0D to 3D morphologies [5,7,8,9,10,11,12]. The 1D titania nanostructures include TiO2 nanotubes (TNT) that are promising nanostructured photocatalysts mainly due to their excellent electron transport [13,14,15]. In the form of self-organized arrays (i.e., supported layers), TNT exhibit enhanced charge carriers separation, thus leading to exceptional photocatalytic properties [13,14,15,16]. The preparation TNT layers can be conducted by anodic oxidation of a Ti electrode in a fluoride-containing electrolyte [17,18,19]. The variation in applied voltage, fluoride concentration and anodization time can be tuned to design TNT of specific length and wall thickness [17,18,19,20]. In addition, by extending one of these experimental parameters, the TNT morphology can be transformed in a porous nanostructure, which also exhibits interesting photocatalytic properties as reported in our recent study [20]. Indeed, by increasing the anodization voltage, it is possible to form a porous sponge-like structure.
The treatment of water by photocatalysis is one of the most investigated alternatives to enhance the already existing processes which are used in wastewater treatment plants (WWTPs) and in the production of drinking water [21]. Indeed, the photocatalytic process belongs to advanced oxidation processes (AOPs), thus generating reactive oxygen species (ROS) such as hydroxyl radicals (HO•) [22]. Using a semiconductor photocatalyst such as TiO2, UVA light is necessary to generate the electron/hole (e−/h+) pair, from which h+ reacts with water to produce HO•. The HO• react with high kinetic rate and non-selectively with organic molecules including persistent organic pollutants (POPs) and contaminants of emerging concern (CECs) [22]. Among CECs, pharmaceutical and personal care products (PPCPs) are consumed daily and their long-term impact on the natural environmental and human health is not yet clarified [23,24]. Intergovernmental agencies have implemented stringent norms for pollution control and water quality, such as the implementing decision of the European Union (EU) No. 2020/1161 on the directive No. 2008/105/CE [25]. Therefore, the modification of conventional water treatments appears a necessary conditions to fulfill these norms, especially by adding or replacing current chemical oxidation processes with AOPs; thus, TNT photocatalysts are an excellent candidate. In this study, sulfamethoxazole (SMX) is used as a model pollutant since it is a widely used antibiotic. In addition, SMX is one of the most frequently detected pollutants in water around the world [23].
The treatment of air for the removal of nitrous oxide (N2O) using photocatalytic reactions was described back in the 1990s, when the first experiments employed zeolite containing Cu-based photocatalysts [26]. N2O is in the top 3 gases responsible for global warming, since it has been proven to be involved at 6.2% of the total global radiative forcing. The major origin of N2O is from natural processes (nitrification of ammonia, denitrification of nitrates, etc.) but also anthropogenic activities (N-based fertilizers, combustion of fossil fuels, etc.) [27]. Therefore, intense research is being carried out to develop different types of photocatalysts including TiO2 [28,29,30,31,32,33]. However, one significant disadvantage of these already existing photocatalysts is that they are not easy to handle because of their powder form. Using supported TNT photocatalysts, such a drawback might be overcome.
In the present study, the use of anodic TNT layers in wastewater and air treatments is assessed using the degradation of aqueous sulfamethoxazole solution and the reduction of gaseous nitrous oxide, respectively, as model reactions. To the best of our knowledge, it is the first time that anodic TiO2 layers either in the form of nanotubal or sponge-like structure are used in such versatile photocatalytic reactions, and their performance is compared to another TiO2 nanostructure i.e., TiO2 thin layer deposited by the sol–gel method. Such a comparative study will bring further insights into the application of TiO2 nanomaterials for environmental purposes. In addition, the degradation of SMX is also performed in real wastewater and the degradation pathway of SMX is proposed, so the fundamental understanding of the prepared materials is coupled with semi-applied science, which is the early stage of industrial application development.
2. Results and Discussion
2.1. Structural and Optical Properties of Photocatalysts
The XRD patterns (Figure 1) of the F20, F40, F60, F80 and SG samples match with the anatase phase of TiO2 (ICDD card no. 03-065-5714) as reported in our previous studies [20,34]. Very weak diffractions of TiO2 anatase phase are also confirmed in the SG sample, where a textured silicon wafer was used as the substrate. Therefore, additional diffractions of the textured silicon wafers correspond to the Kbeta, Lalpha1 and Lalpha2 lines of Si (111) along with impurities. It is worth noting the diffractions from metallic Ti in the TNT layers since it is the underlying substrate of self-organized and highly oriented TiO2 nanotubes. In addition, the F0 sample only exhibits metallic Ti diffraction (ICDD card no. 00-044-1294), although an ultra-thin layer of TiO2 (several nanometers) might be present due to surface oxidation.
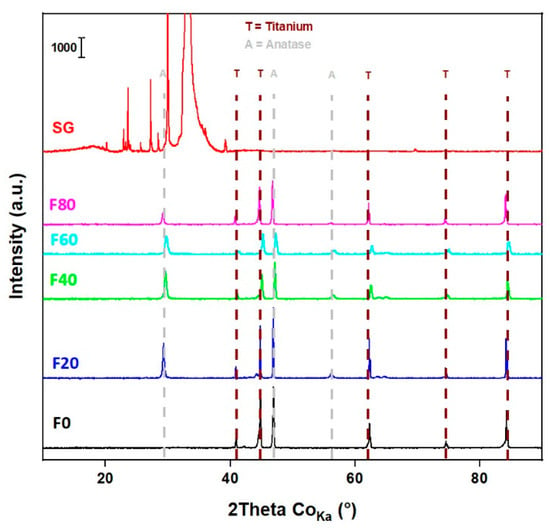
Figure 1.
XRD of the TiO2 nanostructures.
The surface and cross-section morphology of the samples have been characterized by SEM in previous studies [20,34,35] and comprehensively discussed. Briefly, the surface of the F0 sample exhibits the typical morphology of Ti, i.e., a dense structure of microcrystals. As the anodization voltage increases from 20 to 80 V, a porous nanostructure is formed. The F20 sample exhibits the clear nanotubular morphology of a self-organized and highly-ordered TNT layer, while the surface of the TNT in the F40 sample starts to be destroyed. In the F60 and F80 samples, the nanotubular morphology disappears and a porous nanostructure composed of a reminiscent nanotube and a sponge-like structure is formed, respectively.
Concerning the optical properties, the Tauc’s plot (Figure 2) exhibits that the samples showing TNT morphology i.e., F20 and F40, possess the strongest light absorption, along with Eg, of about 3.28 eV. For porous nanostructures such as the F60 and F80 samples, light absorption decreases while the Eg is about 3.35 eV. For the SG sample, light absorption is weaker due to the thickness, which is approx. 5 times thinner than anodized samples. In addition, the F0 sample which is essentially composed of metallic Ti exhibits slight absorption in UVA due to the upper oxidized layer.
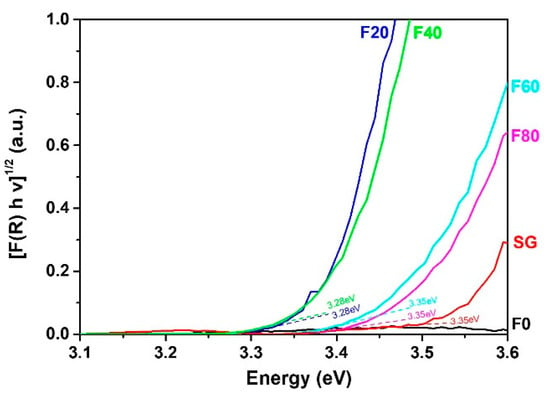
Figure 2.
UV-visible DRS of the TiO2 nanostructures.
2.2. Reduction of N2O
The ability of the TiO2 photocatalysts prepared by two different methods (electrochemical anodization and sol–gel deposition) to reduce nitrous oxide under UVA light is investigated (Figure 3). It is worth noting there is no reference in the literature about testing the photocatalytic decomposition of N2O with the use of a TiO2 nanostructure prepared by electrochemical anodization, thus highlighting the significance of the present study.
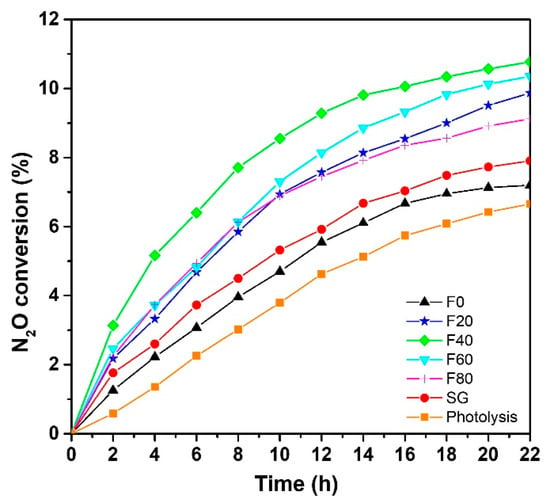
Figure 3.
N2O gas conversion under UVA light.
From Figure 3, it is clear that N2O conversion, which occurs during photocatalytic reactions, is significantly higher than direct photolysis (i.e., without photocatalyst). Comparing the different photocatalysts, the highest N2O conversion is achieved using samples prepared by electrochemical anodization, especially the F40 sample, showing nanotubular morphology. Indeed, such a TNT layer can convert about 10% of N2O after 22 h UVA irradiation. The lowest photocatalytic activities are observed for F0 and SG samples with a conversion about 7.2% and 7.9%, respectively. This is due to two main reasons: (i) these samples exhibit a significantly lower specific surface area compared to nanoporous F20, F40, F60 and F80 and (ii) their thickness is also smaller. Nevertheless, both of these poorly photoactive samples present a higher N2O conversion than simple photolysis (6.6%).
2.3. Degradation of SMX
The degradation curves of SMX using the samples prepared by electrochemical anodization and the sol–gel method are presented in Figure 4, along with the reusability of the best sample (F20) after 3 repeated runs (Figure S1). Globally, a similar trend is observed between N2O reduction and SMX oxidation since the best samples are F20 and F40, i.e., samples with nanotubular morphology. This fact highlights the versatility of anodic TiO2 nanotube layers. The degradation extent of SMX after 4 h UVA irradiation reaches 65% and 62% for in the presence of F20 and F40, respectively. The degradation extent decreases to 52% using F60, since the structure is composed of reminiscent nanotubes. The efficiency of the other samples is relatively poor due to the absence of nanotubular morphology. In other words, the number of catalytic sites and the lifetime of charge carriers is probably significantly reduced. The TOC analysis corroborates the degradation curves. Indeed, for F20, F40 and F60, the mineralization extent after 4 h UVA irradiation reaches 11%, 8% and 3%, respectively, while for the other samples, no mineralization takes place. The potential application of anodic TiO2 layer in water treatment is performed by the degradation of SMX in secondary effluents of wastewater treatment plants (Figure S2). The degradation efficiency decreases from 65 to 45% due to the negative effects of the wastewater matrix, i.e., the dissolved organic matter that plays the role of ROS scavenger. However, the degradation efficiency is still considered to be satisfactory.
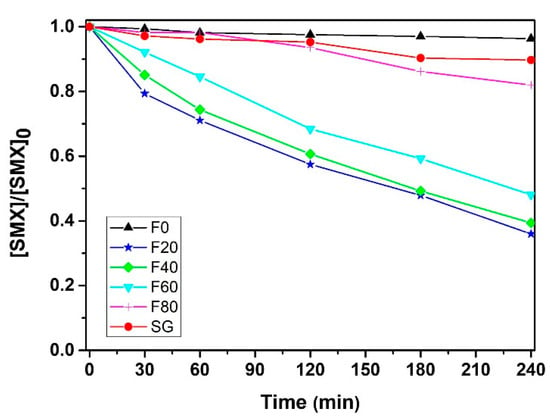
Figure 4.
Degradation of SMX under UVA light.
In order to obtain better insights into the degradation mechanism using F20, the SMX degradation by-products are identified using LC-MS. The transformation products of SMX are presented in Table S1 (Supplementary Materials). The initial SMX molecule displayed a peak at [M + H]+ = 254.0592 and a sodium adduct [M + Na]+ = 276.0410 and [M − H]− = 252.0441. Different degradation pathways are proposed considering the identification of SMX by-products (Figure 5). The formation of P1 occurs through the photo-isomerization of the isoxazole ring, which is also the dominant pathway in the degradation of SMX under UV-mediated degradation [36]. Electrophilic reaction on the aromatic ring leads to the formation of hydroxylated (P2) and dihydroxylated (P3) products, while oxidation of the double bond at the isoxazole ring produced P4. The cleavage of sulfonamide bond by the hydroxyl radical leads to the formation of 3-amino-5-methylisoxazole (P5) and sulfanilic acid (P4). Isoxazole ring rearrangement leads to the formation of P7 that can be further oxidized on the amine ring in the presence of a hydroxyl radical, leading to the formation of P7 and P8. The degradation mechanism confirms the predominant role of hydroxyl radicals.
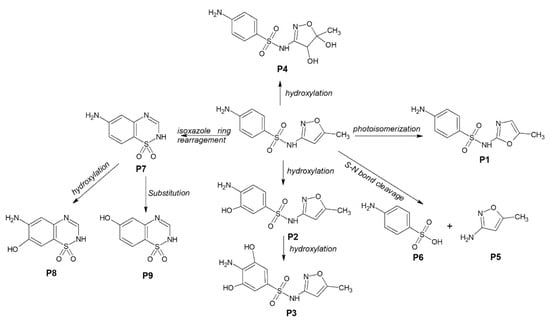
Figure 5.
Proposed mechanism of SMX degradation under UVA light in the presence of anodic TiO2 nanotube layer.
3. Materials and Methods
3.1. Preparation and Characterization of Photocatalysts
The TNT layers are prepared using a similar procedure reported in one of our previous study [34]. Briefly, a disk of 4 cm in diameter of titanium foil (Merck, Darmstadt, Germany, 99.7% with 0.127 mm thickness) is used as a working electrode and dropped in fluoride-based electrolyte based on glycerol. The counter electrode is also a 4 cm diameter Ti foil. The distance between the two electrodes is set at 1.5 cm and electrochemical anodization is performed at different applied voltages from 0 to 80 V (with a 20 V step) for 100 min. The current intensity is kept at 5 A during the procedure. After rinsing the as-prepared TNT followed by annealing at 400 °C for 1 h, TNT layers labelled F0, F20, F40, F60 and F80 are obtained.
For comparison, TiO2 sol–gel films are prepared. To this end, titanium isopropoxide (97.0%; Merck, Darmstadt, Germany) is added to isopropanol (reagent grade, Slavus, Bratislava, Slovakia) containing acetic acid (99.0%, Slavus, Bratislava, Slovakia) and Triton® X-100 (Merck, Darmstadt, Germany) as chelating and structure directing agents, respectively, thus a 0.2 M Ti alkoxide sol–gel is obtained. The sol–gel is deposited by spin-coating (Ossila Ltd.) at 2000 rpm on Si wafer (University Wafer Inc., South Boston, MA, USA) with a diameter of 4 cm. Six-layer films are prepared with intermediate annealing at 300 °C for 10 min and final annealing at 450 °C for 1 h. The TiO2 sol–gel films are labelled SG.
The TNT and sol–gel layers are characterized by DRS (Shimadzu UV-2600, IRS-2600 Plus) and XRD (Rigaku SmartLab) to control the optical energy band gap (Eg) and the crystalline phase composition. The optical energy band gap is determined using the Kubelka-Munk theory (Equation (1)) by assuming an indirect band gap, i.e., z = 2. The value of Eg is determined by the linear extrapolation of the plot with the x-axis. Further characterization details of these reproducible nanomaterials are provided in our previous publications [20,34,35].
where F® is the absorption spectra, h is the Planck constant, Eg is the energy band gap, z is a factor depending on the type of Eg, ν is the incident light frequency and B is a constant.
(F(R) hν)1/z = B (hν − Eg)
3.2. Degradation of N2O in the Gas Phase
The photocatalytic decomposition of gaseous N2O is performed in a custom-made stainless-steel photo-reactor (Figure 6), where the photocatalytic layer is placed at the bottom. After that, the reactor is closed and filled with a N2O/He mixture and pressurized to 1.5 bar (pressure is controlled during the whole experiment). The initial N2O concentration is set at 1030 ppm. The irradiation is generated by a UVA source (UVP Pen-Ray, 8 W Hg lamp; λmax = 365 nm) situated at the top of a photo-reactor, through the quartz glass visor. The N2O concentration is measured using a GC/BID (Gas Chromatography coupled with Barrier discharge Ionization detector, Shimadzu Tracera GC 2010 Plus) in two hour-intervals for 22 h. Each experiment is repeated to check the reproducibility. The conversion of N2O () is calculated using Equation (1), where is the initial mole fraction of N2O and is the mole fraction at different times during the photocatalytic reaction.
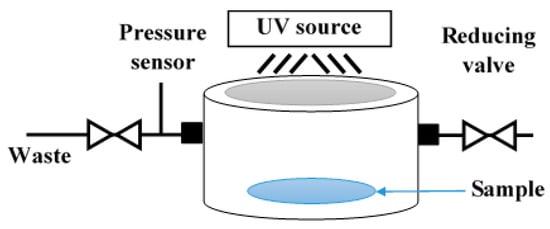
Figure 6.
Scheme of the photo-reactor used to decompose N2O gas.
3.3. Degradation of SMX in Water
Concerning the photocatalytic degradation of SMX solution (50 µM), the photocatalytic layers are placed at the bottom of a homemade photo-reactor equipped of four UVA lamps at the top (Sylvania F15W/350BL; 1.9 mW cm−2 in the range 290–400 nm). Prior to turning on the lamps, the initial pH of SMX solution is adjusted at 7 using HClO4 and NaOH. The photocatalytic degradation is performed under constant air bubbling for 4 h and 500 µL is sampled out every 30 min (filtration through 0.45 µm PTFE filter and quenching into 100 µL methanol). The concentration of SMX is analyzed by HPLC (Shimadzu Nexera XR LC-20AD) equipped with a C18 column (Agilent, EC 250/4.6 nucleodur 100/5). The mobile phase is a mixture of MeOH/H2O in gradient mode from 40:60 (v/v) to 95:5 (v/v), which is achieved in 10 min. The detection wavelength of SMX is fixed at 268 nm. It is worth noting that the degradation experiments are repeated three times to control the reusability of the photocatalytic layers and the variations in degradation efficiency do not exceed 5% (Figure S1).
In addition, to highlight the significance of the present study for potential industrial application, the degradation of SMX is performed in the secondary effluent of municipal wastewater treatment plants collected in Clermont-Ferrand, France. Prior to being used as a wastewater matrix, it is filtered through a 0.45 µm PTFE membrane and further analyzed by total organic analysis (Shimadzu, TOC-L) and ionic chromatography (Thermo Scientific, ICS 5000). The data are presented in Table 1.

Table 1.
Concentration of inorganic ions and inorganic and organic carbon.
The identification of SMX transformation by-products is obtained by ultra-high performance liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry (HRMS) performed on an Orbitrap Q-Exactive (Thermo scientific). The column is a Phenomenex Kinetex C18 (1.7 μm × 100 Å; 100 × 2.1 mm) and the temperature is set at 30 °C. The initial gradient is 5% ACN and 95% water acidified with 1% formic acid, followed by a linear gradient to 99% ACN within 8.5 min and kept constant during 1 min. The flow rate is 0.45 mL min−1 and the injection volume is 5 μL. Ionization is set to 3.2 kV (ESI+) and 3.0 kV (ESI−).
4. Conclusions
The preparation of different TiO2 nanostructures including nanotube layers and thin films has been performed using electrochemical anodization and the sol–gel method, respectively. Their performance in versatile photocatalytic applications has been assessed by investigating their ability to degrade gaseous and aqueous pollutants under UVA light, i.e., N2O and sulfamethoxazole. The best samples in both the photocatalytic reactions are those showing a nanotubular morphology (F20 and F40), probably due to their high specific surface area and excellent charge carriers transport, compared to others samples (F0, F60, F80 and SG) that show non-ordered and less porous nanostructures. In addition, the degradation mechanism of sulfamethoxazole has been proposed and has highlighted the crucial role of hydroxyl radicals. This study is significant since it proves the photocatalytic versatility of anodic TiO2 nanotube layers in different applications such as the remediation of air and water. Indeed, the degradation of sulfamethoxazole has been tested in secondary effluents from a wastewater treatment plant and has confirmed the efficiency of the anodic TiO2 nanotube layers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248959/s1, Figure S1: Reusability of sample F20 in SMX degradation after 3 repeated runs; Figure S2: Degradation of SMX in secondary effluents of wastewater treatment plant using F20 under UVA light; Table S1: LC-MS data of SMX degradation by-products.
Author Contributions
Conceptualization, O.M.; methodology, M.S., M.M., M.B. and O.M.; formal analysis, M.S., S.G., A.M., M.B. and O.M.; investigation, M.S., S.G. and A.M.; data curation, M.S., A.M., O.M. and M.B.; writing—original draft preparation, O.M., M.M., M.S. and S.G.; writing—review and editing, O.M. and M.B.; supervision, O.M. and G.M.; funding acquisition, O.M., M.S. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was co-financed by the Slovak Research and Development Agency (under the contracts No. APVV-21-0039 and No. APVV-21-0053), the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak republic (through the VEGA project No. 1/0062/22) and the Operation Program of Integrated Infrastructure supported by the ERDF for the project “Upscale of Comenius University Capacities and Competence in Research, Development and Innovation” (ITMS 2014+: 313021BUZ3). Sridhar Gowrisankaran also acknowledges the financial support provided by the Comenius University Bratislava through the project No. UK/180/2022. Experimental results were accomplished by using Large Research Infrastructure ENREGAT supported by the Ministry of Education, Youth and Sports of the Czech Republic under project No. LM2018098.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the present manuscript and supplementary materials.
Acknowledgments
The authors acknowledge Guillaume Voyard from CNRS, Institut de Chimie de Clermont-Ferrand, France for the technical support provided for the HPLC analyses.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, A.; Schneider, D.W. Understanding TiO2 Photocatalysis Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Moradeeya, P.G.; Sharma, A.; Kumar, M.A.; Basha, S. Titanium Dioxide Based Nanocomposites—Current Trends and Emerging Strategies for the Photocatalytic Degradation of Ruinous Environmental Pollutants. Environ. Res. 2022, 204, 112384. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Bak, T.; O’Doherty, P.J.; Moffitt, M.C.; Nowotny, J.; Bailey, T.D.; Kersaitis, C. Photocatalysis of Titanium Dioxide for Water Disinfection: Challenges and Future Perspectives. Int. J. Photochem. 2014, 2014, 973484. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lu, Y.; Ren, Z. Mini Review on Photocatalysis of Titanium Dioxide Nanoparticles and Their Solar Applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Motola, M.; Dworniczek, E.; Satrapinskyy, L.; Chodaczek, G.; Grzesiak, J.; Gregor, M.; Plecenik, T.; Nowicka, J.; Plesch, G. UV Light-Induced Photocatalytic, Antimicrobial, and Antibiofilm Performance of Anodic TiO2 Nanotube Layers Prepared on Titanium Mesh and Ti Sputtered on Silicon. Chem. Pap. 2019, 73, 1163–1172. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.C. Using Photocatalyst Metal Oxides as Antimicrobial Surface Coatings to Ensure Food Safety—Opportunities and Challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A Review of Solar and Visible Light Active TiO2 Photocatalysis for Treating Bacteria, Cyanotoxins and Contaminants of Emerging Concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Motola, M.; Zazpe, R.; Hromadko, L.; Prikryl, J.; Cicmancova, V.; Rodriguez-Pereira, J.; Sopha, H.; Macak, J.M. Anodic TiO2 Nanotube Walls Reconstructed: Inner Wall Replaced by ALD TiO2 Coating. Appl. Surf. Sci. 2021, 549, 149306. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins Dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the Antimicrobial Mechanism of TiO2-Based Nanocomposite Films in a Pathogenic Bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Ghicov, A.; Schmuki, P. Dye-Sensitized Anodic TiO2 Nanotubes. Electrochem. Commun. 2005, 7, 1133–1137. [Google Scholar] [CrossRef]
- Regonini, D.; Chen, G.; Leach, C.; Clemens, F.J. Comparison of Photoelectrochemical Properties of TiO2 Nanotubes and Sol-Gel. Electrochim. Acta 2016, 213, 31–36. [Google Scholar] [CrossRef]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.M.; Taveira, L.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and Limits of the Photoelectrochemical Response from Anodic TiO2 Nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TIO2—New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem.—Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Sopha, H.; Baudys, M.; Krbal, M.; Zazpe, R.; Prikryl, J.; Krysa, J.; Macak, J.M. Scaling up Anodic TiO2 Nanotube Layers for Gas Phase Photocatalysis. Electrochem. Commun. 2018, 97, 91–95. [Google Scholar] [CrossRef]
- Hanif, M.B.; Sihor, M.; Liapun, V.; Makarov, H.; Monfort, O.; Motola, M. Porous vs. Nanotubular Anodic TiO2: Does the Morphology Really Matters for the Photodegradation of Caffeine? Coatings 2022, 12, 1002. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Devipriya, S.; Yesodharan, S. Photocatalytic Degradation of Pesticide Contaminants in Water. Sol. Energy Mater. Sol. Cells 2005, 86, 309–348. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Wong, D.; Ntchantcho, R.; Pizarro, J.; Mart, J.; Echeverr, S.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Černanský, S.; Fehér, M.; Birošová, L.; Gál, M. Pharmaceuticals, Drugs, and Resistant Microorganisms—Environmental Impact on Population Health. Curr. Opin. Environ. Sci. Health 2019, 9, 40–48. [Google Scholar] [CrossRef]
- Decision 2020/1161/EU Commission Implementing Decision (EU) 2020/1161-4 August 2020. Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2020, 257, 32–35. [Google Scholar]
- Ebitani, K.; Morokuma, M.; Kim, J.H.; Morikawa, A. Photocatalytic Decomposition of Nitrous Oxide on Cu Ion-Containing ZSM-5 Catalyst. J. Catal. 1993, 141, 725–728. [Google Scholar] [CrossRef]
- deRichter, R.; Caillol, S. Fighting Global Warming: The Potential of Photocatalysis against CO2, CH4, N2O, CFCs, Tropospheric O3, BC and Other Major Contributors to Climate Change. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Sano, T.; Negishi, N.; Mas, D.; Takeuchi, K. Photocatalytic Decomposition of N2O on Highly Dispersed Ag+ Ions on TiO2 Prepared by Photodeposition. J. Catal. 2000, 194, 71–79. [Google Scholar] [CrossRef]
- Obalová, L.; Reli, M.; Lang, J.; Matějka, V.; Kukutschová, J.; Lacný, Z.; Kočí, K. Photocatalytic Decomposition of Nitrous Oxide Using TiO2 and Ag-TiO2 Nanocomposite Thin Films. Catal. Today 2013, 209, 170–175. [Google Scholar] [CrossRef][Green Version]
- Kočí, K.; Krejčíková, S.; Šolcová, O.; Obalová, L. Photocatalytic Decomposition of N2O on Ag-TiO2. Catal. Today 2012, 191, 134–137. [Google Scholar] [CrossRef]
- Matějová, L.; Polách, L.; Lang, J.; Šihor, M.; Reli, M.; Brunátová, T.; Daniš, S.; Peikertová, P.; Troppová, I.; Kočí, K. Novel TiO2 Prepared from Titanyl Sulphate by Using Pressurized Water Processing and Its Photocatalytic Activity Evaluation. Mater. Res. Bull. 2017, 95, 30–46. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupková, J.; Kustrowski, P.; Praus, P. Photocatalytic Decomposition of N2O over TiO2/g-C3N4 Photocatalysts Heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, M.; Liao, L.; Hu, W.; Liu, Z.; Liu, Z.; Guo, L.; Li, K.; Cui, Y.; Lin, F.; et al. 100% N2O Inhibition in Photocatalytic NOx Reduction by Carbon Particles over Bi2WO6/TiO2 Z-Scheme Heterojunctions. Chem. Eng. J. 2023, 453, 139892. [Google Scholar] [CrossRef]
- Sihor, M.; Hanif, M.B.; Thirunavukkarasu, G.K.; Liapun, V.; Edelmannova, M.F.; Roch, T.; Satrapinskyy, L.; Pleceník, T.; Rauf, S.; Hensel, K.; et al. Anodization of Large Area Ti: A Versatile Material for Caffeine Photodegradation and Hydrogen Production. Catal. Sci. Technol. 2022, 12, 5045–5052. [Google Scholar] [CrossRef]
- Monfort, O.; Roch, T.; Gregor, M.; Satrapinskyy, L.; Raptis, D.; Lianos, P.; Plesch, G. Photooxidative Properties of Various BiVO4/TiO2 Layered Composite Films and Study of Their Photocatalytic Mechanism in Pollutant Degradation. J. Environ. Chem. Eng. 2017, 5, 5143–5149. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W.; Sun, W.; Yang, C.; Lu, Z.; Li, C. Mechanisms and Toxicity Evaluation of the Degradation of Sulfamethoxazole by MPUV/PMS Process. Chemosphere 2018, 212, 365–375. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).