Daidzein Protects Caco-2 Cells against Lipopolysaccharide-Induced Intestinal Epithelial Barrier Injury by Suppressing PI3K/AKT and P38 Pathways
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity of LPS, Daidzein, LY294002, and SB203580
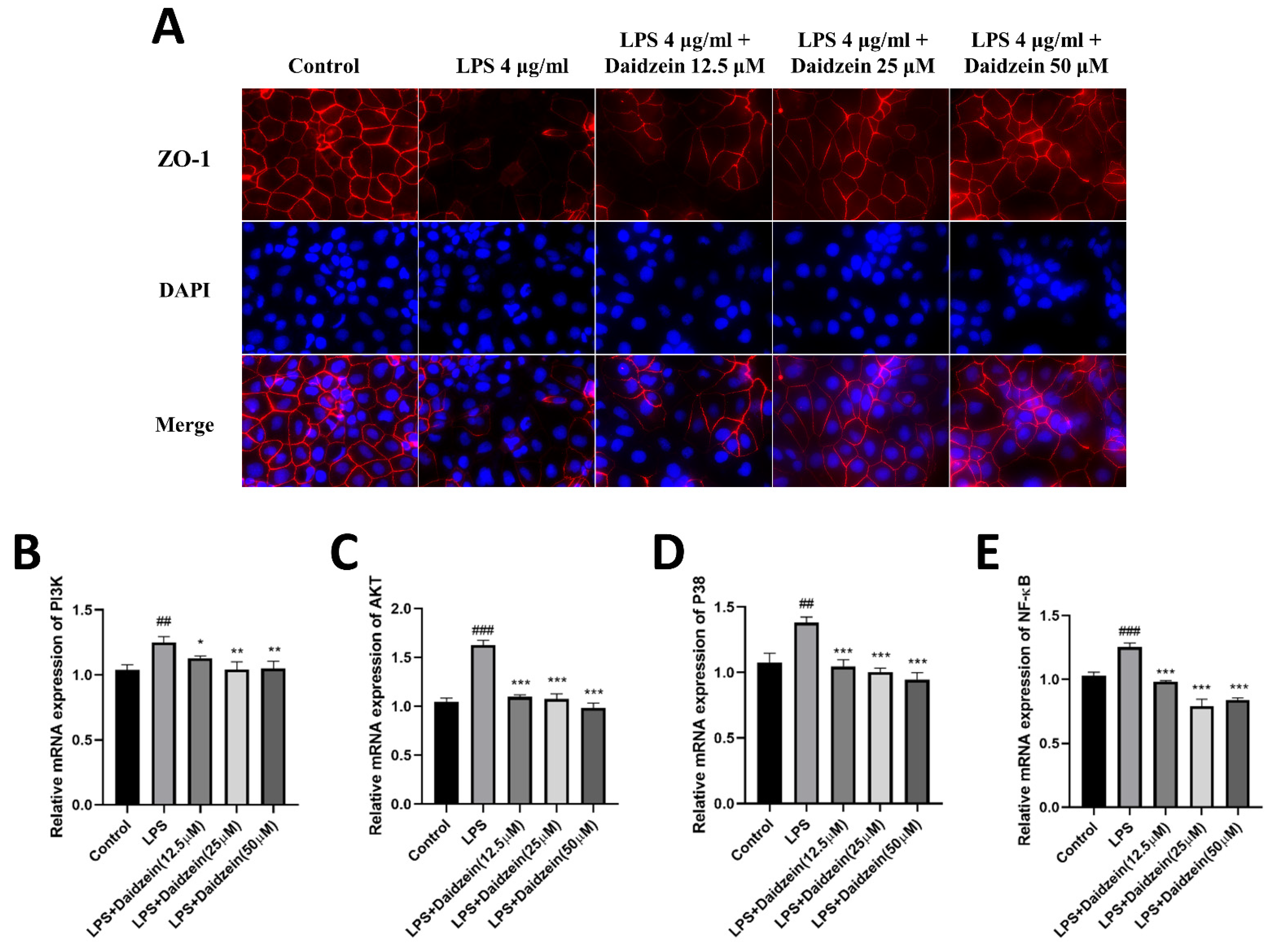
2.2. Daidzein Improved LPS-Induced Integrity Disruption in Caco-2 Cell Monolayer
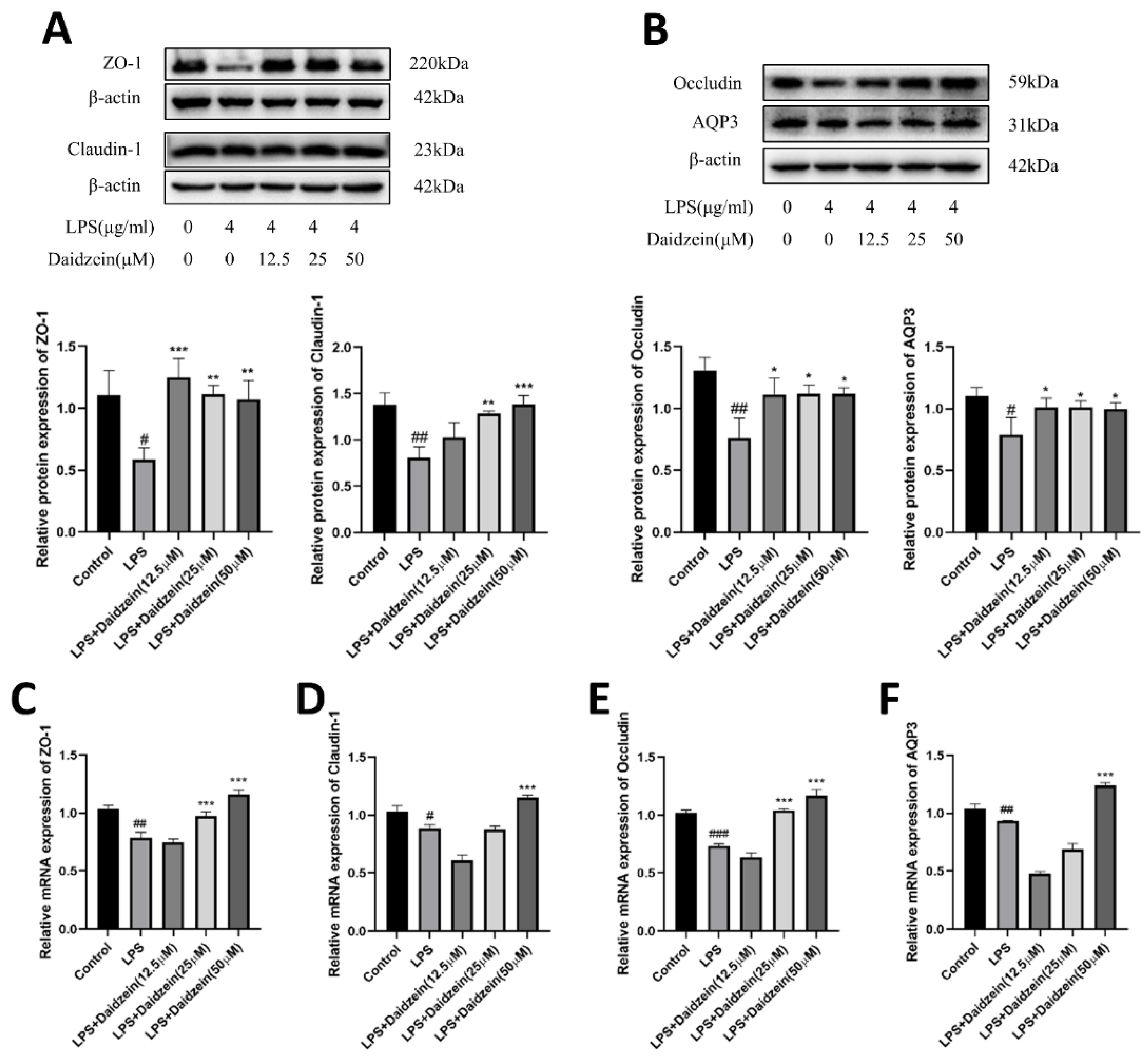
2.3. Daidzein Increased LPS-Induced Downregulated Expression of TJ and AQP3 Proteins and mRNA
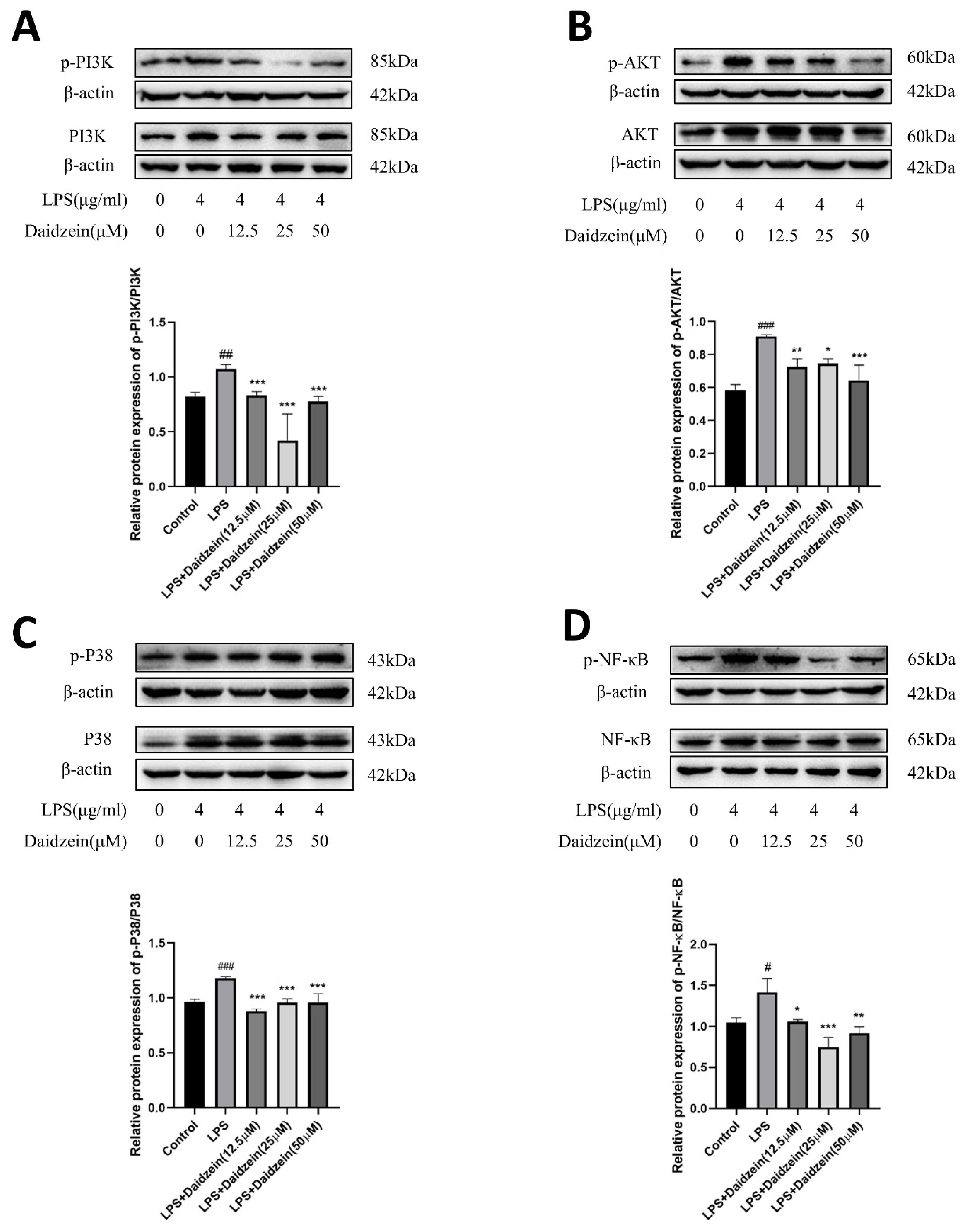
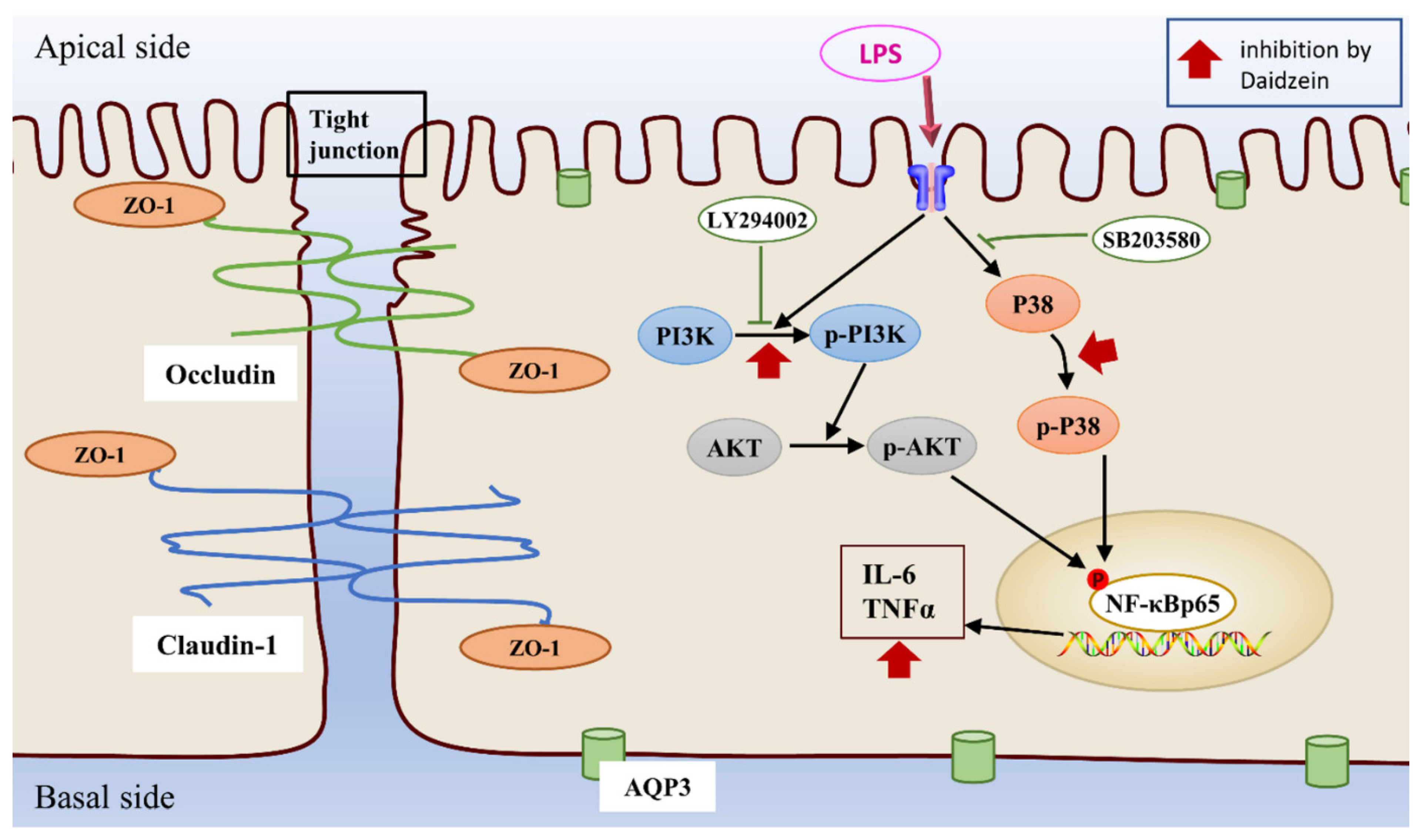
2.4. Daidzein Inhibited LPS-Induced PI3K/AKT and P38 Pathway Activation in Caco-2 Cells
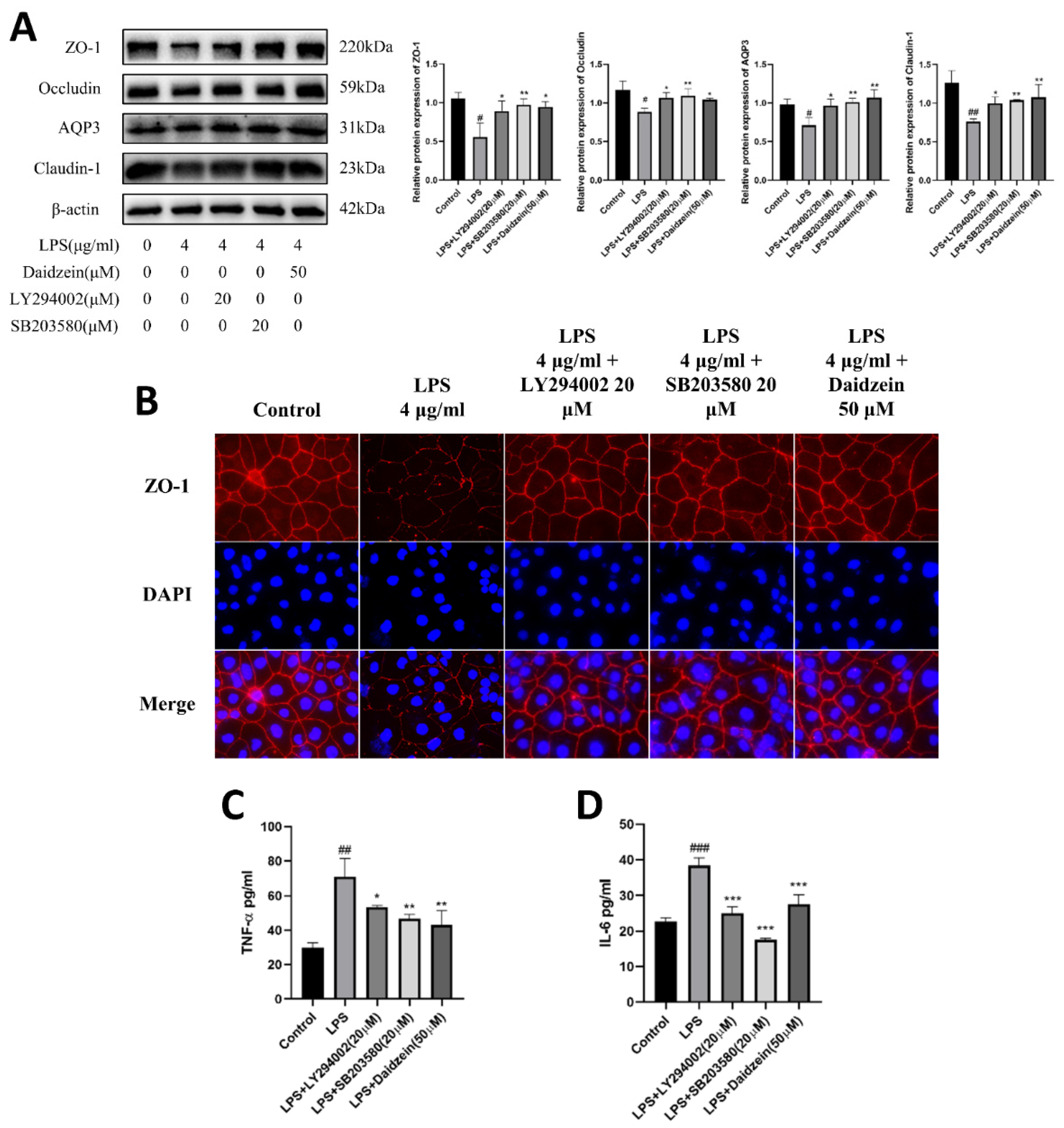
2.5. Daidzein Improved LPS-Induced Downregulation Expression of TJ and AQP3 Proteins by Inhibiting PI3K/AKT and P38 Pathways
2.6. Daidzein Suppressed LPS-Induced Inflammation through PI3K/AKT and P38 Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Solution Preparation
4.3. Cell Viability Assays
4.4. Intestinal Epithelial Barrier Function Measurement
4.5. Enzyme-Linked Immunosorbent Assays (ELISA)
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Immunofluorescent (IF) Analysis
4.8. Western Blotting Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Platts-Mills, J.A.; Nasrin, D.; Roose, A.; Blackwelder, W.C.; Levine, M.M. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017, 35, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.; Gill, R. Infectious diarrhea. Gut Microbes 2014, 1, 4–21. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Weil, A.; Ryan, E.T.; Calderwood, S.B.; Harris, J.B.; Chowdhury, F.; Begum, Y.; Qadri, F.; LaRocque, R.C.; Turnbaugh, P.J. Gut microbial succession follows acute secretory diarrhea in humans. mBio 2015, 6, e315–e381. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Moura-Neto, V.; Bolick, D.T.; Guerrant, R.L.; Fawad, J.A.; Shin, J.H.; Medeiros, P.H.Q.S.; Ledwaba, S.E.; Kolling, G.L.; Martins, C.S.; et al. S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides difficile Infection by Modulating Inflammatory Response. Front. Cell. Infect. Mi. 2021, 11, 739874. [Google Scholar] [CrossRef]
- Chen, J.; Wan, C.; Gong, S.; Fang, F.; Sun, M.; Qian, Y.; Huang, Y.; Wang, B.; Xu, C.; Ye, L.; et al. Chinese clinical practice guidelines for acute infectious diarrhea in children. World J. Pediatr. 2018, 14, 429–436. [Google Scholar] [CrossRef]
- Barrett, K.E. New ways of thinking about (and teaching about) intestinal epithelial function. Adv. Physiol. Educ. 2008, 32, 25–34. [Google Scholar] [CrossRef]
- Runkle, E.A.; Mu, D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef]
- Mukiza, C.N.; Dubreuil, J.D. Escherichia coli Heat-Stable Toxin b Impairs Intestinal Epithelial Barrier Function by Altering Tight Junction Proteins. Infect. Immun. 2013, 81, 2819–2827. [Google Scholar] [CrossRef]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Aspects Med. 2012, 33, 642–650. [Google Scholar] [CrossRef]
- Ricanek, P.; Lunde, L.; Frye, S.; Morth, J.; Rydning, A.; Vatn, M.; Amiry-Moghaddam, M.; Stoen, M.; Nygaard, S.; Toenjum, T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin. Exp. Gastroenterol. 2015, 8, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Awomoyi, A.; Thomas, K.E.; Phalipon, A.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Qureshi, N.; Sztein, M.B.; Vogel, S.N. Differential Activation of Human TLR4 by Escherichia coli and Shigella flexneri 2a Lipopolysaccharide: Combined Effects of Lipid a Acylation State and TLR4 Polymorphisms on Signaling. J. Immunol. 2008, 180, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef]
- Tunisi, L.; Forte, N.; Fernández-Rilo, A.C.; Mavaro, I.; Capasso, R.; D’Angelo, L.; Milić, N.; Cristino, L.; Di Marzo, V.; Palomba, L. Orexin-A Prevents Lipopolysaccharide-Induced Neuroinflammation at the Level of the Intestinal Barrier. Front. Endocrinol. 2019, 10, 219. [Google Scholar] [CrossRef]
- Li, F.; Huang, L.; Dong, C.; Wang, J.; Wu, H.; Shuang, S. Down-regulation of aquaporin3 expression by lipopolysaccharidevia p38/c-Jun N-terminal kinase signaling pathway in HT-29 human colon epithelial cells. World J. Gastroenterol. 2015, 21, 4547–4554. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef]
- Kim, S.; Kawaguchi, K.; Hayashi, H.; Furusho, K.; Maruyama, M. Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients 2019, 11, 1746. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential Effects of Flavonoids on Barrier Integrity in Human Intestinal Caco-2 Cells. J. Agr. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Ou, W.; Hu, H.; Yang, P.; Dai, J.; Ai, Q.; Zhang, W.; Zhang, Y.; Mai, K. Dietary daidzein improved intestinal health of juvenile turbot in terms of intestinal mucosal barrier function and intestinal microbiota. Fish Shellfish Immun. 2019, 94, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, D.; Liang, L.; Song, L.; Zhao, W. Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected Caco-2 cells. Arch. Virol. 2015, 160, 1421–1433. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Wang, J.; Yu, P.; Liu, Q.; Zeng, D.; Song, H.; Kuang, Z. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can. J. Physiol. Pharm. 2015, 93, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef] [PubMed]
- El Feghaly, R.E.; Stauber, J.L.; Deych, E.; Gonzalez, C.; Tarr, P.I.; Haslam, D.B. Markers of Intestinal Inflammation, Not Bacterial Burden, Correlate with Clinical Outcomes in Clostridium difficile Infection. Clin. Infect. Dis. 2013, 56, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, R.; Chen, J.; Wu, Q.; Kuang, Z. Baicalin Protects against TNF-α-Induced Injury by Down-Regulating miR-191a That Targets the Tight Junction Protein ZO-1 in IEC-6 Cells. Biol. Pharm. Bull. 2017, 40, 435–443. [Google Scholar] [CrossRef]
- Lu, X.; Li, C.; Li, C.; Li, P.; Fu, E.; Xie, Y.; Jin, F. Heat-Labile Enterotoxin-Induced PERK-CHOP Pathway Activation Causes Intestinal Epithelial Cell Apoptosis. Front. Cell. Infect. Microbiol. 2017, 7, 244. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Bojarski, C.; Zeissig, S.; Heller, F.; Gitter, A.H.; Fromm, M. Disrupted barrier function through epithelial cell apoptosis. Ann. N. Y. Acad. Sci. 2006, 1072, 288–299. [Google Scholar] [CrossRef]
- Lv, Z.; Dai, H.; Wei, Q.; Jin, S.; Wang, J.; Wei, X.; Yuan, Y.; Yu, D.; Shi, F. Dietary genistein supplementation protects against lipopolysaccharide-induced intestinal injury through altering transcriptomic profile. Poult. Sci. 2020, 99, 3411–3427. [Google Scholar] [CrossRef]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef]
- Byun, E.B.; Sung, N.Y.; Yang, M.S.; Lee, B.S.; Song, D.S.; Park, J.N.; Kim, J.H.; Jang, B.S.; Choi, D.S.; Park, S.H.; et al. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-kappa B and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem. Toxicol. 2014, 74, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, C.; Tang, Q.; Zheng, W.; Feng, Z.; Yu, X.; Guo, X.; Wang, J. Paeonol Inhibits IL-1beta-Induced Inflammation via PI3K/Akt/NF-kappa B Pathways: In Vivo and Vitro Studies. Inflammation 2017, 40, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Yu, Q.; Sun, Q.; Xu, Y.; Gu, Q.; Xu, X. H-RN, a novel antiangiogenic peptide derived from hepatocyte growth factor inhibits inflammation in vitro and in vivo through PI3K/AKT/IKK/NF-kappa B signal pathway. Biochem. Pharmacol. 2014, 89, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Qian, D.; Jiang, S.; Shang, E.; Zhu, Z.; Duan, J. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 3634. [Google Scholar] [CrossRef] [PubMed]
- Heit, B.; Tavener, S.; Raharjo, E.; Kubes, P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 2002, 159, 91–102. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M.; Porn-Ares, M.I.; Grethe, S.; Smith, D.; Zheng, L.; Andersson, T. p38 Mitogen-activated protein kinase and phosphatidylinositol 3-kinase activities have opposite effects on human neutrophil apoptosis. FASEB J. 2002, 16, 129–131. [Google Scholar] [CrossRef]
- Berra, E.; Diaz-Meco, M.T.; Moscat, J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J. Biol. Chem. 1998, 273, 10792–10797. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Lei, S.; Zhao, B.; Xia, Z. LY294002 prevents lipopolysaccharide induced hepatitis in a murine model by suppressing IkappaB phosphorylation. Mol. Med. Rep. 2016, 13, 811–816. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.N.; Wang, H.; Ma, J.Y.; Zhai, S.S.; Duan, J. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-kappa B signaling pathway. Mol. Immunol. 2020, 120, 13–22. [Google Scholar] [CrossRef]



| Gene | Upstream Primer Sequence | Downstream Primer Sequence |
|---|---|---|
| ZO-1 | AAACAAACCCAGCATCATCAAC | TGTGCCCTGGGTGACTAACG |
| Occludin | TTCCTATAAATCCACGCCGG | TGTCTCAAAGTTACCACCGCTG |
| Claudin-1 | GCCAGGTACGAATTTGGTCAG | TTGGTGTTGGGTAAGAGGTTGT |
| AQP3 | GACCCTCATCCTGGTGATGTTT | GCCCAGAGTGACAGCAAAGC |
| PI3K | ACTGCCGAGAGATTTTCCCAC | TCACTCATCTGTCGCAGGCA |
| AKT | TACTCTTTCCAGACCCACGACC | CCCGGTACACCACGTTCTTCT |
| P38 | GCTCTCCAGACCATTTCAGTCC | CATGAGATGGGTCACCAGATACAC |
| NF-κB | TCCCATCTTTGACAATCGTGC | AGCCTGGTCCCGTGAAATAC |
| Gapdh | GGAAGCTTGTCATCAATGGAAATC | TGATGACCCTTTTGGCTCCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wei, X.; Ding, M.; Luo, Z.; Tan, X.; Zheng, Z. Daidzein Protects Caco-2 Cells against Lipopolysaccharide-Induced Intestinal Epithelial Barrier Injury by Suppressing PI3K/AKT and P38 Pathways. Molecules 2022, 27, 8928. https://doi.org/10.3390/molecules27248928
Zhang B, Wei X, Ding M, Luo Z, Tan X, Zheng Z. Daidzein Protects Caco-2 Cells against Lipopolysaccharide-Induced Intestinal Epithelial Barrier Injury by Suppressing PI3K/AKT and P38 Pathways. Molecules. 2022; 27(24):8928. https://doi.org/10.3390/molecules27248928
Chicago/Turabian StyleZhang, Baoping, Xiaohan Wei, Mengze Ding, Zhenye Luo, Xiaomei Tan, and Zezhong Zheng. 2022. "Daidzein Protects Caco-2 Cells against Lipopolysaccharide-Induced Intestinal Epithelial Barrier Injury by Suppressing PI3K/AKT and P38 Pathways" Molecules 27, no. 24: 8928. https://doi.org/10.3390/molecules27248928
APA StyleZhang, B., Wei, X., Ding, M., Luo, Z., Tan, X., & Zheng, Z. (2022). Daidzein Protects Caco-2 Cells against Lipopolysaccharide-Induced Intestinal Epithelial Barrier Injury by Suppressing PI3K/AKT and P38 Pathways. Molecules, 27(24), 8928. https://doi.org/10.3390/molecules27248928






