Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product
Abstract
1. Introduction
2. Results
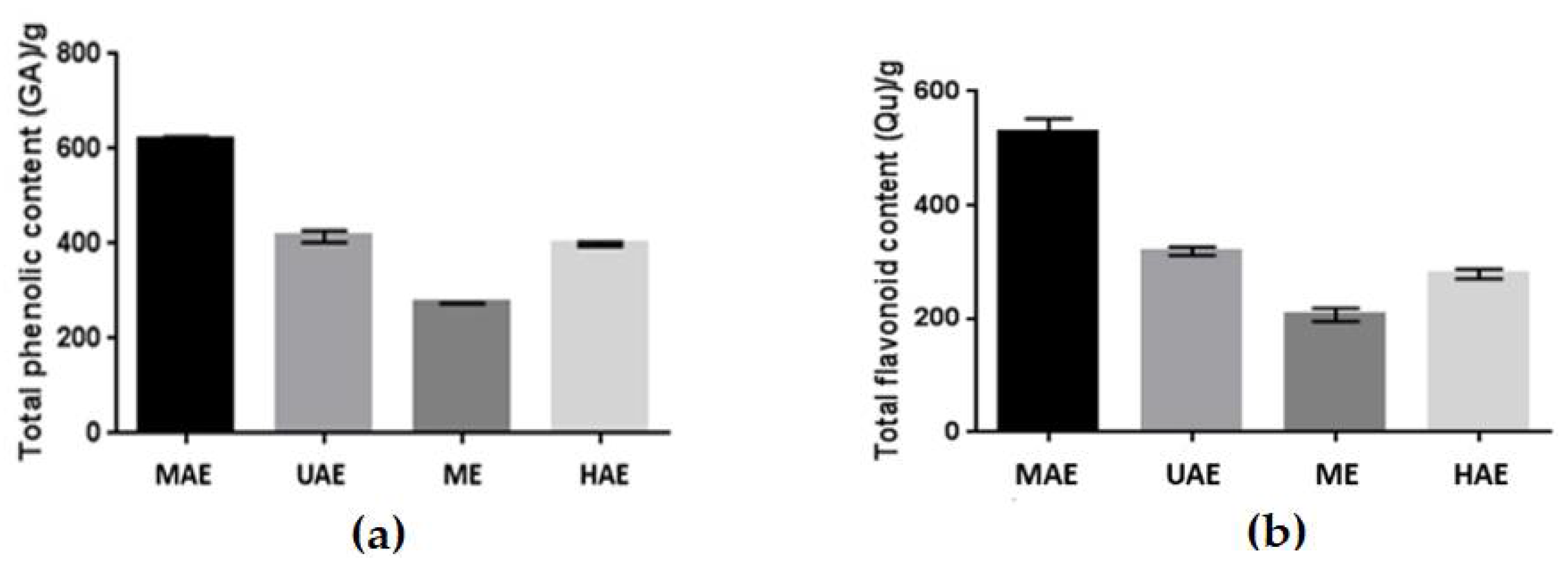
2.1. Phytochemical Composition of the Extracts and Quantitative Analysis of Main Constituents
| TR (min.) | Observed Ion Mass [M + H]+/(Fragments) | ppm | Formula | Identified | Ref. |
|---|---|---|---|---|---|
| 5.50 | 433.22431 (415, 455) | 4.47 | C24H32O7 | Schisandrol A | [18,22] |
| 7.10 | 417.19097 (399) | 0.46 | C23H28O7 | Schisandrol B | [18,22] |
| 9.80 | 501.24861 (401,483,523,539) | 0.63 | C28H36O8 | Micrantherin A | [18] |
| 11.30 | 530.25361 (431,548,569) | 4.87 | C29H37O9 | Angeloylgomisin Q | [18] |
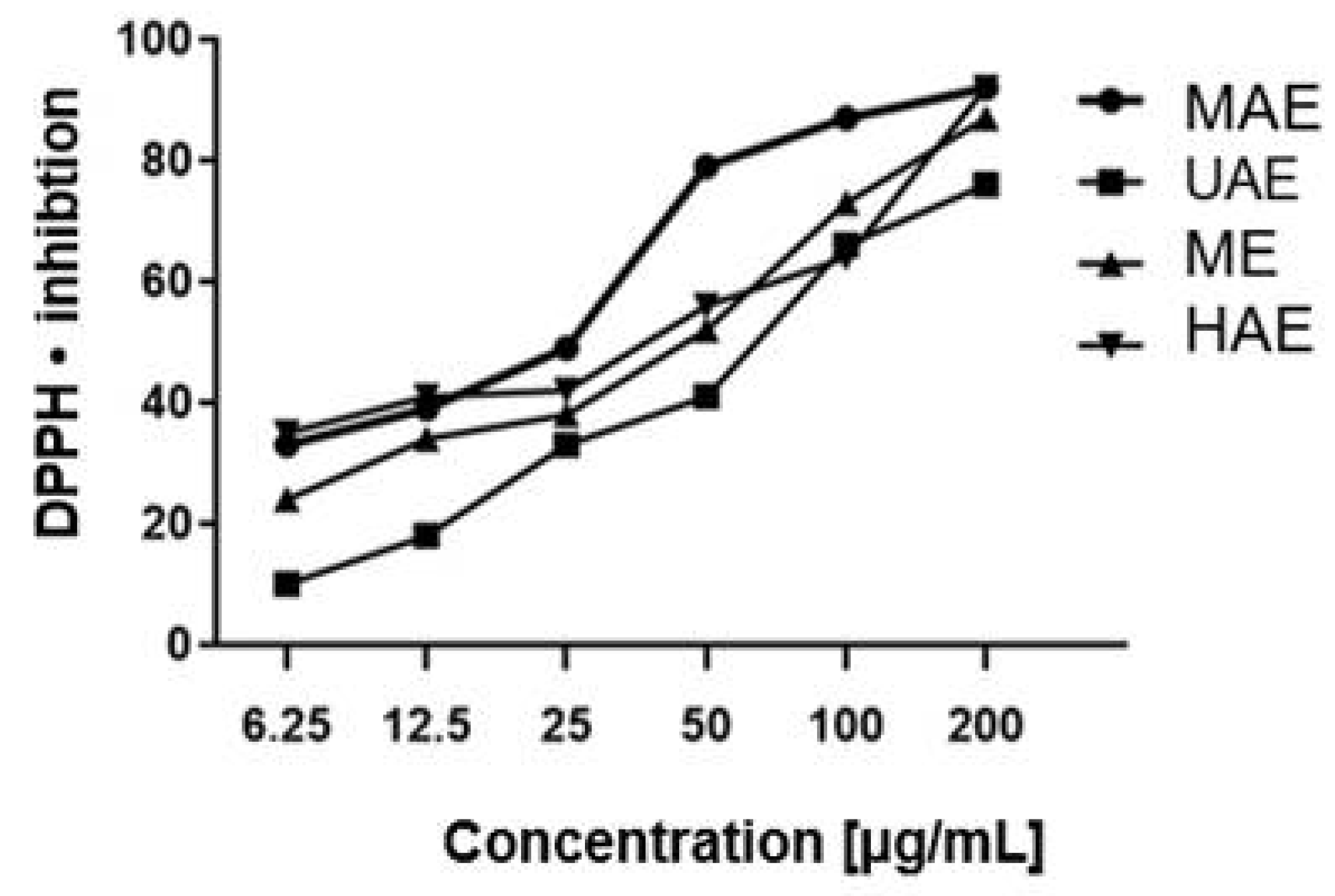
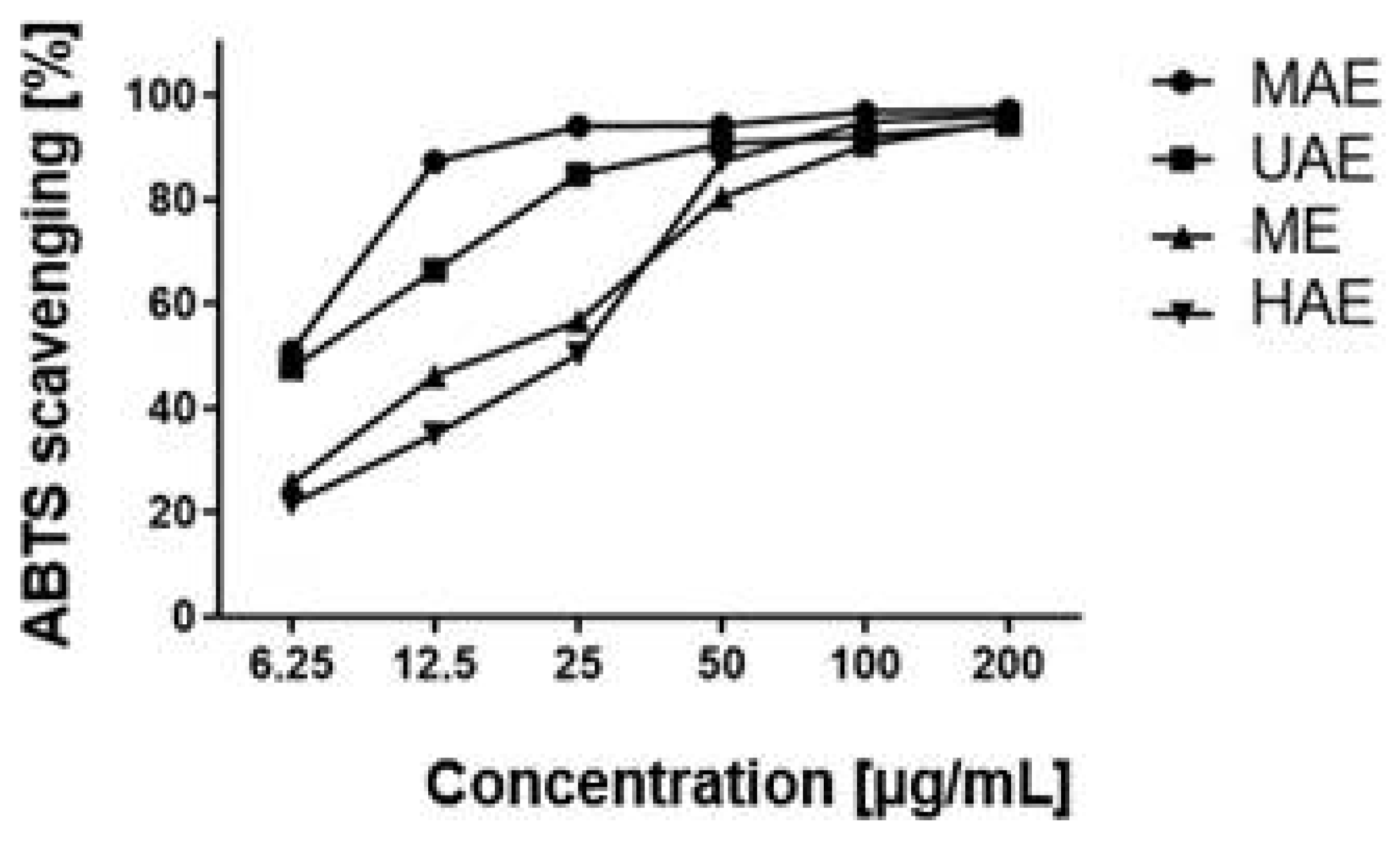
2.2. Antioxidant Activity
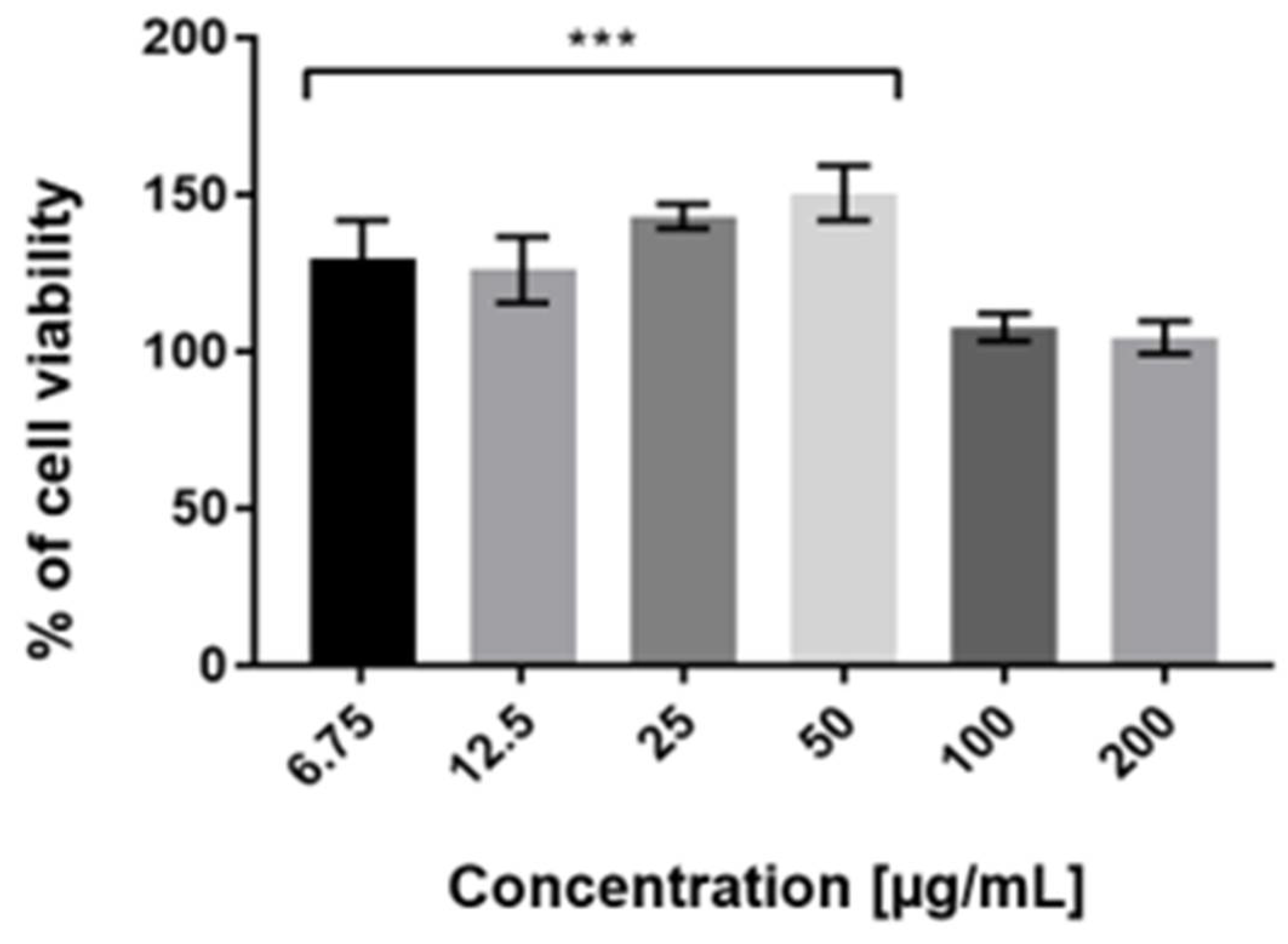
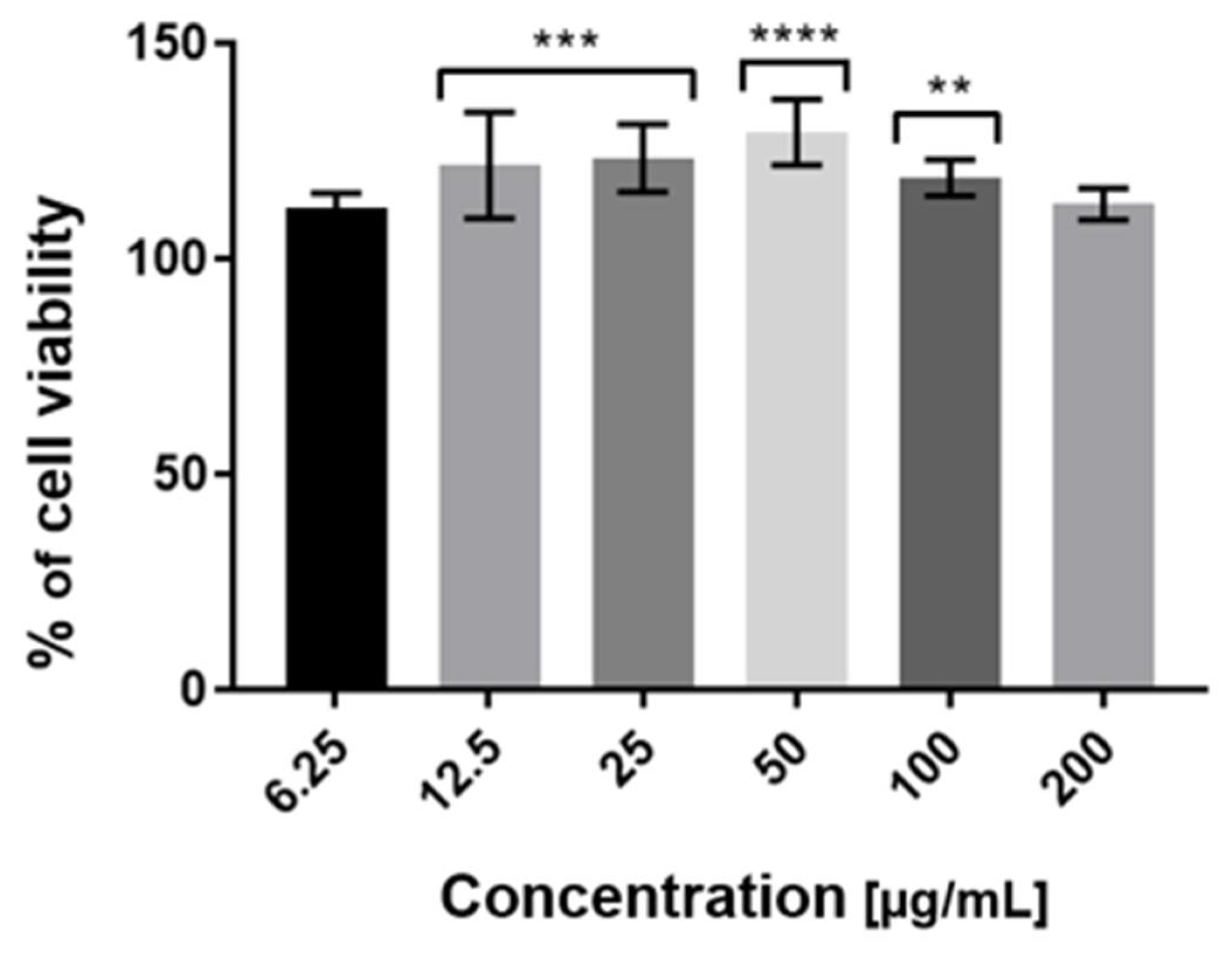
2.3. Cytotoxicity Assessment
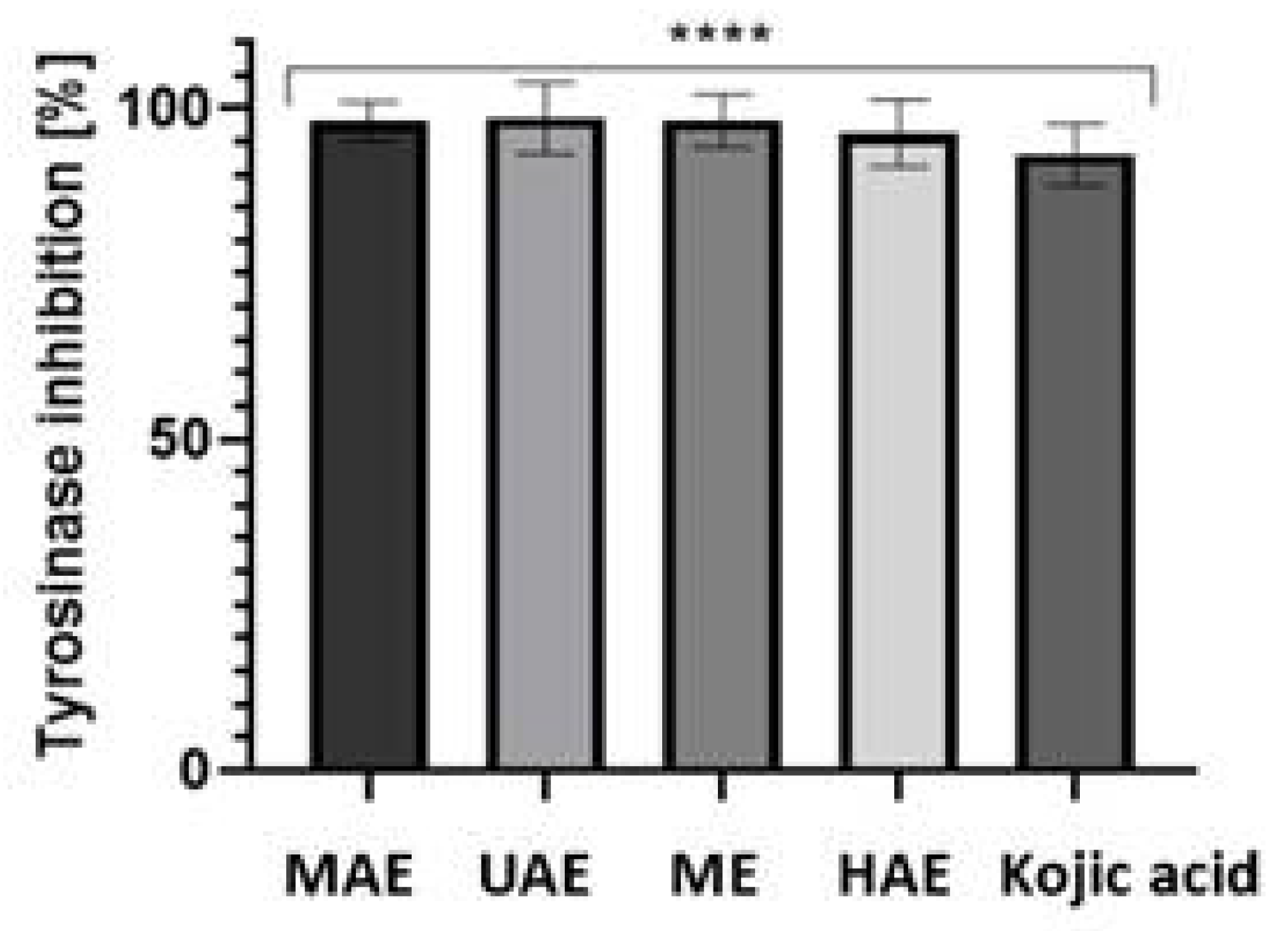
2.4. Tyrosinase Activity
2.5. Irritant Potential of Model Body Wash Gels
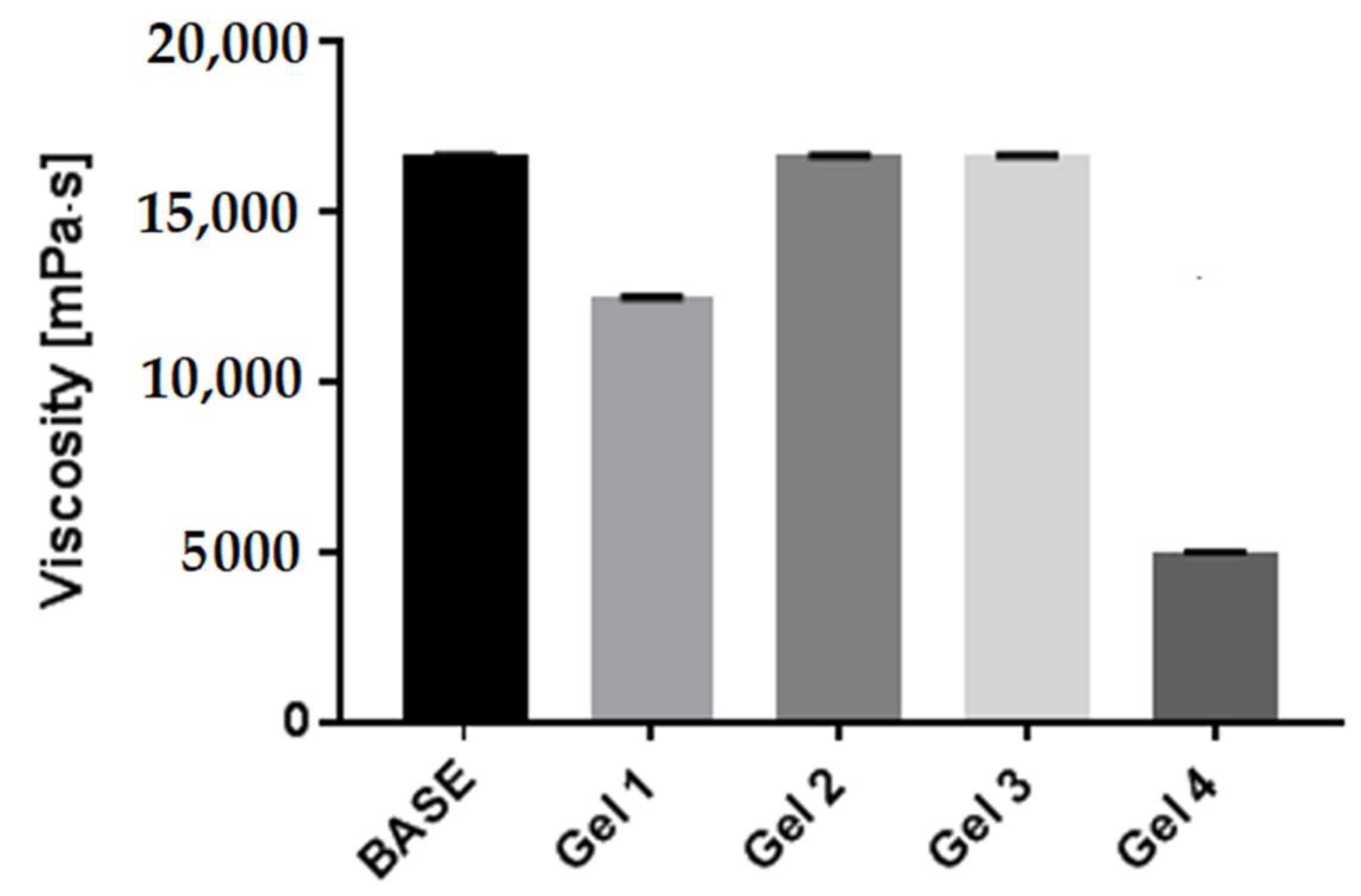
2.6. Rheological Properties of Model Body Wash Gels
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction Procedure
4.1.1. Ultrasound-Assisted Extraction Method (UAE)
4.1.2. Microwave-Assisted Extraction Method (MAE)
4.1.3. Heat-Assisted Extraction Method (HAE)
4.1.4. Maceration with Stirring (ME)
4.2. Total Phenolic Content Determination
4.3. Total Flavonoids Content Determination
4.4. UHPLC-MS Analysis
4.5. Antioxidant Assay
4.5.1. DPPH Radical Scavenging Assay
4.5.2. ABTS•+ Radical Scavenging Assay
4.6. Tyrosinase Inhibitory Activity Measurement
4.7. Cell Culture
4.8. Cell Viability Assay
4.8.1. Neutral Red Uptake Assay
4.8.2. Alamar Blue Assay
4.9. Technology for Obtaining Prototypical Body Wash Gels
4.10. Zein Test
4.11. Viscosity Measurements
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Oh, E.Y.; Jang, J.Y.; Choi, Y.H.; Choi, Y.W.; Choi, B.T. Inhibitory effects of 1-O-methyl-fructofuranose from Schisandra chinensis fruit on melanogenesis in B16F0 melanoma cells. J. Ethnopharmacol. 2010, 132, 219–224. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Bulter, H. Poucher’s Perfumes, Cosmetics and Soaps, 10th ed.; Kluwer Academic Publishers: London, UK, 2010. [Google Scholar]
- Mostafa, E.; Maher, A.; Mostafa, D.; Gad, S.; Nawwar, M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Barel, M.; Paye, M. Handbook of Cosmetic Science and Technology, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Opletal, L.; Sovova, H.; Bartlowa, M. Dibenzo [a,c] cyclooctadienelignans of the genus Schizandra: Importance, isolation and determination. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, D.-F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef]
- Ranouille, E.; Boutot, C.; Bony, E.; Bombarde, O.; Grosjean, S.; Lazewski, A.; Berthon, J.-Y.; Filaire, E. Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study. Cosmetics 2018, 5, 36. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, J.J.; Jeong, K.Y.; Hank, S.K.; Jeong, T.H.; Yun, M.Y. Antioxidant activity and inhibition of MMP-1 expression of Schizandrae fructus extract. Kor. J. Pharmacogn. 2013, 1, 47–52. [Google Scholar]
- Lam, P.Y.; Yan, C.W.; Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Schisandrin B protects against solar irradiation-induced oxidative stress in rat skin tissue. Fitoterapia 2011, 82, 393–400. [Google Scholar] [CrossRef]
- Kang, Y.H.; Shin, H.M. Inhibitory effects of Schizandra chinensis extract on atopic dermatitis in NC/Nga mice. Immunopharmacol. Immunotoxicol. 2011, 34, 292–298. [Google Scholar] [CrossRef]
- Yan, Z.-F.; Guo, J.; Tian, F.-H.; Mao, X.-X.; Li, Y.; Li, C.-T. Active compounds from Schisandra chinensis exhibiting tyrosinase activity and melanin content inhibition in B16 melanoma cells. Biotechnol. Bioprocess Eng. 2015, 20, 814–823. [Google Scholar] [CrossRef]
- Lamer-Zarawska, E.; Chwała, C.; Gwardys, A. Rośliny w Kosmetyce i Kosmetologii Przeciwstarzeniowej; PZWL: Warszawa, Poland, 2012. [Google Scholar]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crișan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Masike, K.; Mhlongo, M.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Exhaustive Qualitative LC-DAD-MSn Analysis of Arabica Green Coffee Beans: Cinnamoyl-glycosides and Cinnamoylshikimic Acids as New Polyphenols in Green Coffee. J. Agric. Food Chem. 2016, 64, 9663–9674. [Google Scholar] [CrossRef]
- Liao, J.; Zang, J.; Yuan, F.; Liu, S.; Zhang, Y.; Li, H.; Piao, Z.; Li, H. Identification and analysis of anthocyanin components in fruit color variation in Schisandra chinensis. J. Sci. Food Agric. 2015, 96, 3213–3219. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Li, X.; Qi, Y.; Peng, Y.; Zhang, B.; Xiao, P. Chemical analysis of twelve lignans in the fruit of Schisandra sphenanthera by HPLC–PAD-MS. Phytomedicine 2012, 19, 1234–1241. [Google Scholar] [CrossRef]
- Teng, H.; Lee, W.Y. Antibacterial and antioxidant activities and chemical compositions of volatile oils extracted from Schisandra chinensis Baill. seeds using simultaneous distillation extraction method, and comparison with Soxhlet and microwave-assisted extraction. Biosci. Biotechnol. Biochem. 2014, 78, 79–85. [Google Scholar] [CrossRef]
- Adamska-Szewczyk, A.; Zgórka, G. Plant polyphenols in cosmetics—A review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Lee, D.; Kim, Y.-M.; Chin, Y.-W.; Kang, K. Schisandrol A Exhibits Estrogenic Activity via Estrogen Receptor α-Dependent Signaling Pathway in Estrogen Receptor-Positive Breast Cancer Cells. Pharmaceutics 2021, 13, 1082. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in Human Health and Nutrition: A Review of Current Knowledge and Therapeutic Perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Wermerris, W.; Nicholson, R. Phenolic Compounds Biochemistry; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Winckler, J. Vital staining of lysosomes and other cell organelles of the rat with neutral red (author’s transl). Prog. Histochem. Cytochem. 1974, 6, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A New rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, D.H.; Bin Park, C.; Park, T.S.; Park, B.J. Anti-Inflammatory and Skin-Moisturizing Effects of a Flavonoid Glycoside Extracted from the Aquatic Plant Nymphoides indica in Human Keratinocytes. Molecules 2018, 23, 2342. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 19. [Google Scholar] [CrossRef]
- Kang, B.Y.; Kim, S.; Lee, K.-H.; Lee, Y.S.; Hong, I.; Lee, M.-O.; Min, D.; Chang, I.; Hwang, J.S.; Park, J.S.; et al. Transcriptional profiling in human HaCaT keratinocytes in response to kaempferol and identification of potential transcription factors for regulating differential gene expression. Exp. Mol. Med. 2008, 40, 208–219. [Google Scholar] [CrossRef]
- Gao, C.; Chen, H.; Niu, C.; Hu, J.; Cao, B. Protective effect of Schizandrin B against damage of UVB irradiated skin cells depend on inhibition of inflammatory pathways. Bioengineered 2016, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, J.; Kern, D.; Knaggs, H.E. Assessment of Human Skin Gene Expression by Different Blends of Plant Extracts with Implications to Periorbital Skin Aging. Int. J. Mol. Sci. 2018, 19, 3349. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Gao, W.; Wang, D.; Liu, Q.; Zheng, S.; Wang, Y. The Protecting Effect of Deoxyschisandrin and Schisandrin B on HaCaT Cells against UVB-Induced Damage. PLoS ONE 2015, 10, e0127177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Han, J.-M.; Tran, V.H.; Jeong, H.; Kim, E.S. A Study on the Fabrication of an Effective Natural Substance Based on Schisandra chinensis Extracted Fermentation. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Frenk, E. Treatment of melasma with depigmenting agents. In Melasma: New Approaches to Treatment; Martin Dunitz: London, UK, 1995; pp. 9–15. [Google Scholar]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Kopustinskiene, D.; Bernatoniene, J. Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Quirin, K.W. Supercritical schisandra extracts—A new concept for personal care cosmetics. Cosmet Sci. Technol. 2008, 1, 28. [Google Scholar]
- Chen, Y.-C.; Liaw, C.-C.; Cheng, Y.-B.; Lin, Y.-C.; Chen, C.-H.; Huang, Y.-T.; Liou, S.-S.; Chen, S.-Y.; Chien, C.-T.; Lee, G.-C.; et al. Anti-liver fibrotic lignans from the fruits of Schisandra arisanensis and Schisandra sphenanthera. Bioorg. Med. Chem. Lett. 2013, 23, 880–885. [Google Scholar] [CrossRef]
- Yang, B.; Liu, X.; Gao, Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov. Food Sci. Emerg. Technol. 2009, 10, 610–615. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation. Nat. Prod. Isol. 2012, 864, 89–115. [Google Scholar] [CrossRef]
- Antoniou, C.; Kyratzis, A.; Rouphael, Y.; Stylianou, S.; Kyriacou, M.C. Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods 2020, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Boucherle, B.; Rey, A.; Rome, M.; Fuzzati, N.; Peuchmaur, M. Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 2020, 148, 104798. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Matejic, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.M.; Ranđelović, V.N.; Krivošej, Z.D.; Marin, P.D. Total phenolic and flavonoid content, antioxidant and antimicrobial activity of extracts from Tordylium maximum. J. Appl. Pharm. Sci. 2013, 3, 55–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Frątczak, A.; Byłka, W. Ocena właściwości hamujących tyrozynyzę przez wybrane wyciągi roślinne. Pol. J. Cosmetol. 2016, 19, 51–55. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A new fuorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Bujak, T.; Wasilewski, T.; Szmuc, E. Inulin as an effectiveness and safe ingredient in cosmetics. Pol. J. Chem. Technol. 2019, 21, 44–49. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef] [PubMed]

| TR (min.) | Observed Ion Mass [M-H]-/(Fragments) | Δppm | Formula | Identified * | Ref. |
|---|---|---|---|---|---|
| 1.56 | 191.05701 | 4.68 | C7H12O6 | quinic acid | str |
| 8.01 | 153.01946 (109) | 0.83 | C7H6O4 | protocatechuic acid | str |
| 10.37 | 353.08872 (179) | 2.58 | C16H18O9 | neochlorogenic acid | [18], str |
| 13.33 | 337.09293 (163) | 0.12 | C16H18O8 | 3-p-coumaryl quinic acid | [18] |
| 15.52 | 353.08895 (179) | 3.23 | C16H18O9 | chlorogenic acid | [18], str |
| 18.51 | 337.09457 (173) | 4.97 | C16H18O8 | 4-p-coumaryl quinic acid | [18] |
| 19.37 | 337.09338 (191) | 1.45 | C16H18O8 | 5-p-coumaryl quinic acid | [18] |
| 31.72 | 609.14762 (300,463) | 2.48 | C27H30O16 | quercetin-3-O-rutinoside | [18], str |
| 32.17 | 463.08881 (300) | 1.32 | C21H20O12 | quercetin-3-O-galactoside | [18] |
| 32.75 | 463.08864 (300) | 0.95 | C21H20O12 | quercetin-3-O-glucoside | [18], str |
| 37.48 | 593.15299 (285) | 3.02 | C27H30O15 | kaempferol-3-O-rutinoside | [18], str |
| 38.30 | 447.09354 (285) | 0.57 | C21H20O11 | kaempferol-3-O-glucoside | [18], str |
| MAE | UAE | HAE | ME | |
|---|---|---|---|---|
| Phenolics acids | ||||
| Protocatechuic | 2.07 ± 0.11 | 0.92 ± 0.04 | 1.65 ± 0.07 | 1.07 ± 0.08 |
| Chlorogenic (total) | 0.22 ± 0.02 | nd | 0.18 ± 0.01 | nd |
| p-coumaryl quinic 1 (total) | 11.53 ± 0.52 | 6.31 ± 0.32 | 12.04 ± 0.81 | 8.49 ± 0.54 |
| Flavonoids | ||||
| Quercetin-3-O-rutinoside | 0.58 ± 0.03 | 0.22 ± 0.01 | 0.46 ± 0.03 | 0.37 ± 0.03 |
| Quercetin-3-O-galactoside 2 | 0.42 ± 0.02 | 0.19 ± 0.02 | 0.31 ± 0.02 | 0.23 ± 0.02 |
| Quercetin-3-O-glucoside | 0.85 ± 0.03 | 0.22 ± 0.02 | 0.47 ± 0.03 | 0.21 ± 0.02 |
| Kaempferol-3-O-rutinoside | 0.98 ± 0.04 | 0.27 ± 0.01 | 0.70 ± 0.04 | 0.35 ± 0.02 |
| Kaempferol-3-O-glucoside | 0.54 ± 0.04 | 0.24 ± 0.02 | 0.54 ± 0.02 | 0.14 ± 0.01 |
| Anthocyanin | ||||
| cyanidin 3-O-xylosyl-rutinoside 3 | 0.18 ± 0.02 | 0.10 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.02 |
| Ingredient (INCI) | Ingredient Content (%) |
|---|---|
| Aqua | to 100.0 |
| Lauryl Glucoside | 7.0 |
| Schisandra chinensis extract | 3.0 |
| Cocamidopropyl Betaine | 5.0 |
| Cocamide DEA | 1.5 |
| Sodium Benzoate and Potassium Sorbate | 1.0 |
| PEG-75 Lanolin | 0.5 |
| Lactic Acid | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product. Molecules 2022, 27, 8877. https://doi.org/10.3390/molecules27248877
Zagórska-Dziok M, Wójciak M, Ziemlewska A, Nizioł-Łukaszewska Z, Hoian U, Klimczak K, Szczepanek D, Sowa I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product. Molecules. 2022; 27(24):8877. https://doi.org/10.3390/molecules27248877
Chicago/Turabian StyleZagórska-Dziok, Martyna, Magdalena Wójciak, Aleksandra Ziemlewska, Zofia Nizioł-Łukaszewska, Uliana Hoian, Katarzyna Klimczak, Dariusz Szczepanek, and Ireneusz Sowa. 2022. "Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product" Molecules 27, no. 24: 8877. https://doi.org/10.3390/molecules27248877
APA StyleZagórska-Dziok, M., Wójciak, M., Ziemlewska, A., Nizioł-Łukaszewska, Z., Hoian, U., Klimczak, K., Szczepanek, D., & Sowa, I. (2022). Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product. Molecules, 27(24), 8877. https://doi.org/10.3390/molecules27248877






