Non-Covalent Dimer as Donor Chromophore for Constructing Artificial Light-Harvesting System in Water
Abstract
1. Introduction
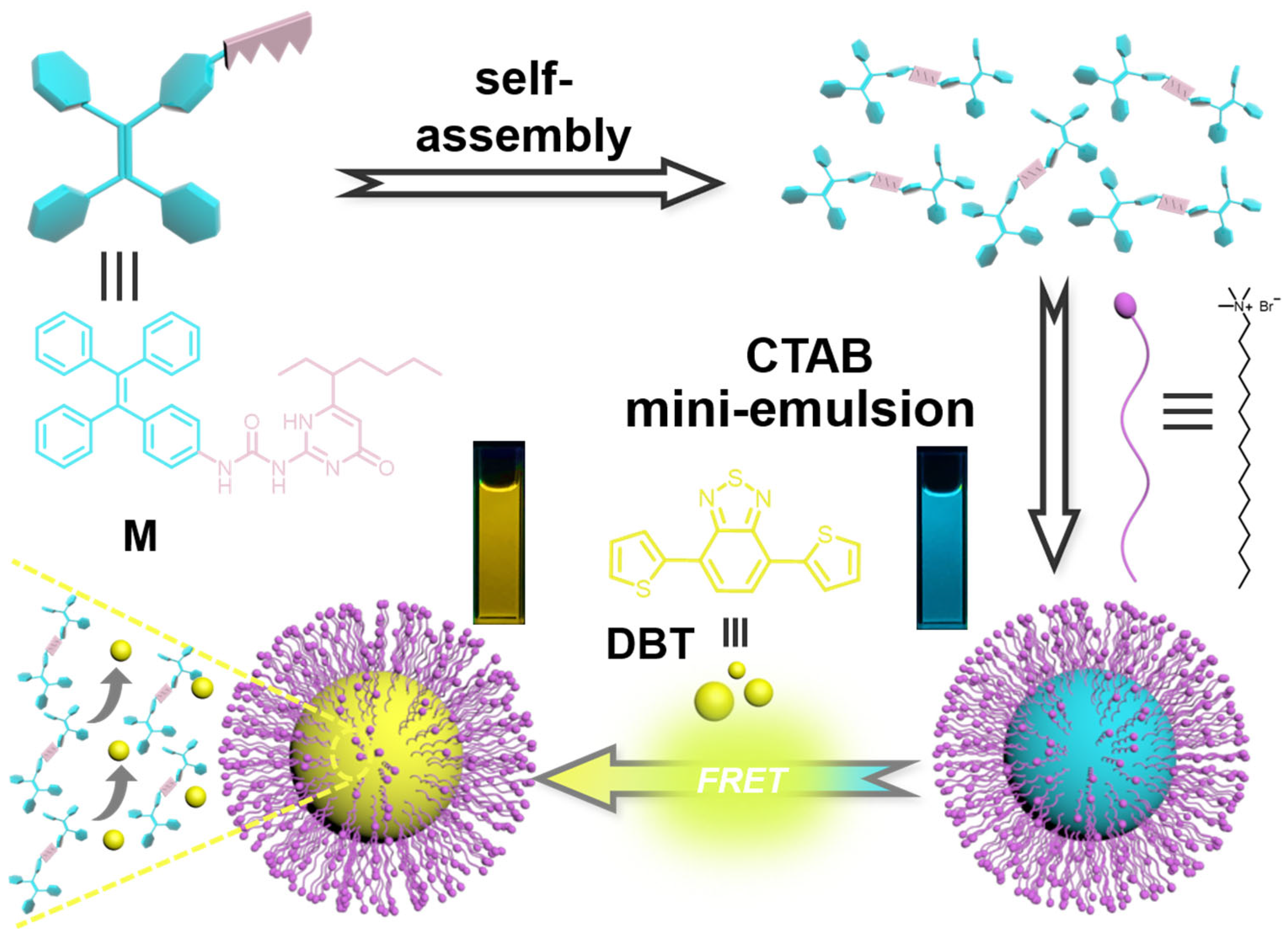
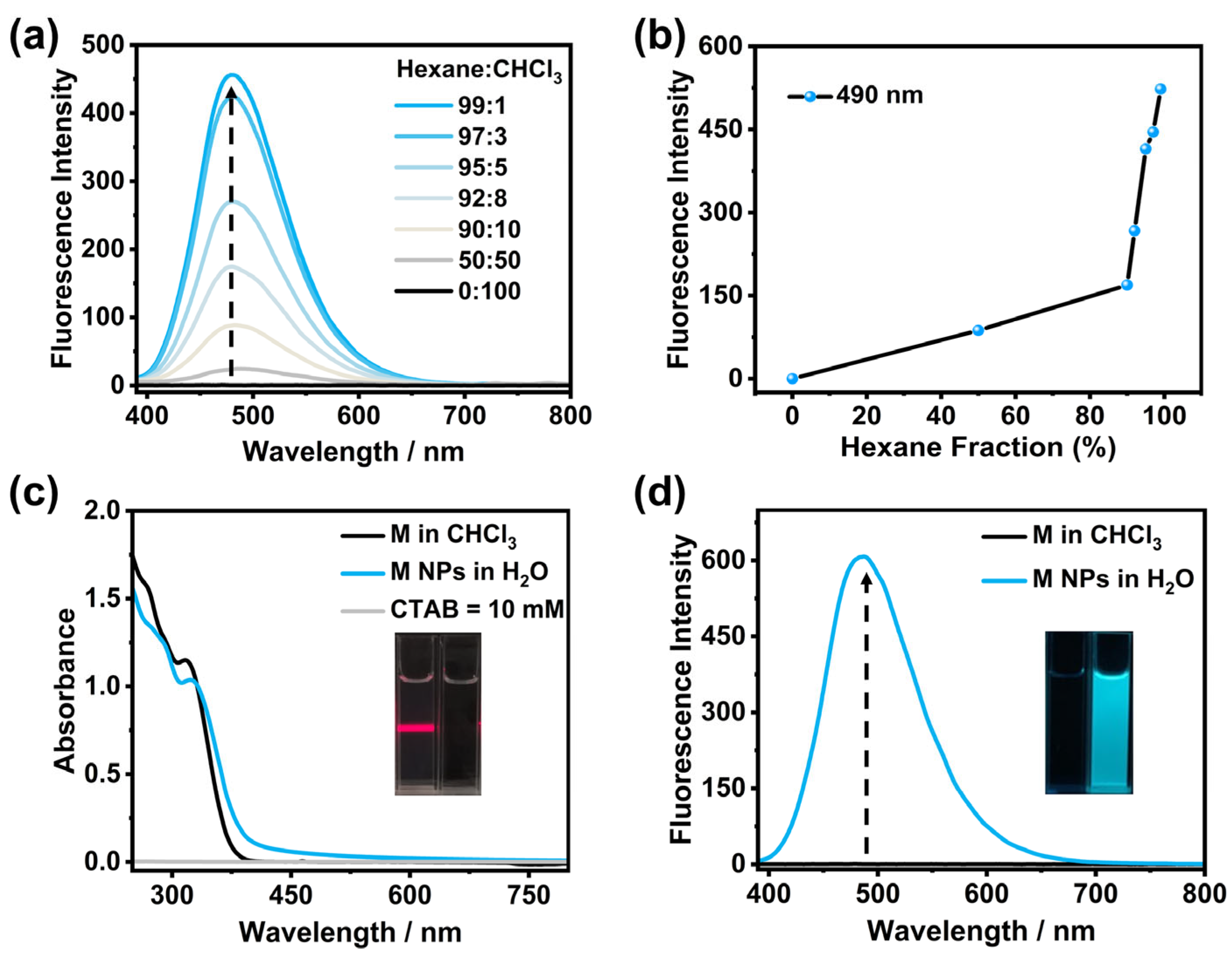
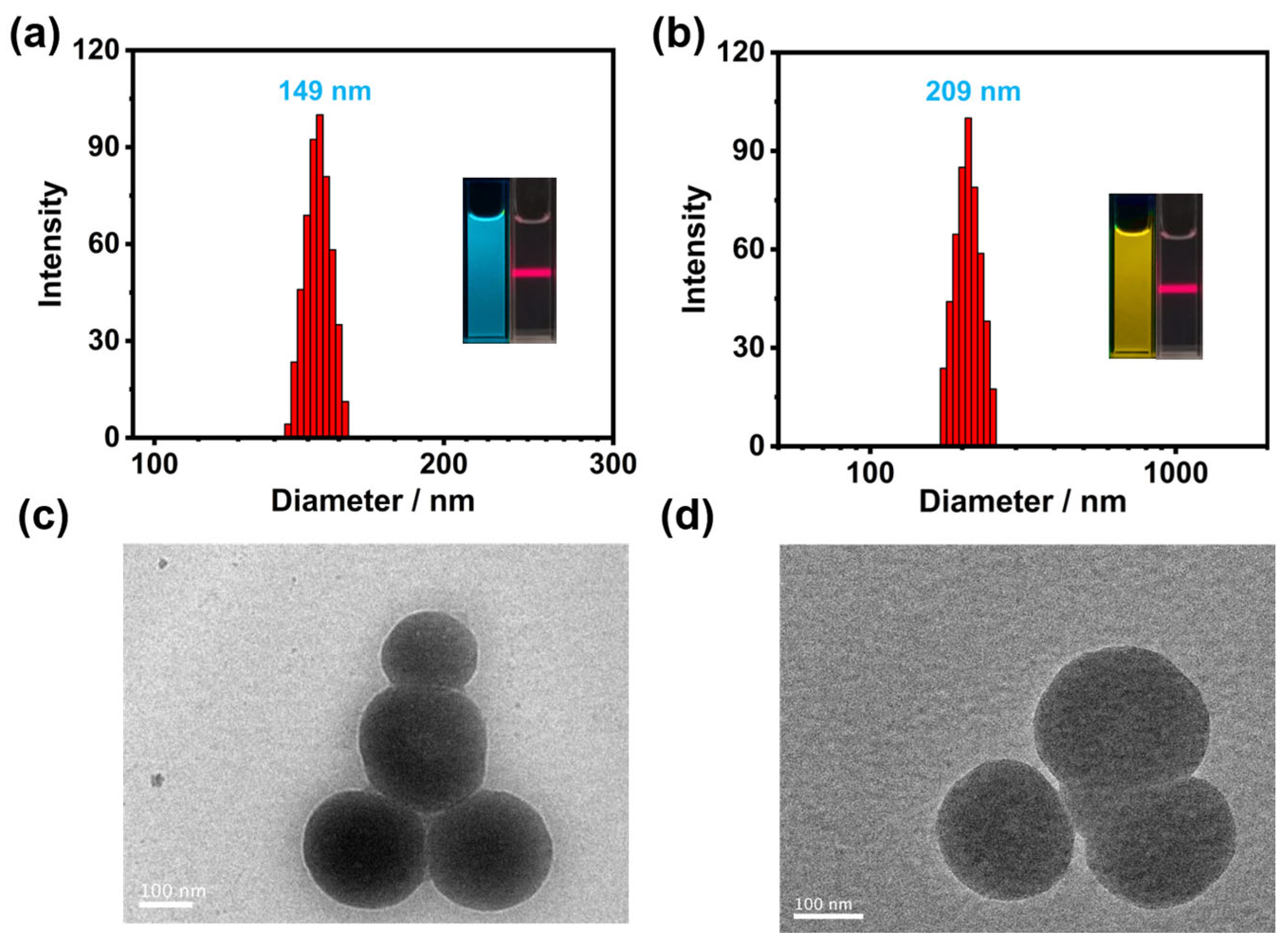
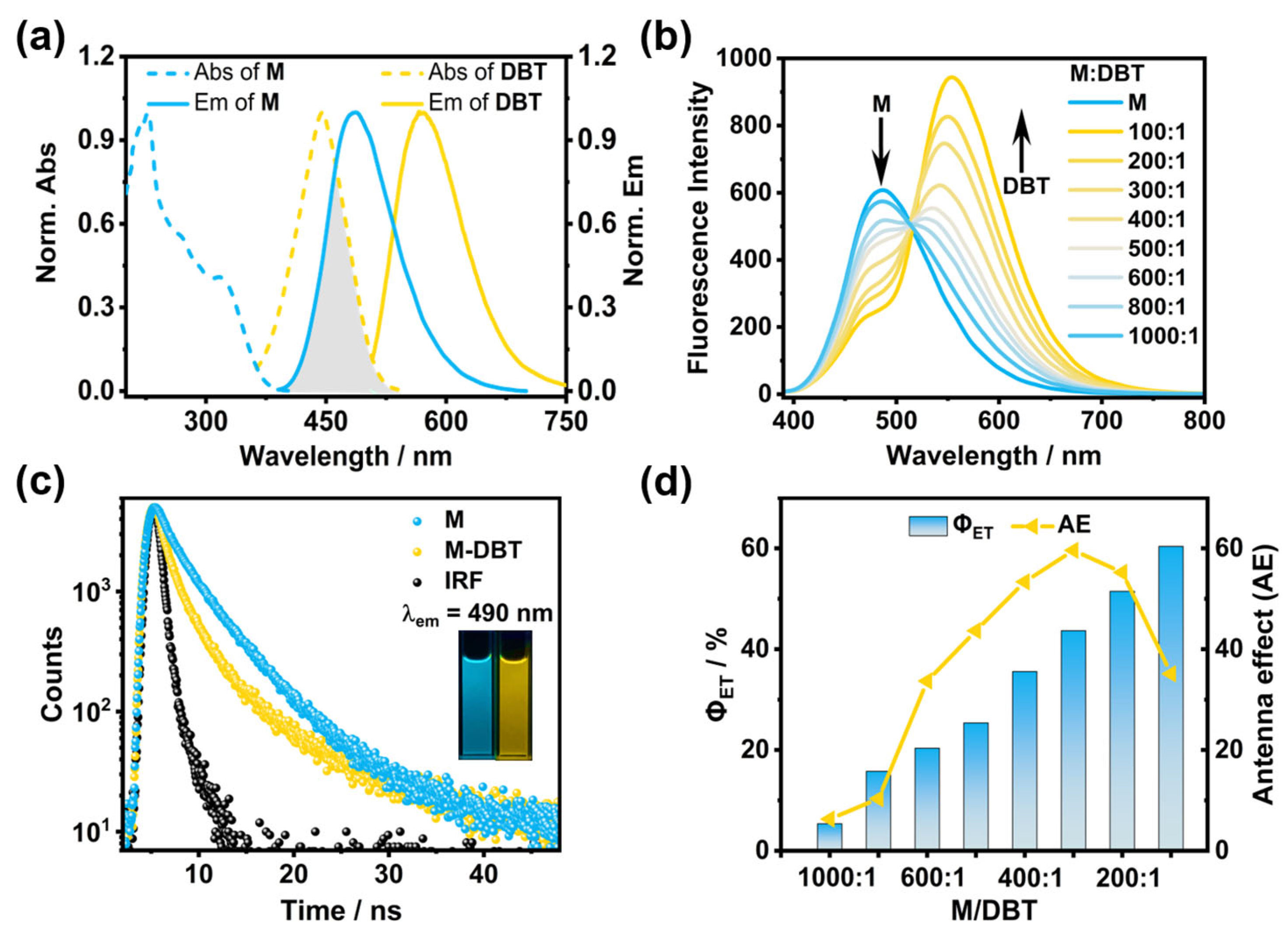
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
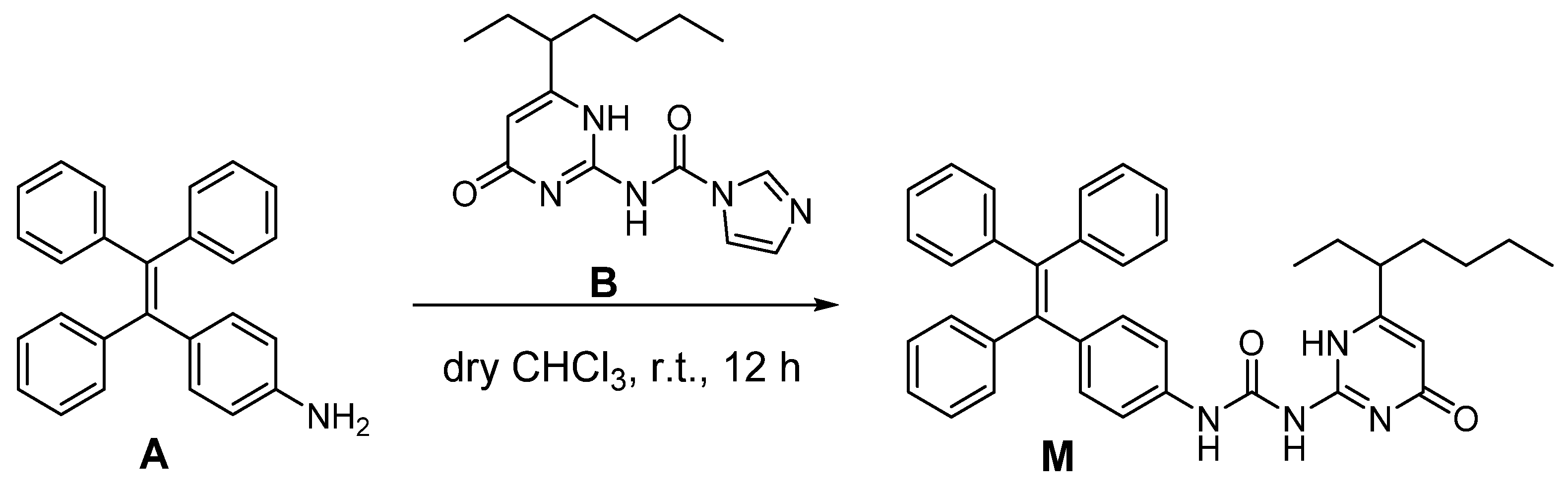
3.3. Synthesis of M
3.4. Preparation of NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, K.; Velmurugan, K.; Li, B.; Hu, X.Y. Artificial light-harvesting systems based on macrocycle-assisted supramolecular assembly in aqueous media. Chem. Commun. 2021, 57, 13641–13654. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zhong, W.; Zhou, L.; Xu, L.; Sun, X.-Q.; Elmes, R.B.P.; Hu, X.-Y.; Wang, L. Artificial light-harvesting systems fabricated by supramolecular host–guest interactions. Chin. Chem. Lett. 2019, 30, 31–36. [Google Scholar] [CrossRef]
- Otsuki, J. Supramolecular approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 2018, 6, 6710–6753. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Niu, L.-Y.; Chen, Y.-Z.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Biological Applications of Supramolecular Assemblies Designed for Excitation Energy Transfer. Chem. Rev. 2015, 115, 7502–7542. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Mahata, K.; Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 2013, 42, 1847–1870. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Qian, H.; Shen, Y.; Wei, C.; Ren, D.; Zhang, L.; Li, Z.-Y.; Wang, L.; Sun, X.-Q. A tunable artificial light-harvesting system based on host-guest interaction exhibiting ultrahigh antenna effect and narrowed emission band. Mater. Today Chem. 2022, 24, 100833. [Google Scholar] [CrossRef]
- Wang, Y.; Han, N.; Li, X.-L.; Wang, R.Z.; Xing, L.-B. Novel Strategy of Constructing Artificial Light-Harvesting System with Two-Step Sequential Energy Transfer for Efficient Photocatalysis in Water. ACS Appl. Mater. Interfaces 2022, 14, 45734–45741. [Google Scholar] [CrossRef]
- Sun, G.; Cai, L.; Cui, H.; Hu, Y.; Wang, J.; Wang, M.; Zhu, J.; Sun, T.; Tang, Y. Naphthalenyl-phenylacrylonitrile-based supramolecular aqueous artificial light-harvesting system for photochemical catalysis. Dye. Pigm. 2022, 201, 110257. [Google Scholar] [CrossRef]
- Chen, X.-M.; Cao, K.-W.; Bisoyi, H.K.; Zhang, S.; Qian, N.; Guo, L.; Guo, D.-S.; Yang, H.; Li, Q. Amphiphilicity-Controlled Polychromatic Emissive Supramolecular Self-Assemblies for Highly Sensitive and Efficient Artificial Light-Harvesting Systems. Small 2022, 18, e2204360. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, N.; Hou, W.; Wang, C.Y.; Wang, Y.; Tang, J.; Yang, Y.-W. Efficient Aggregation-Induced Emission Manipulated by Polymer Host Materials. Adv. Mater. 2019, 31, 1903962. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Chen, Y.; Yu, J.; Cheng, N.; Liu, Y. A Supramolecular Artificial Light-Harvesting System with an Ultrahigh Antenna Effect. Adv. Mater. 2017, 29, 1701905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, W.; Li, S.; Xia, Y.; Li, X.; Li, Y.; Yi, T. Artificial Light-Harvesting Metallacycle System with Sequential Energy Transfer for Photochemical Catalysis. J. Am. Chem. Soc. 2021, 143, 1313–1317. [Google Scholar] [CrossRef]
- Li, Y.; Rajasree, S.S.; Lee, G.Y.; Yu, J.; Tang, J.-H.; Ni, R.; Li, G.; Houk, K.N.; Deria, P.; Stang, P.J. Anthracene–Triphenylamine-Based Platinum(II) Metallacages as Synthetic Light-Harvesting Assembly. J. Am. Chem. Soc. 2021, 143, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Z.; Hou, Y.; Wang, H.; Li, X.; He, G.; Zhang, M. Aqueous Platinum(II)-Cage-Based Light-Harvesting System for Photocatalytic Cross-Coupling Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 8862–8866. [Google Scholar] [CrossRef]
- Sautter, A.; Kaletaş, B.K.; Schmid, D.G.; Dobrawa, R.; Zimine, M.; Jung, G.; van Stokkum, I.H.M.; De Cola, L.; Williams, R.M.; Würthner, F. Ultrafast Energy-Electron Transfer Cascade in a Multichromophoric Light-Harvesting Molecular Square. J. Am. Chem. Soc. 2005, 127, 6719–6729. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Ge, Z.; Gao, Z.; Liao, R.; Wang, F. A bioinspired sequential energy transfer system constructed via supramolecular copolymerization. Nat. Commun. 2022, 13, 3546. [Google Scholar] [CrossRef]
- Diao, K.; Whitaker, D.J.; Huang, Z.; Qian, H.; Ren, D.; Zhang, L.; Li, Z.-Y.; Sun, X.-Q.; Xiao, T.; Wang, L. An ultralow-acceptor-content supramolecular light-harvesting system for white-light emission. Chem. Commun. 2022, 58, 2343–2346. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, L.; Wu, H.; Qian, H.; Ren, D.; Li, Z.-Y.; Sun, X.-Q. Supramolecular polymer-directed light-harvesting system based on a stepwise energy transfer cascade. Chem. Commun. 2021, 57, 5782–5785. [Google Scholar] [CrossRef]
- Wang, X.-H.; Lou, X.-Y.; Lu, T.; Wang, C.; Tang, J.; Liu, F.; Wang, Y.; Yang, Y.W. Supramolecular Engineering of Efficient Artificial Light-Harvesting Systems from Cyanovinylene Chromophores and Pillar [5]arene-Based Polymer Hosts. ACS Appl. Mater. Interfaces 2021, 13, 4593–4604. [Google Scholar] [CrossRef]
- Xiao, T.; Wu, H.; Sun, G.; Diao, K.; Wei, X.; Li, Z.-Y.; Sun, X.-Q.; Wang, L. An efficient artificial light-harvesting system with tunable emission in water constructed from a H-bonded AIE supramolecular polymer and Nile Red. Chem. Commun. 2020, 56, 12021–12024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.-X.; Niu, L.-Y.; Yang, Q.-Z. Aggregation-Induced Emission Materials with Narrowed Emission Band by Light-Harvesting Strategy: Fluorescence and Chemiluminescence Imaging. Chem. Mater. 2019, 31, 3573–3581. [Google Scholar] [CrossRef]
- Song, Q.; Goia, S.; Yang, J.; Hall, S.C.L.; Staniforth, M.; Stavros, V.G.; Perrier, S. Efficient Artificial Light-Harvesting System Based on Supramolecular Peptide Nanotubes in Water. J. Am. Chem. Soc. 2021, 143, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; Duff, M.R. DNA-Based Supramolecular Artificial Light Harvesting Complexes. J. Am. Chem. Soc. 2009, 131, 16024–16026. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Yu, J.-L.; Wu, M.-X.; Xue, Z.-Y.; Xia, Q.-Q.; Liu, X.; Wang, X.-H. Supramolecular Assembly-Induced Emission Enhancement Vesicles Regulated by Pincer-Like Hosts Containing Pillar [5]arenes. Adv. Opt. Mater. 2022, 10, 2201496. [Google Scholar] [CrossRef]
- Wang, W.M.; Dai, D.; Wu, J.R.; Wang, C.-Y.; Wang, Y.; Yang, Y.-W. Recyclable Supramolecular Assembly-Induced Emission System for Selective Detection and Efficient Removal of Mercury(II). Chem. Eur. J. 2021, 27, 11879–11887. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Yang, Y.-W. Manipulating Aggregation-Induced Emission with Supramolecular Macrocycles. Adv. Opt. Mater. 2018, 6, 1800668. [Google Scholar] [CrossRef]
- Yuan, Y.-X.; Jia, J.-H.; Song, Y.-P.; Ye, F.-Y.; Zheng, Y.-S.; Zang, S.-Q. Fluorescent TPE Macrocycle Relayed Light-Harvesting System for Bright Customized-Color Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 5389–5399. [Google Scholar] [CrossRef]
- Ma, C.-Q.; Li, X.-L.; Han, N.; Wang, Y.; Wang, R.-Z.; Yu, S.; Wang, Y.-B.; Xing, L.-B. A novel polyelectrolyte-based artificial light-harvesting system for photocatalysis of cross-dehydrogenation coupling. J. Mater. Chem. A 2022, 10, 16390–16395. [Google Scholar] [CrossRef]
- Xiao, T.; Shen, Y.; Bao, C.; Diao, K.; Ren, D.; Qian, H.; Zhang, L. Efficient artificial light-harvesting system constructed from supramolecular polymers with AIE property. RSC Adv. 2021, 11, 30041–30045. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-X.; Li, W.-J.; Jia, P.-P.; Wang, X.-Q.; Xu, L.; Yang, H.-B. Supramolecular Artificial Light-Harvesting Systems with Aggregation-Induced Emission. Adv. Opt. Mater. 2020, 8, 2000265. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Cheng, L.; Qin, C.; Nian, H.; Zhang, H.; Yu, Y.; Cao, L. Aggregation-Induced Emission and Light-Harvesting Function of Tetraphenylethene-Based Tetracationic Dicyclophane. J. Am. Chem. Soc. 2019, 141, 8412–8415. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Bao, C.; Zhang, L.; Diao, K.; Ren, D.; Wei, C.; Li, Z.-Y.; Sun, X.-Q. An artificial light-harvesting system based on the ESIPT–AIE–FRET triple fluorescence mechanism. J. Mater. Chem. A 2022, 10, 8528–8534. [Google Scholar] [CrossRef]
- Xiao, T.; Wei, X.; Wu, H.; Diao, K.; Li, Z.-Y.; Sun, X.-Q. Acetal-based spirocyclic skeleton bridged tetraphenylethylene dimer for light-harvesting in water with ultrahigh antenna effect. Dye. Pigm. 2021, 188, 109161. [Google Scholar] [CrossRef]
- Xiao, T.; Li, S.-L.; Zhang, Y.; Lin, C.; Hu, B.; Guan, X.; Yu, Y.; Jiang, J.; Wang, L. Novel self-assembled dynamic [2]catenanes interlocked by the quadruple hydrogen bonding ureidopyrimidinone motif. Chem. Sci. 2012, 3, 1417–1421. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, L.; Götz, J.; Cheng, M.; Würthner, F.; Gu, J.; Feng, X.; Li, Z.-Y.; Sun, X.-Q.; Wang, L. Supramolecular polymerization and cyclization of dioxynaphthalene motif bridged bifunctional UPys: Minor variations in the molecular skeleton and drastic differences in self-assembly. Mater. Chem. Front. 2019, 3, 2738–2745. [Google Scholar] [CrossRef]
- Xiao, T.; Zhong, W.; Qi, L.; Gu, J.; Feng, X.; Yin, Y.; Li, Z.-Y.; Sun, X.-Q.; Cheng, M.; Wang, L. Ring-opening supramolecular polymerization controlled by orthogonal non-covalent interactions. Poly. Chem. 2019, 10, 3342–3350. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, L.; Wang, J.; Li, Z.-Y.; Sun, X.-Q.; Wang, L. Biomimetic folding of small organic molecules driven by multiple non-covalent interactions. Org. Chem. Front. 2019, 6, 936–941. [Google Scholar] [CrossRef]
- Xiao, T.; Zhong, W.; Yang, W.; Qi, L.; Gao, Y.; Sue, A.C.H.; Li, Z.-Y.; Sun, X.-Q.; Lin, C.; Wang, L. Reversible hydrogen-bonded polymerization regulated by allosteric metal templation. Chem. Commun. 2020, 56, 14385–14388. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Wang, L.; Tang, H.; Cao, D. Effect of scaffold structures on the artificial light-harvesting systems: A case study with an AIEE-active pillar [5]arene dyad. Chem. Commun. 2019, 55, 5910–5913. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Q.; Xu, J.F.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Water-dispersible nanospheres of hydrogen-bonded supramolecular polymers and their application for mimicking light-harvesting systems. Chem. Commun. 2014, 50, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Auweter, H.; Haberkorn, H.; Heckmann, W.; Horn, D.; Lüddecke, E.; Rieger, J.; Weiss, H. Supramolecular Structure of Precipitated Nanosize β-Carotene Particles. Angew. Chem. Int. Ed. 1999, 38, 2188–2191. [Google Scholar] [CrossRef]
- Lian, Z.; Qiao, F.; Jiang, M.; Wang, R.-Z.; Xing, L.-B.; Liu, S.; Wang, S. Quadruple hydrogen bonded hyperbranched supramolecular polymers with aggregation-induced emission for artificial light-harvesting. Dye. Pigm. 2019, 171, 107774. [Google Scholar] [CrossRef]
- Li, W.-B.; Luo, W.-J.; Li, K.-X.; Yuan, W.-Z.; Zhang, Y.-M. Aggregation-induced phosphorescence and mechanochromic luminescence of a tetraphenylethene-based gold(I) isocyanide complex. Chin. Chem. Lett. 2017, 28, 1300–1305. [Google Scholar] [CrossRef]
- Keizer, H.M.; Sijbesma, R.P.; Meijer, E.W. The Convenient Synthesis of Hydrogen-Bonded Ureidopyrimidinones. Eur. J. Org. Chem. 2004, 2004, 2553–2555. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Shen, Z.; Wang, R.; Zhang, W.; Núñez-Delgado, A.; Han, N.; Xing, L.-B. Supramolecular assemblies working as both artificial light-harvesting system and nanoreactor for efficient organic dehalogenation in aqueous environment. J. Colloid Interface Sci. 2022, 617, 118–128. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Dai, X.; Li, J.; Li, P.; Liu, Y. Sulfonatocalix [4]arene-based light-harvesting amphiphilic supramolecular assemblies for sensing sulfites in cells. J. Mater. Chem. C 2020, 9, 1958–1965. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Qian, H.; Wu, Z.; Zhang, Q.; Li, S.; Cheng, M.; Xiao, T. Non-Covalent Dimer as Donor Chromophore for Constructing Artificial Light-Harvesting System in Water. Molecules 2022, 27, 8876. https://doi.org/10.3390/molecules27248876
Zhang L, Qian H, Wu Z, Zhang Q, Li S, Cheng M, Xiao T. Non-Covalent Dimer as Donor Chromophore for Constructing Artificial Light-Harvesting System in Water. Molecules. 2022; 27(24):8876. https://doi.org/10.3390/molecules27248876
Chicago/Turabian StyleZhang, Liangliang, Hongwei Qian, Zhiying Wu, Qiaona Zhang, Shengke Li, Ming Cheng, and Tangxin Xiao. 2022. "Non-Covalent Dimer as Donor Chromophore for Constructing Artificial Light-Harvesting System in Water" Molecules 27, no. 24: 8876. https://doi.org/10.3390/molecules27248876
APA StyleZhang, L., Qian, H., Wu, Z., Zhang, Q., Li, S., Cheng, M., & Xiao, T. (2022). Non-Covalent Dimer as Donor Chromophore for Constructing Artificial Light-Harvesting System in Water. Molecules, 27(24), 8876. https://doi.org/10.3390/molecules27248876







