Phenotypic and Target-Directed Screening Yields New Acaricidal Alternatives for the Control of Ticks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Collection and Natural Extracts
2.2. Phenotypic Screening
2.2.1. Effect of Compounds on BME26 Cell Cultures
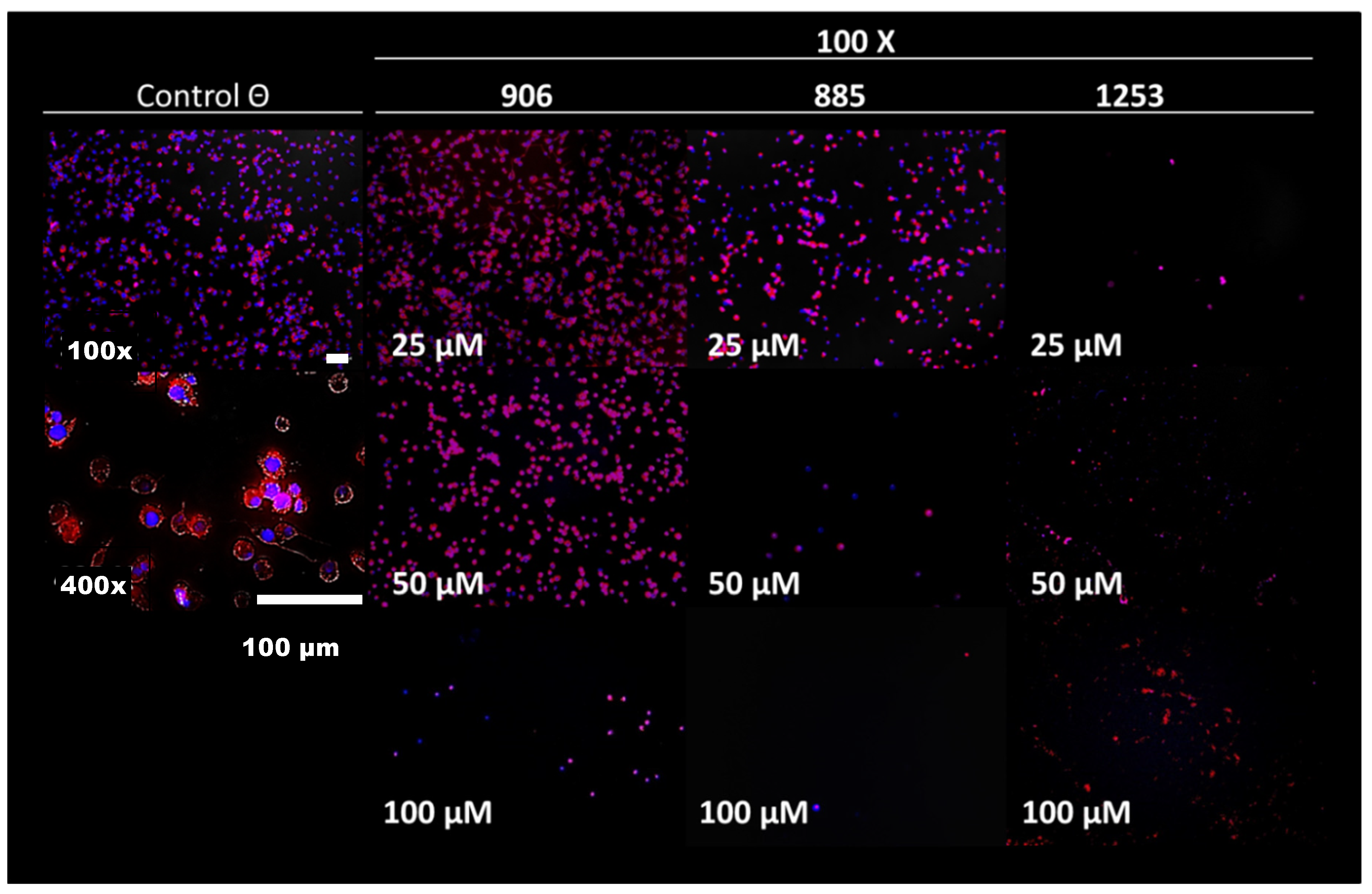
2.2.2. Effect of Compounds on BME26 Cell Morphology [31]
2.3. Effect of Compounds on the Enzymatic Activity of TIM
2.4. Nonspecific Cytotoxicity
2.4.1. Cytotoxicity Assay on Murine Macrophages
2.4.2. Cytotoxicity Assay on Bovine Spermatozoa
2.5. Adult Immersion Test (AIT)
2.6. Larval Immersion Test (LIT)
2.7. Toxicology and Pharmacokinetic Profiles
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenwood, P.L. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 2021, 15, 100295. [Google Scholar]
- Miraballes, C.; Barros, A.T.M.; Lucas, M.; Klafke, G.M.; Domingues, L.N.; Riet-Correa, F. Susceptibility of field populations of Haematobia irritans to fipronil in Uruguay. Pesqui. Vet. Bras. 2021, 41, e06821. [Google Scholar] [CrossRef]
- Guglielmone, A.A. Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 1995, 57, 109–119. [Google Scholar]
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar]
- Agwunobi, D.O.; Yu, Z.; Liu, J. A retrospective review on ixodid tick resistance against synthetic acaricides: Implications and perspectives for future resistance prevention and mitigation. Pestic. Biochem. Physiol. 2021, 173, 104776. [Google Scholar]
- Cuore, U.; Cardozo, H.; Trelles, A.; Nari, A.; Solari, M.A. Primer Diagnóstico Características de los garrapaticidas utilizados en Uruguay. Eficacia y poder residual. Veterinaria 2008, 43, 13–24. [Google Scholar]
- Valsoni, L.M.; de Freitas, M.G.; Echeverria, J.T.; Borges, D.G.L.; Tutija, J.; de Almeida Borges, F. Resistance to all chemical groups of acaricides in a single isolate of Rhipicephalus microplus in Mato Grosso do Sul, Brazil. Int. J. Acarol. 2020, 46, 276–280. [Google Scholar]
- Castro Janer, E.; Klafke, G.M.; Capurro, M.L.; Schumaker, T.T.S. Cross-resistance between fipronil and lindane in Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2015, 210, 77–83. [Google Scholar]
- Torrents, J.; Morel, N.; Rossner, M.V.; Martínez, N.C.; Toffaletti, J.R.; Nava, S. In vitro diagnosis of resistance of the cattle tick Rhipicephalus (Boophilus) microplus to fipronil in Argentina. Exp. Appl. Acarol. 2020, 82, 397–403. [Google Scholar]
- Nath, S.; Mandal, S.; Pal, S.; Jadhao, S.; Ottalwar, N.; Sanyal, P. Impact and Management of Acaricide Resistance- Pertaining to Sustainable Control of Ticks. Int. J. Livest. Res. 2018, 8, 46. [Google Scholar]
- Klafke, G.; Webster, A.; Dall Agnol, B.; Pradel, E.; Silva, J.; de La Canal, L.H.; Becker, M.; Osório, M.F.; Mansson, M.; Barreto, R.; et al. Multiple resistance to acaricides in field populations of Rhipicephalus microplus from Rio Grande do Sul state, Southern Brazil. Ticks Tick. Borne. Dis. 2017, 8, 73–80. [Google Scholar]
- Le Gall, V.L.; Klafke, G.M.; Torres, T.T. Detoxification mechanisms involved in ivermectin resistance in the cattle tick, Rhipicephalus (Boophilus) microplus. Sci. Rep. 2018, 8, 12401. [Google Scholar]
- Rodríguez-Vivas, R.I.; Pérez-Cogollo, L.C.; Rosado-Aguilar, J.A.; Ojeda-Chi, M.M.; Trinidad-Martinez, I.; Miller, R.J.; Li, A.Y.; Pérez de León, A.; Guerrero, F.; Klafke, G. Rhipicephalus (Boophilus) microplus resistant to acaricides and ivermectin in cattle farms of Mexico. Rev. Bras. Parasitol. Vet. 2014, 23, 113–122. [Google Scholar]
- Reck, J.; Klafke, G.M.; Webster, A.; Agnol, B.D.; Scheffer, R.; Souza, U.A.; Corassini, V.B.; Vargas, R.; Silveira, J.; Ricardo, J.; et al. First report of fluazuron resistance in Rhipicephalus microplus: A field tick population resistant to six classes of acaricides. Vet. Parasitol. 2014, 201, 128–136. [Google Scholar]
- Yessinou, R.E.; Akpo, Y.; Ossè, R.; Adoligbe, C.; Cassini, R.; Akogbeto, M.; Farougou, S. Molecular characterization of pyrethroids resistance mechanisms in field populations of Rhipicephalus microplus (Acari: Ixodidae) in district of Kpinnou and Opkara, Benin. Int. J. Acarol. 2018, 44, 198–203. [Google Scholar]
- Abdulla, M.H.; Ruelas, D.S.; Wolff, B.; Snedecor, J.; Lim, K.C.; Xu, F.; Renslo, A.R.; Williams, J.; McKerrow, J.H.; Caffrey, C.R. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis. 2009, 3, e478. [Google Scholar]
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest Manag. Sci. 2021, 77, 64–76. [Google Scholar]
- Brandhorst, T.T.; Kean, I.R.L.; Lawry, S.M.; Wiesner, D.L.; Klein, B.S. Phenylpyrrole fungicides act on triosephosphate isomerase to induce methylglyoxal stress and alter hybrid histidine kinase activity. Sci. Rep. 2019, 9, 5047. [Google Scholar]
- Olivares-Illana, V.; Rodríguez-Romero, A.; Becker, I.; Berzunza, M.; García, J.; Pérez-Montfort, R.; Cabrera, N.; López-Calahorra, F.; de Gómez-Puyou, M.T.; Gómez-Puyou, A. Perturbation of the dimer interface of triosephosphate isomerase and its effect on Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2007, 1, e01–e08. [Google Scholar]
- Reyes-Vivas, H.; Diaz, A.; Peon, J.; Mendoza-Hernandez, G.; Hernandez-Alcantara, G.; De la Mora-De la Mora, I.; Enriquez-Flores, S.; Dominguez-Ramirez, L.; Lopez-Velazquez, G. Disulfide bridges in the mesophilic triosephosphate isomerase from Giardia lamblia are related to oligomerization and activity. J. Mol. Biol. 2007, 365, 752–763. [Google Scholar]
- Yadav, A.K.; Kumar, K.; Verma, M.; Kumar, M.; Kumar, M. Designing of Species-specific inhibition: The cysteine residues of triosephosphate isomerase. Basic Res. J. Med. Clin. Sci. 2014, 3, 62–66. [Google Scholar]
- Téllez-Valencia, A.; Olivares-Illana, V.; Hernández-Santoyo, A.; Pérez-Montfort, R.; Costas, M.; Rodríguez-Romero, A.; López-Calahorra, F.; Tuena De Gómez-Puyou, M.; Gómez-Puyou, A. Inactivation of triosephosphate isomerase from Trypanosoma cruzi by an agent that perturbs its dimer interface. J. Mol. Biol. 2004, 341, 1355–1365. [Google Scholar]
- Fort, R.S.; Barnech, J.M.T.; Dourron, J.; Colazzo, M.; Aguirre-crespo, F.J. Isolation and Structural Characterization of Bioactive Molecules on Prostate Cancer from Mayan Traditional Medicinal Plants. Pharmaceuticals 2018, 11, 78. [Google Scholar]
- Matiadis, D.; Karagiaouri, M.; Mavroidi, B.; Nowak, K.E.; Katsipis, G.; Pelecanou, M.; Pantazaki, A.; Sagnou, M. Synthesis and antimicrobial evaluation of a pyrazoline-pyridine silver(I) complex: DNA-interaction and anti-biofilm activity. BioMetals 2021, 34, 67–85. [Google Scholar]
- Li, P.Z.; Liu, Z.Q. Ferrocenyl-substituted curcumin: Can it influence antioxidant ability to protect DNA? Eur. J. Med. Chem. 2011, 46, 1821–1826. [Google Scholar]
- Álvarez, G.; Perdomo, C.; Coronel, C.; Aguilera, E.; Varela, J.; Aparicio, G.; Zolessi, F.R.; Cabrera, N.; Vega, C.; Rolón, M.; et al. Multi-anti-parasitic activity of arylidene ketones and thiazolidene hydrazines against Trypanosoma cruzi and Leishmania spp. Molecules 2017, 22, 709. [Google Scholar]
- Lawrence, C.; Mason, T. Zebrafish Housing Systems: A Review of Basic Operating Principles and Considerations for Design and Functionality. ILAR J. 2012, 53, 179–191. [Google Scholar]
- Cerecetto, H.; González, M. Anti-T. cruzi agents: Our experience in the evaluation of more than five hundred compounds. Mini Rev. Med. Chem. 2008, 8, 1355–1383. [Google Scholar]
- Cabrera, M.; Simoens, M.; Falchi, G.; Lavaggi, M.L.; Piro, O.E.; Castellano, E.E.; Vidal, A.; Azqueta, A.; Sagrera, G.; Seoane, G.; et al. Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure–activity relationships. Bioorganic Med. Chem. 2007, 15, 3356–3367. [Google Scholar]
- Esteves, E.; Lara, F.A.; Lorenzini, D.M.; Costa, G.H.; Fukuzawa, A.H.; Pressinotti, L.N.; Silva, J.R.; Ferro, J.A.; Kurtti, T.J.; Munderloh, U.G.; et al. Cellular and molecular characterization of an embryonic cell line (BME26) from the tick Rhipicephalus (Boophilus) microplus. Insect. Biochem. Mol. Biol. 2008, 38, 568–580. [Google Scholar]
- Saramago, L.; Gomes, H.; Aguilera, E.; Cerecetto, H.; González, M.; Cabrera, M.; Alzugaray, M.; da Silva Vaz Junior, I.; Nunes da Fonseca, R.; Aguirre-López, B.; et al. Novel and Selective Rhipicephalus microplus Triosephosphate Isomerase Inhibitors with Acaricidal Activity. Vet. Sci. 2018, 5, 74. [Google Scholar]
- Ferraro, F.; Corvo, I.; Bergalli, L.; Ilarraz, A.; Cabrera, M.; Gil, J.; Susuki, B.M.; Caffrey, C.R.; Timson, D.J.; Robert, X.; et al. Novel and selective inactivators of Triosephosphate isomerase with anti-trematode activity. Sci. Rep. 2020, 10, 2587. [Google Scholar]
- Oba, P.; Pereira, M. A ensaios in vitro pelos critérios de oba drummond de chlorpyrifos sobre linhagem supostamente resistente de boophilus microplus. Rev. Fac. Med. Vet. Zootec. Univ. S. Paulo. 1976, 13, 409–420. [Google Scholar]
- Castro-Janer, E.; Rifran, L.; González, P.; Niell, C.; Piaggio, J.; Gil, A.; Schumaker, T.T.S. Determination of the susceptibility of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) to ivermectin and fipronil by Larval Immersion Test (LIT) in Uruguay. Vet. Parasitol. 2011, 178, 148–155. [Google Scholar]
- Herz, W.; Gage, D.; Kumar, N. Damsinic acid and ambrosanolides from vegetative ambrosia hispida. Phytochemistry 1981, 20, 1601–1604. [Google Scholar]
- Maldini, M.; Montoro, P.; Pizza, C. Phenolic compounds from Byrsonima crassifolia L. bark: Phytochemical investigation and quantitative analysis by LC-ESI MS/MS. J. Pharm. Biomed. Anal. 2011, 56, 1–6. [Google Scholar]
- Rastogi, T.; Leder, C.; Kümmerer, K. Re-Designing of Existing Pharmaceuticals for Environmental Biodegradability: A Tiered Approach with β-Blocker Propranolol as an Example. Environ. Sci. Technol. 2015, 49, 11756–11763. [Google Scholar]
| Collection Code | Scientific Name/(Spanish or Mayan Name)/Tissues Employed/Solvent | % of Growth Inhibition | % of Growth Inhibition for Mammalian Cells [23] | Plant Visualization |
|---|---|---|---|---|
| T2 | Leucaena leucocephala (Huachi in Mayan) leaves and branches by MeOH | 70 ± 7 | 80 |  1 |
| T8 | Leucaena leucocephala (Huachi in Mayan) leaves and branches by CH2Cl2 | 72 ± 8 | 80 | |
| T3 | Cnidoscolus chayamansa (Chaya in Mayan) leaves by MeOH | 63 ± 5 | 25 |  |
| T4 | Cnidoscolus chayamansa (Chaya in Mayan) leaves by CH2Cl2 | 100 ± 9 | 75 | |
| T25 | Ipomoea pes-caprae (Riñonera in Spanish) leaves and branches by MeOH | 70 ± 7 | 0 |  |
| T15 | Ambrosia hispida (K’an lool xiiw in Mayan) leaves and branches by MeOH | 93 ± 5 | ND |  |
| T26 | Ambrosia hispida (K’an lool xiiw in Mayan) leaves and branches by CH2Cl2 | 47 ± 4 | ND | |
| T19 | Malmea depressa (Elemuy in Mayan) leaves and branches by MeOH | 63 ± 7 | 75 |  2 |
| T22 | Cecropia obtusifolia (Guarumbo in Mayan) leaves by CH2Cl2 | 100 ± 9 | 0 |  |
| T28 | Byrsonima crassifolia (Nance in Mayan) tree bark by CH2Cl2 | 48 ± 7 | 50 |  3 |
| T44 | Ruellia nudiflora (Engelm. & A. Gray) Urb. (Xana mukuy in Mayan) leaves and branches by MeOH | 100 ± 8 | ND |  |
| Chemical Collection Code | Structure | % of Growth Inhibition | IC50 (µM) | IC50 (µM) Mammalian Cells |
|---|---|---|---|---|
| 906 | 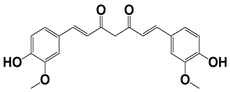 | 95 | 24 ± 3 | <25 |
| 795 |  | 80 | 20 ± 3 | >50 |
| 796 | 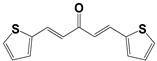 | 93 | 15 ± 3 | >50 |
| 809 | 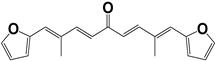 | 50 | >100 | >50 |
| 133 | 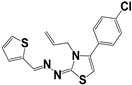 | 71 | >50 | >50 |
| 266 | 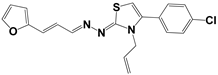 | 77 | >100 | >50 |
| 903 | 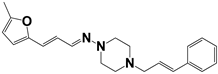 | 75 | >50 | >50 |
| 912 | 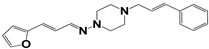 | 52 | >50 | >50 |
| 715 |  | 64 | >50 | ND |
| 183 |  | 62 | ND | <25 |
| 181 | 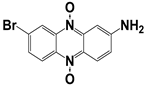 | 90 | ND | <25 |
| 885 |  | 91 | 17 ± 4 | >50 |
| 1253 |  | 95 | 12 ± 1 | >50 |
| Chemical Collection Code | Structure | % Inhibition of Enzymatic Activity of RmTIM at 10 µM * or 100 µM ** | IC50 (µM) | % Inhibition of Enzymatic Activity of HsTIM at 10 µM | EC50 (µM) for Mammalian Cells |
|---|---|---|---|---|---|
| 910 | 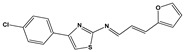 | 60 ** | 23 ± 2 | 0 | >50 |
| 1367 | 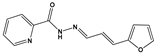 | 82 * | <10 | 0 | >50 |
| 1366 |  | 38 ** | ND | ND | ND |
| 1378 | 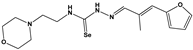 | 77 ** | 30 | 0 | ND |
| 1404 | 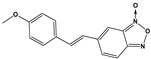 | 100 * | <10 | ND | >50 |
| 799 |  | 88 * | <10 | 0 | >50 |
| 1387 | 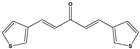 | 100 * | <10 | 0 | >50 |
| 1088 | 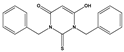 | 50 | >50 | ND | ND |
| 1408 | 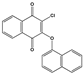 | 72 ** | >50 | ND | ND |
| 1385 |  | 89 * | <10 | ND | >50 |
| 1386 | 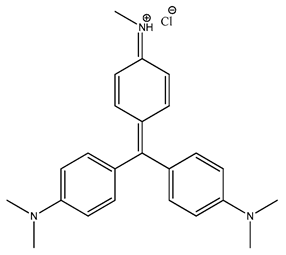 | 100 * | <10 | ND | ND |
| 879 | 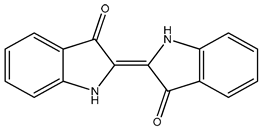 | 95 * | <10 | 0 | >50 |
| Mar106 | 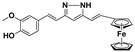 | 69 ** | 25 ± 4 | 0 | >25 |
| Mar105 |  | 65 | 10 ± 1 | 0 | >25 |
| Dm97 | 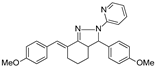 | 0 ** | ND | ND | ND |
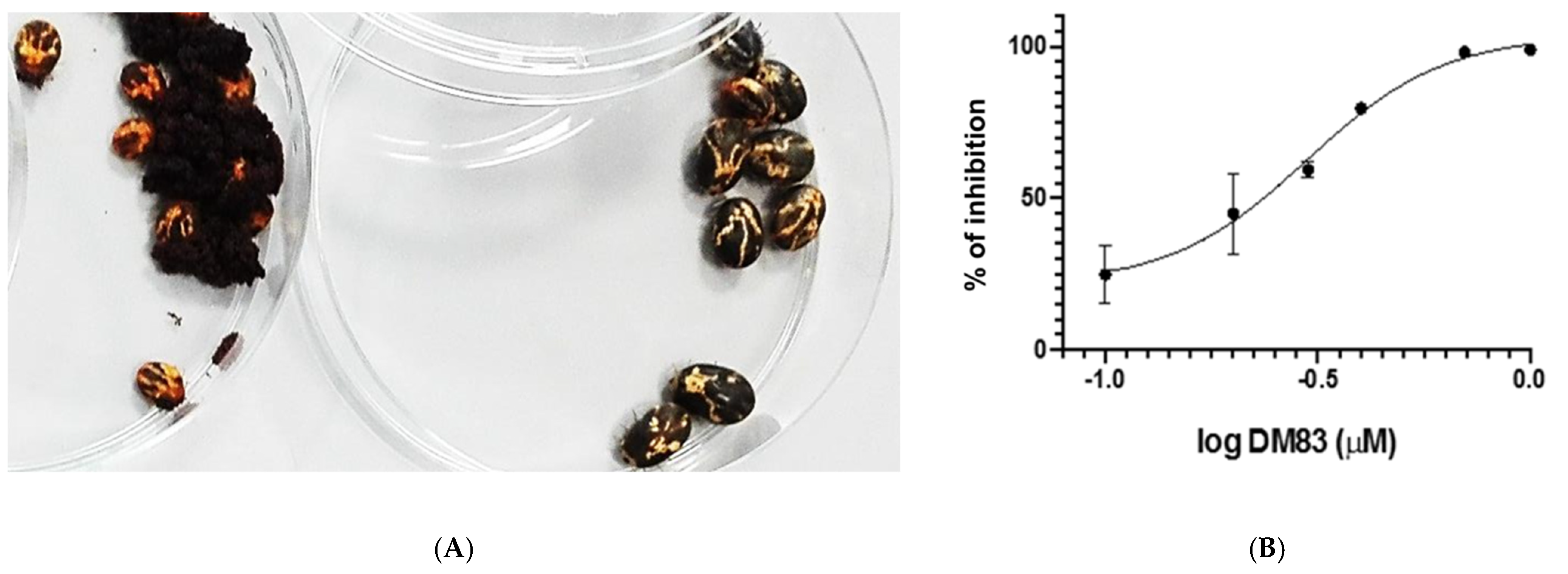
| Dm83 |  | 99 * | 0.30 ± 0.05 | 0 | >50 |
| Collection Code | % of Mortality LIT | % of Mortality AIT |
|---|---|---|
| T2 | ND | 30 ± 8 |
| T8 | ND | 42 ± 7 |
| T3 | ND | 46 ± 9 |
| T4 | ND | 0 |
| T25 | ND | 30 ± 5 |
| T15 | ND | 64 ± 8 |
| T26 | ND | 64 ± 4 |
| T19 | ND | 32 ± 8 |
| T22 | ND | 0 |
| T28 | ND | 64 ± 9 |
| T44 | ND | 70 ± 2 |
| 906 | ND | 60 ± 8 |
| 796 | 36 ± 8 | 20 ± 1 |
| 795 | ND | 70 ± 7 |
| 903 | ND | 10 ± 1 |
| 912 | ND | 30 ± 8 |
| 885 | 100 ± 9 | 100 ± 9 |
| 1253 | 50 ± 8 | ND |
| Dm83 | 69 ± 7 | 50 ± 8 |
| Ivermectin | 100 | ND |
| Amitraz | 100 | 100 |
| Chemical Collection Code | Mutagenicity by Ames Test | °LD50 (mg/kg) | Consensus Log Po/w | Solubility mg/mL | GI ** Absorption | •Log Kp cm/s | Metabolic Stability |
|---|---|---|---|---|---|---|---|
| 906 | Negative | 5000 | 3.0 | 4 × 10−2 | High | −6.3 | Low |
| 796 | Negative * | >2000+ | 3.7 | 4 × 10−2 | High | −5.3 | Medium |
| 885 | Negative | 2448 | 2.4 | 0.1 | High | −5.7 | Medium |
| 1253 | Positive | 2015 | 1.4 | 0.2 | High | −6.1 | Low |
| DM83 | Negative | 1700 | >5 | <3 × 10−4 | High | −4.9 | Medium |
| Amitraz | Negative | 400 | 4.8 | 1.6 × 10−3 | High | −4.2 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saporiti, T.; Cabrera, M.; Bentancur, J.; Ferrari, M.E.; Cabrera, N.; Pérez-Montfort, R.; Aguirre-Crespo, F.J.; Gil, J.; Cuore, U.; Matiadis, D.; et al. Phenotypic and Target-Directed Screening Yields New Acaricidal Alternatives for the Control of Ticks. Molecules 2022, 27, 8863. https://doi.org/10.3390/molecules27248863
Saporiti T, Cabrera M, Bentancur J, Ferrari ME, Cabrera N, Pérez-Montfort R, Aguirre-Crespo FJ, Gil J, Cuore U, Matiadis D, et al. Phenotypic and Target-Directed Screening Yields New Acaricidal Alternatives for the Control of Ticks. Molecules. 2022; 27(24):8863. https://doi.org/10.3390/molecules27248863
Chicago/Turabian StyleSaporiti, Tatiana, Mauricio Cabrera, Josefina Bentancur, María Elisa Ferrari, Nallely Cabrera, Ruy Pérez-Montfort, Francisco J. Aguirre-Crespo, Jorge Gil, Ulises Cuore, Dimitris Matiadis, and et al. 2022. "Phenotypic and Target-Directed Screening Yields New Acaricidal Alternatives for the Control of Ticks" Molecules 27, no. 24: 8863. https://doi.org/10.3390/molecules27248863
APA StyleSaporiti, T., Cabrera, M., Bentancur, J., Ferrari, M. E., Cabrera, N., Pérez-Montfort, R., Aguirre-Crespo, F. J., Gil, J., Cuore, U., Matiadis, D., Sagnou, M., & Alvarez, G. (2022). Phenotypic and Target-Directed Screening Yields New Acaricidal Alternatives for the Control of Ticks. Molecules, 27(24), 8863. https://doi.org/10.3390/molecules27248863









