In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells
Abstract
1. Introduction
2. Results
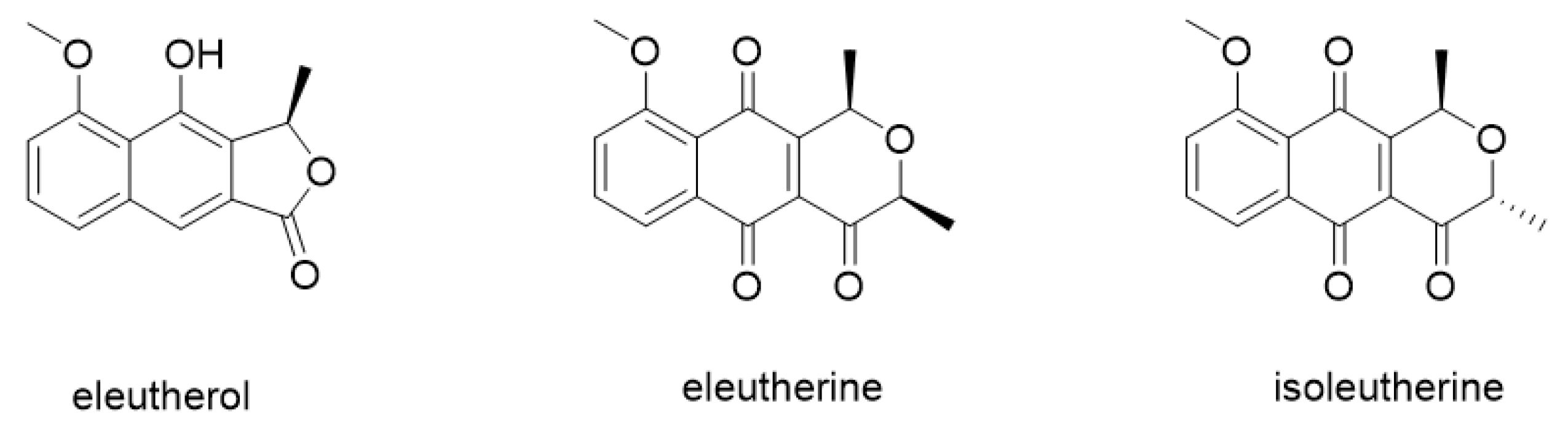
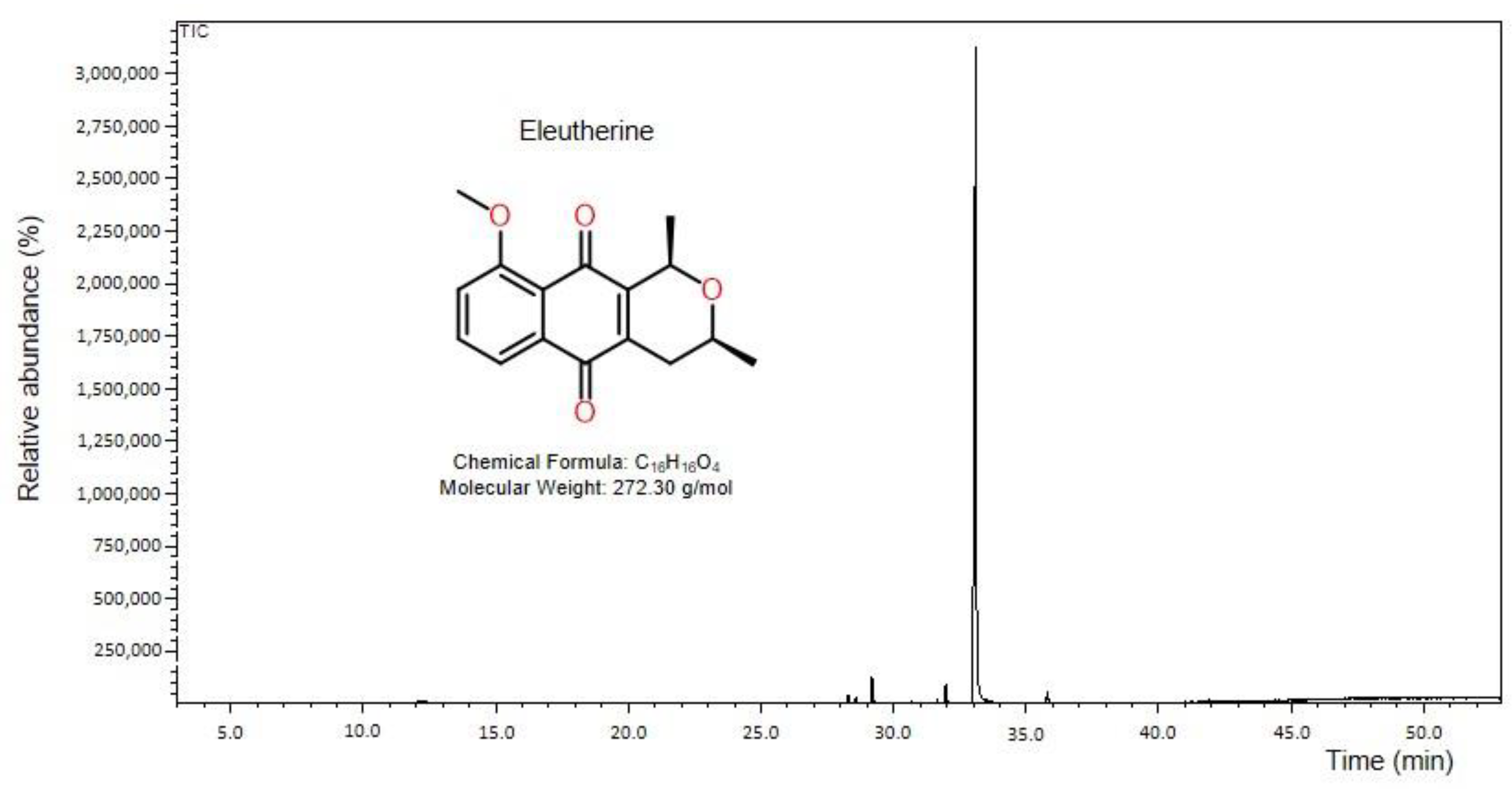
2.1. Characterization of the Compound Isolate
2.2. Cytotoxic Effect of Eleutherine Treatment on C6 Cells
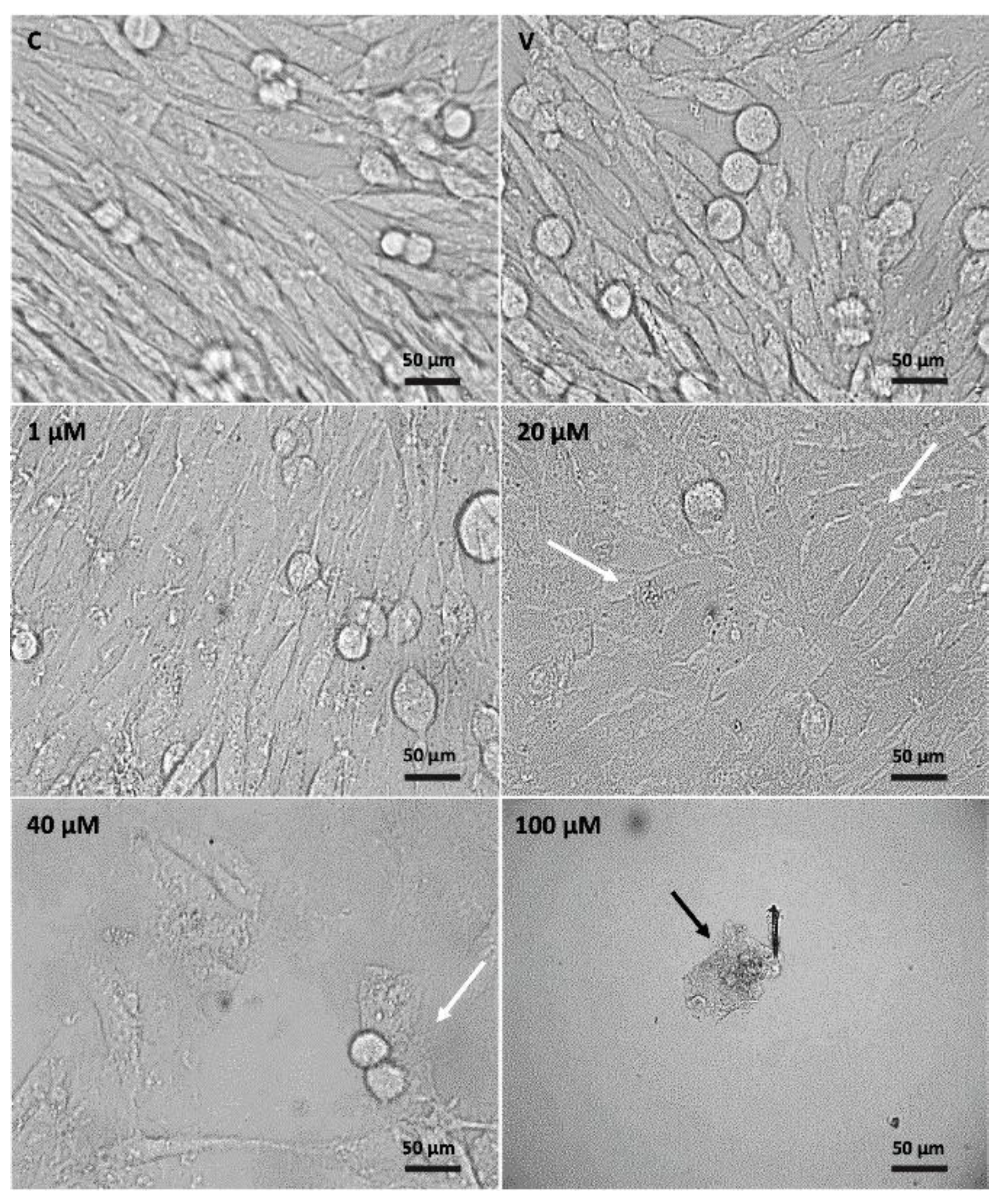
2.3. Morphological Changes in C6 Cells after Eleutherine Treatment
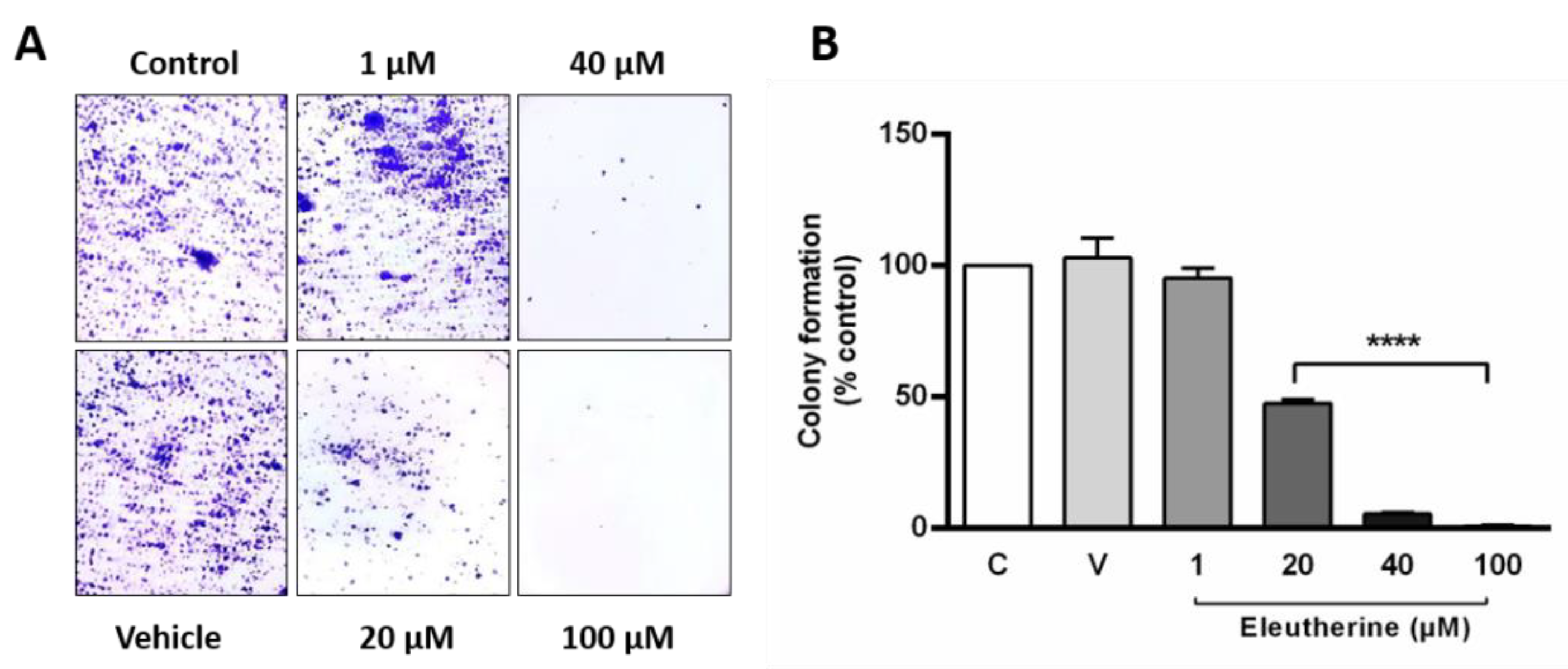
2.4. Eleutherine Reduces Colony Formation by C6 Cells
2.5. Eleutherine Induces C6 Cell Apoptosis
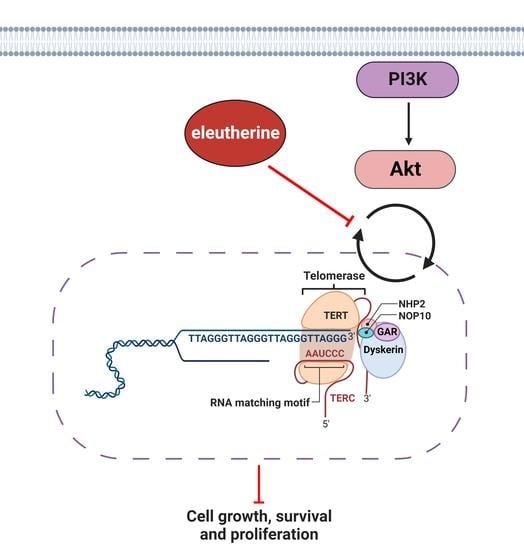
2.6. Eleutherine Reduces the Expression of pAKT in C6 Cells
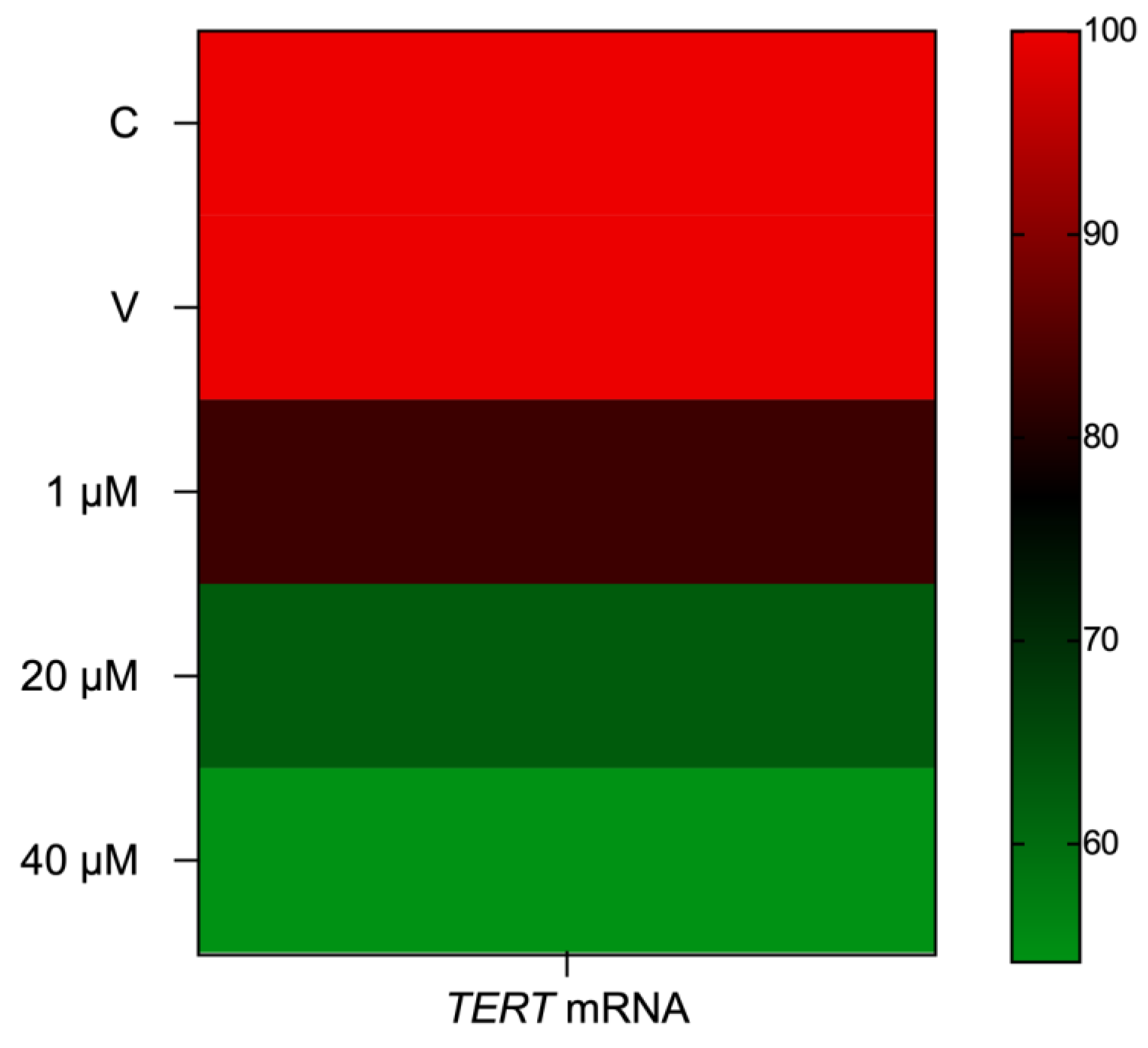
2.7. Eleutherine Reduces Telomerase (TERT) mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Production of Raw Extract
4.3. Extraction and Isolation
4.4. Cell Culture
4.5. Cell Cytotoxicity Assay
4.6. Morphological Analysis of Eleutherine-Treated C6 Glioma Cells
4.7. Wound Healing/Scratch Migration Assay
4.8. Colony Formation Assay
4.9. Annexin V-FITC–Propidium Iodide (PI) Assay
4.10. Western Blotting
4.11. Real-Time Quantitative PCR Assay
4.12. Ethics Statement
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartzbaum, J.; Fisher, J.L.; Aldape, K.D.; Wrensch, M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006, 2, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Glioblastoma: From Molecular Pathology to Targeted Treatment. Annu. Rev. Pathol. 2014, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Hu, M.G.; liu, Y.Y.; Li, R.T.; Yu, M.; Xu, X.D.; Ma, G.X. New Naphthalene Derivatives from the Bulbs of Eleutherine americana with Their Protective Effect on the Injury of HUVECs. Molecules 2018, 23, 2111. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, C.R.; Brakel, B.A.; Tatari, N.; Savage, N.; Salim, S.K.; Venugopal, C.; Singh, S.K. Advances in Immunotherapy for Adult Glioblastoma. Cancers 2021, 13, 3400. [Google Scholar] [CrossRef]
- Behin, A.; Xuan-Hoang, K.; Carpentier, A.F.; Delattre, J.Y. Primary brain tumors in adults. Lancet 2003, 361, 323–331. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumors in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, 1–96. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osόrio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. Codon Publ. 2017, 11, 197–241. [Google Scholar]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Oh, J.; Sahgal, A.; Sanghera, P.; Tsao, M.N.; Davey, P.; Lam, K.; Symons, S.; Aviv, R.; Perry, J.R. Glioblastoma: Patterns of Recurrence and Efficacy of Salvage Treatments. Can. J. Neurol. Sci. 2011, 38, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Harmouch, E.; Seitlinger, J.; Chaddad, H.; Ubeaud-Sequier, G.; Barths, J.; Saidu, S.; Désaubry, L.; Grandemange, L.; Massfelder, T.; Fuhrmann, G.; et al. Flavagline synthetic derivative induces senescence in glioblastoma cancer cells without being toxic to healthy astrocytes. Sci. Rep. 2020, 10, 13750. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Bolyard, C.; Lee, T.J.; Kaur, B.; Yoo, J.Y. Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy. Cancers 2021, 13, 3562. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A. Cell biology-metabolic crosstalk in glioma. Int. J. Biochem. Cell Biol. 2017, 89, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Shabani, S.; Awad, A.J.; Kaushal, M.; Doan, N. Molecular Markers of Therapy-Resistant Glioblastoma and Potential Strategy to Combat Resistance. Int. J. Mol. Sci. 2018, 19, 1765. [Google Scholar] [CrossRef]
- Barros, M.B.L.; Pinheiro, D.R.; Borges, B.N. Mitochondrial DNA Alterations in Glioblastoma (GBM). Int. J. Mol. Sci. 2021, 22, 5855. [Google Scholar] [CrossRef]
- Ferreira, W.A.S.; Amorim, C.K.N.; Burbano, R.R.; Villacis, R.A.R.; Marchi, F.A.; Medina, T.S.; de Lima, M.M.C.; de Oliveira, E.H.C. Genomic and transcriptomic characterization of the human glioblastoma cell line AHOL1. Braz. J. Med. Biol. Res. 2021, 54, 9571. [Google Scholar] [CrossRef]
- Mckinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, 1560. [Google Scholar] [CrossRef]
- Campos, A.; Vendramini-Costa, D.B.; Fiorito, G.F.; Ruiz, A.L.T.G.; Carvalho, J.E.; Souza, G.M.R.; Delle-Monache, F.; Cechinel-Filho, V. Antiproliferative effect of extracts and pyranonaphthoquinones obtained from Cipura paludosa bulbs. Pharm. Biol. 2016, 54, 1022–1026. [Google Scholar] [CrossRef]
- Gomes, A.R.Q.; Galucio, N.C.R.; Albuquerque, K.C.O.; Brígido, H.P.C.; Varela, E.L.P.; Castro, A.L.G.; Vale, V.V.; Bahia, M.O.; Burbano, R.M.R.; Molfeta, F.A.; et al. Toxicity evaluation of Eleutherine plicata Herb. extracts and possible cell death mechanism. Toxicol. Rep. 2021, 8, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Mahabusarakam, W.; Hemtasin, C.; Chakthong, S.; Voravuthikunchai, S.P.; Olawumi, I.B. Naphthoquinones, Anthraquinones and Naphthalene Derivatives from the Bulbs of Eleutherine americana. Planta Med. 2010, 76, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yue, Y.; Qin, J.; Xiao, X.; Ren, Q.; Xiao, B. Plumbagin suppresses the migration and invasion of glioma cells via downregulation of MMP-2/9 expression and inaction of PI3K/Akt signaling pathway in vitro. J. Pharmacol. Sci. 2017, 134, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Matias, D.; Balça-Silva, J.; Dubois, L.G.; Pontes, B.; Ferrer, V.P.; Rosário, L.; Anália, C.; Echevarria, J.L.; Ribeiro, A.B.S.; Lopes, M.C.; et al. Dual treatment with shikonin and temozolomide reduces glioblastoma tumor growth, migration and glial-to-mesenchymal transition. Cell Oncol. (Dordr.) 2017, 40, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.S.; Guimarães, G.H.C.; De Faria, F.C.C.; Longo, G.M.C.; Lopes, G.P.F.; Netto, C.D.; Costa, P.R.R.; Maia, R.C. LQB-118 compound inhibits migration and induces cell death in glioblastoma cells. Oncol. Rep. 2020, 43, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bastow, K.F. Novel Mechanisms of DNA Topoisomerase I—Inhibition by Pyranonaphthoquinone Derivatives Eleutherin, a Lapachone, and B Lapachone. Biochem. Pharmacol. 2000, 60, 1367–1379. [Google Scholar] [CrossRef]
- Andoh, T.; Ishida, R. Catalytic inhibitors of DNA topoisomerase II. Biochim. Biophys. Acta 1998, 1400, 155–171. [Google Scholar] [CrossRef]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva-Jr, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. J. Pharm. Soc. Jpn. 2020, 68, 46–57. [Google Scholar] [CrossRef]
- Kumar, P.; Mallya, P.; Jain, V. Naphthoquinones in the Treatment of Cancer. J. Pharm. Sci. Res. 2020, 12, 587–590. [Google Scholar]
- Tewierik, L.M.; Dimitriadis, C.; Donner, C.D.; Gill, M.; Willems, B. Total synthesis of enantiopure 1,3-dimethylpyranonaphthoquinones including ventiloquinones E, G, L and eleutherin. Org. Biomol. Chem. 2006, 4, 3311–3318. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Miao, R.; Gao, X.; Huang, D.; Hu, Z.; Ji, N.; Nan, Y.; Jiang, F.; Gou, X. Shikonin inhibits proliferation and induces apoptosis in glioma cells via downregulation of CD147. Mol. Med. Rep. 2019, 19, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, M.; Hao, C.; Yang, W. Shikonin induces tumor apoptosis in glioma cells via endoplasmic reticulum stress, and Bax/Bak mediated mitochondrial outer membrane permeability. J. Ethnopharmacol. 2020, 263, 113059. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Cai, Q.; Yao, L.; Mao, Y.; Ming, Y.; Ouyang, G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol. Int. 2006, 30, 221–226. [Google Scholar] [CrossRef]
- Deleyrolle, L.P.; Reynolds, B.A. Identifying and enumerating neural stem cells: Application to aging and cancer. Prog. Brain Res. 2009, 175, 43–51. [Google Scholar]
- Rajendran, V.; Jain, M.V. In Vitro Tumorigenic Assay: Colony Forming Assay for Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 89–95. [Google Scholar]
- Zhang, F.Y.; Hu, Y.; Que, Z.Y.; Wang, P.; Liu, Y.H.; Wang, Z.H.; Xue, Y.X. Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine. Int. J. Mol. Sci. 2015, 16, 23823–23848. [Google Scholar] [CrossRef]
- Costa, D.C.; Rangel, L.P.R.; Martins-Dinis, M.M.D.C.; Ferreti, G.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, Lapachone and their analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef]
- Rhun, E.L.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Behrooz, A.B.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef] [PubMed]
- Dratwa, M.; Wysoczanska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, E.; Kageler, L.; Wen, P.Y.; Meyerson, M. Telomerase as a therapeutic target in glioblastoma. Neuro Oncol. 2021, 23, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.M.; Oliveira, I.M.; Chiela, E.C.F.; Viau, C.M.; Saffi, J.; Horn, F.; Rosa, R.M.; Guecheva, T.N.; Henriques, J.A.P. Diphenyl Ditelluride-Induced Cell Cycle Arrest and Apoptosis: A Relation with Topoisomerase I Inhibition. Basic Clin. Pharmacol. Toxicol. 2015, 116, 273–280. [Google Scholar] [CrossRef]
- Brazvan, B.; Ebrahimi, A.K.; Velaei, K.; Mehdipour, A.; Aliyari, Z.S.; Ebrahimi, A.; Ghorbani, M.; Cheraghi, O.; Charoudeh, H.N. Telomerase activity and telomere on stem progeny senescence. Biomed. Pharmacother. 2018, 102, 9–17. [Google Scholar] [CrossRef]
- Dogan, F.; Avci, C.B. Correlation between Telomerase and mTOR pathway in Cancer Stem Cells. Gene 2018, 641, 235–239. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Ahmadian, S.; Zarghami, N.; Kahroba, H. Fundamental insights into the interaction between telomerase/TERT and intracellular signaling pathways. Biochimie 2021, 181, 12–24. [Google Scholar] [CrossRef]
- Baliza, D.D.M.S.; Silva, J.F.M.S.; Ferreira, E.M.S.; Ferreira, I.M.; Silva, E.O.; Nascimento, J.L.M.; Pimenta, R.S. Screening of Endophytes for Anticancer Compounds. In Endophytic Microbes: Isolation, Identification, and Bioactive Potentials; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Arocho, A.; Chen, B.; Ladanyi, M.; Pan, Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn. Mol. Pathol. 2006, 15, 56–61. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinkai, V.M.T.; Sampaio, I.M.O.; dos Santos, E.G.; Galué-Parra, A.J.; Ferreira, D.P.; Baliza, D.D.M.S.; Ramos, N.F.; Pimenta, R.S.; Burbano, R.M.R.; Sena, C.B.C.; et al. In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells. Molecules 2022, 27, 8850. https://doi.org/10.3390/molecules27248850
Shinkai VMT, Sampaio IMO, dos Santos EG, Galué-Parra AJ, Ferreira DP, Baliza DDMS, Ramos NF, Pimenta RS, Burbano RMR, Sena CBC, et al. In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells. Molecules. 2022; 27(24):8850. https://doi.org/10.3390/molecules27248850
Chicago/Turabian StyleShinkai, Victoria Mae Tsuruzaki, Izana Marize Oliveira Sampaio, Eline Gomes dos Santos, Adan Jesús Galué-Parra, Dionisia Pelaes Ferreira, Drielly Dayanne Monteiro Santos Baliza, Neidiane Farias Ramos, Raphael Sanzio Pimenta, Rommel Mario Rodriguez Burbano, Chubert Bernardo Castro Sena, and et al. 2022. "In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells" Molecules 27, no. 24: 8850. https://doi.org/10.3390/molecules27248850
APA StyleShinkai, V. M. T., Sampaio, I. M. O., dos Santos, E. G., Galué-Parra, A. J., Ferreira, D. P., Baliza, D. D. M. S., Ramos, N. F., Pimenta, R. S., Burbano, R. M. R., Sena, C. B. C., Macchi, B. M., Ferreira, I. M., Silva, E. O., & do Nascimento, J. L. M. (2022). In Vitro Cytotoxic Effects and Mechanisms of Action of Eleutherine Isolated from Eleutherine plicata Bulb in Rat Glioma C6 Cells. Molecules, 27(24), 8850. https://doi.org/10.3390/molecules27248850