Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages
Abstract
1. Introduction
2. Results and Discussion
2.1. GBP Ameliorated LPS-Mediated Inflammation in Macrophages
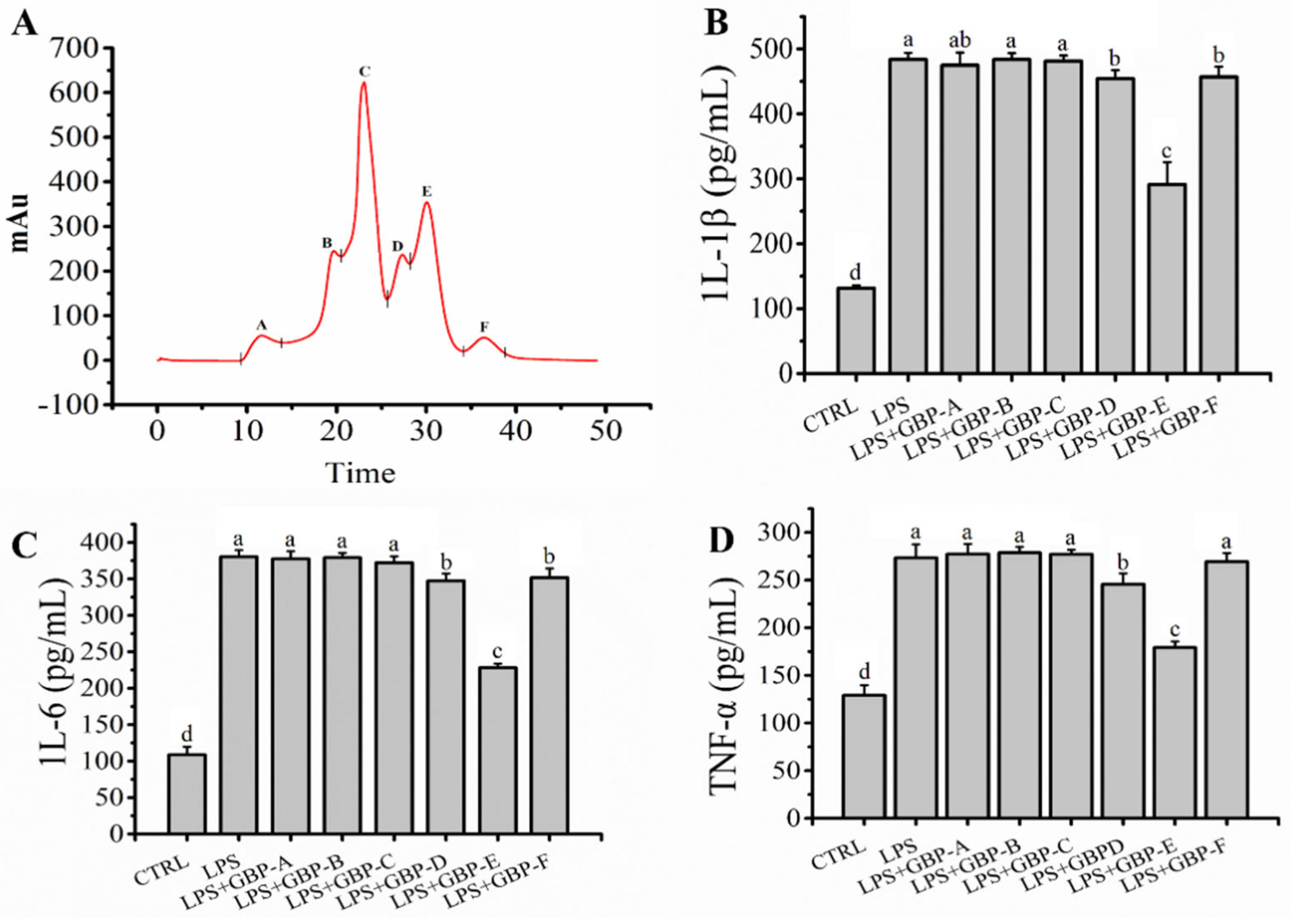
2.2. GBP Was Separated by SEC and Ameliorated LPS Mediated Inflammation in RAW264.7 Macrophages
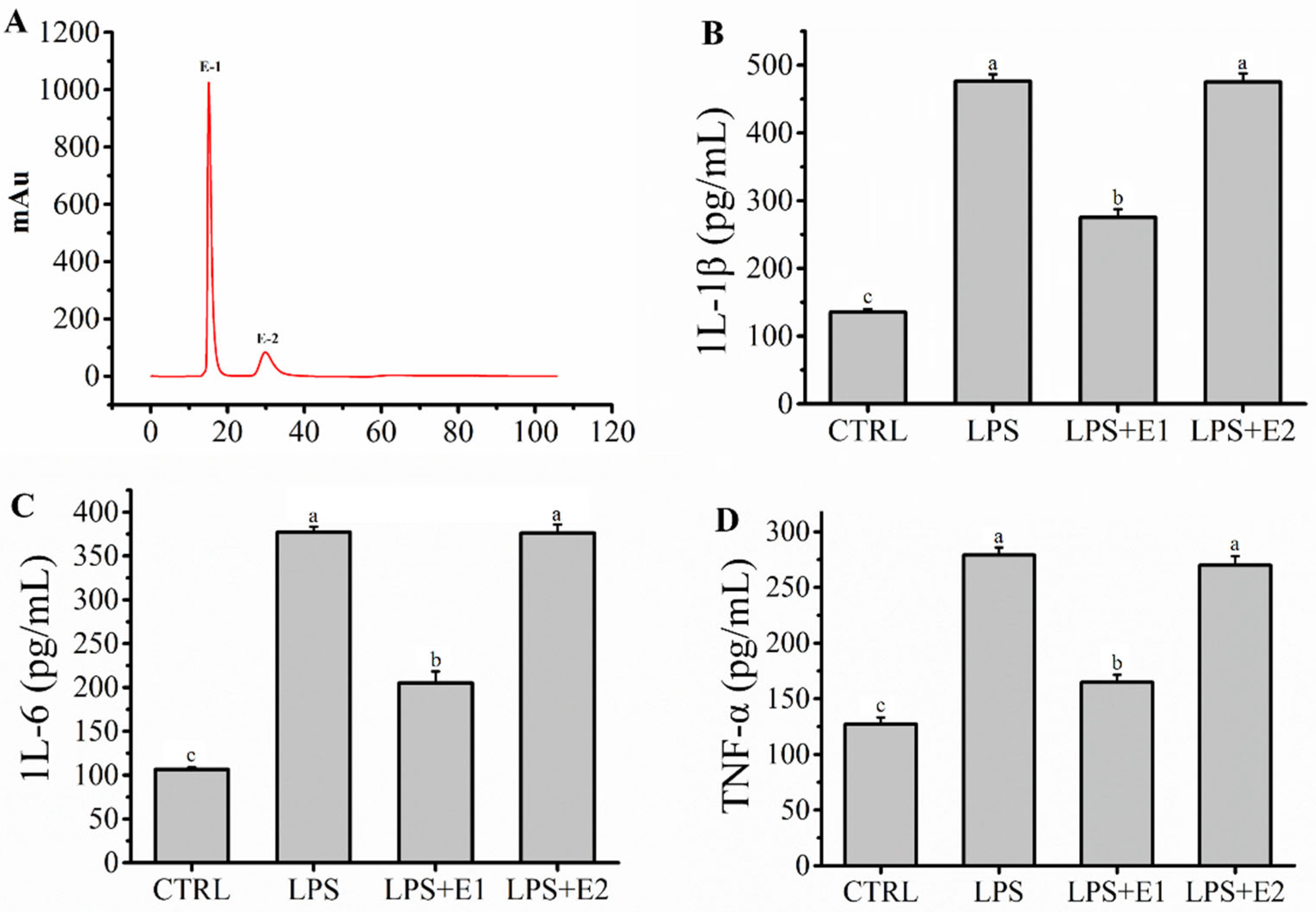
2.3. Fraction E Was Separated by IEC and Ameliorated Inflammation Mediated by LPS in RAW264.7 Macrophages
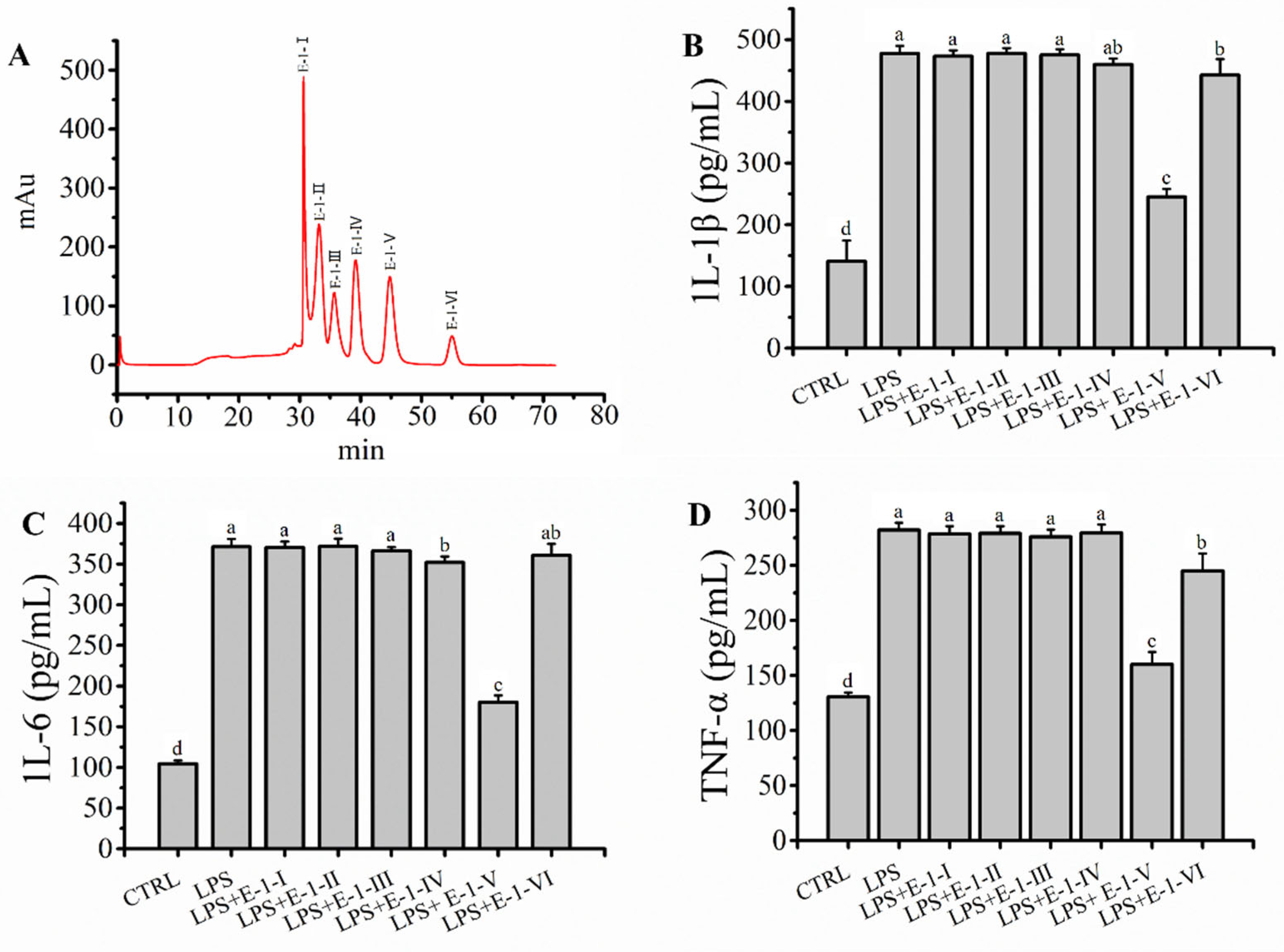
2.4. Fraction E-1 Was Separated by RP-LC and Ameliorated LPS-Mediated Inflammation in RAW264.7 Macrophages
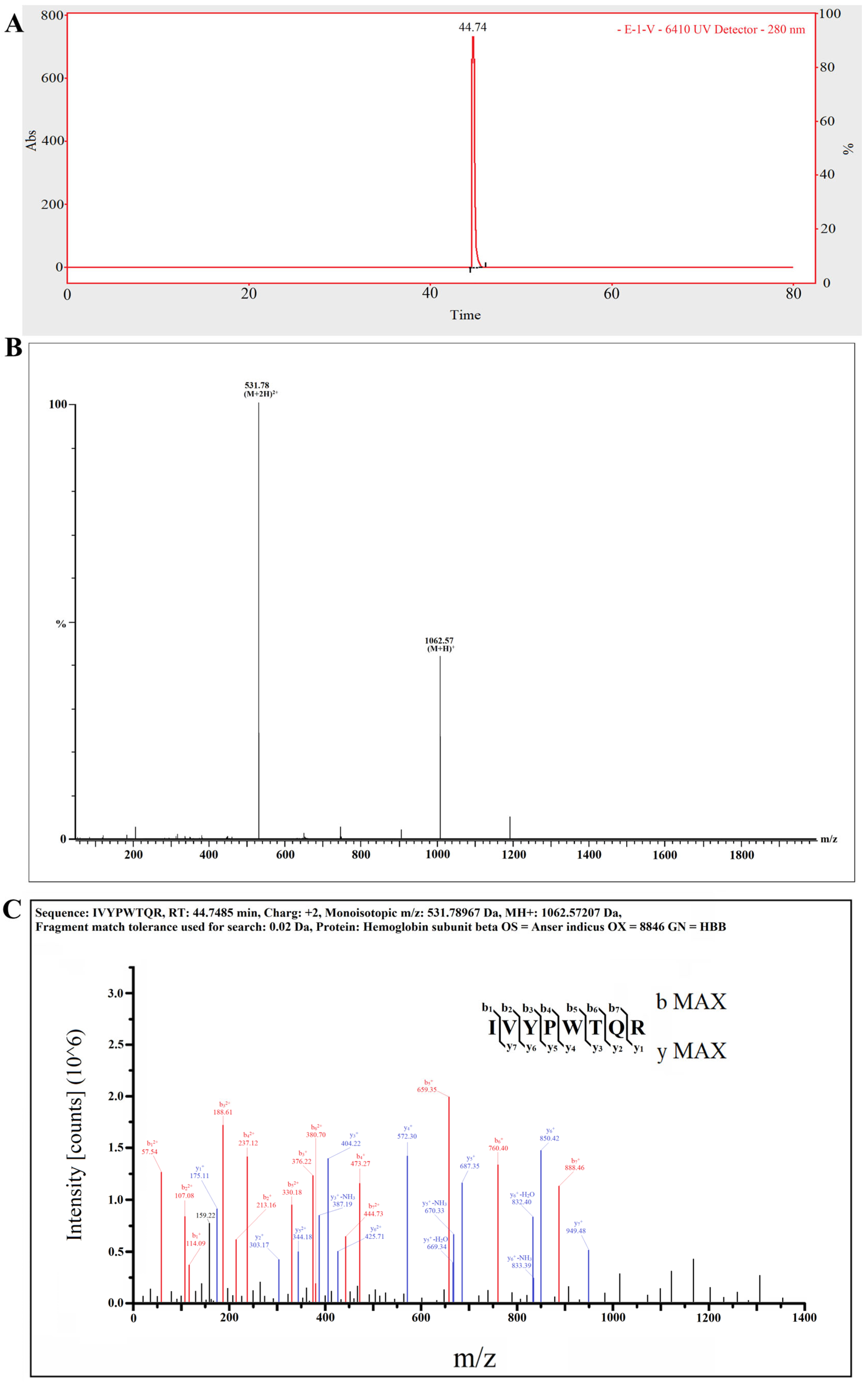
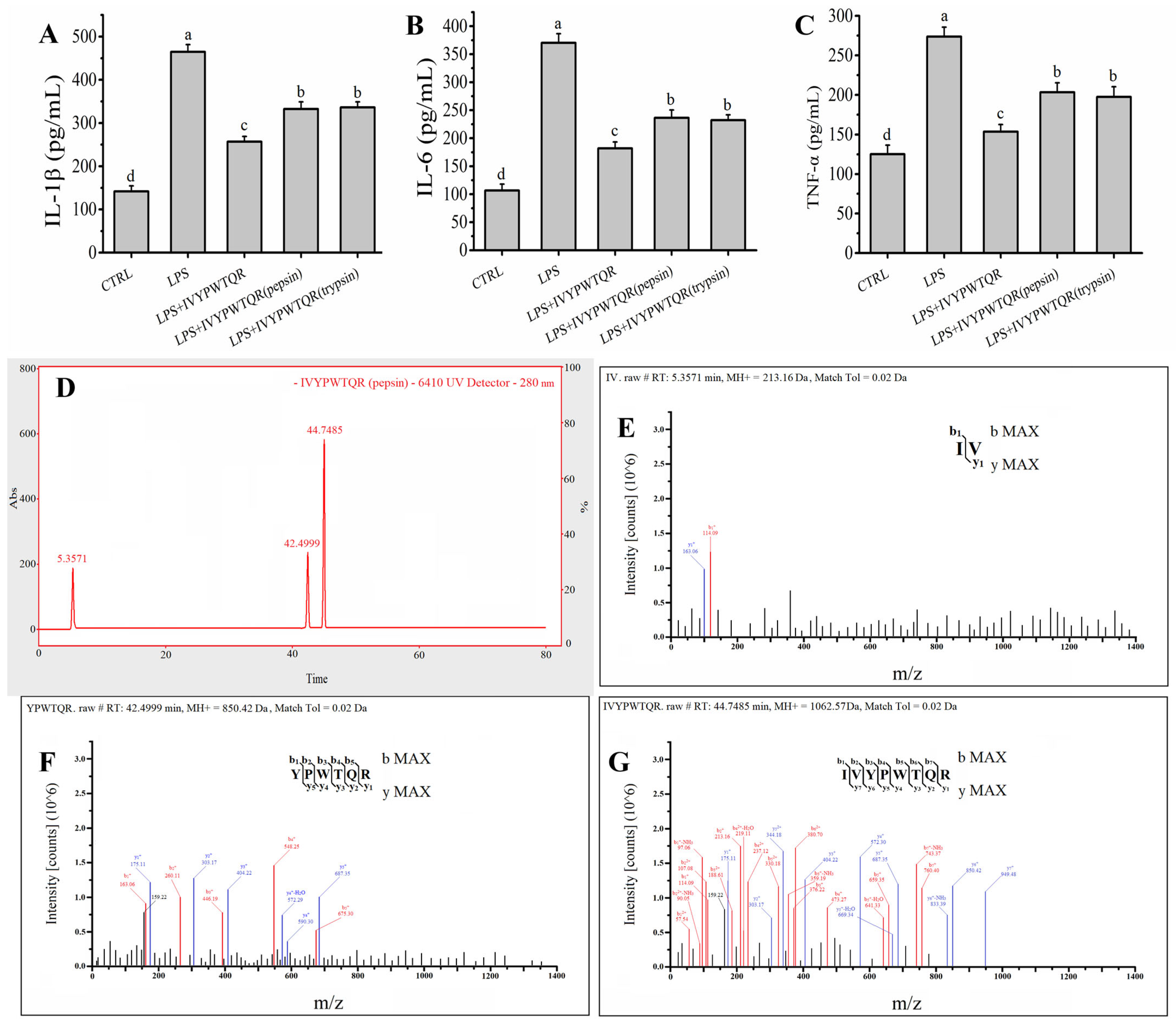
2.5. The Target Anti-Inflammatory Peptide Was Determined by LC–MS/MS
2.6. Effects of Pepsin–Trypsin Simulated GI Digestion on the Stability of IVYPWTQR
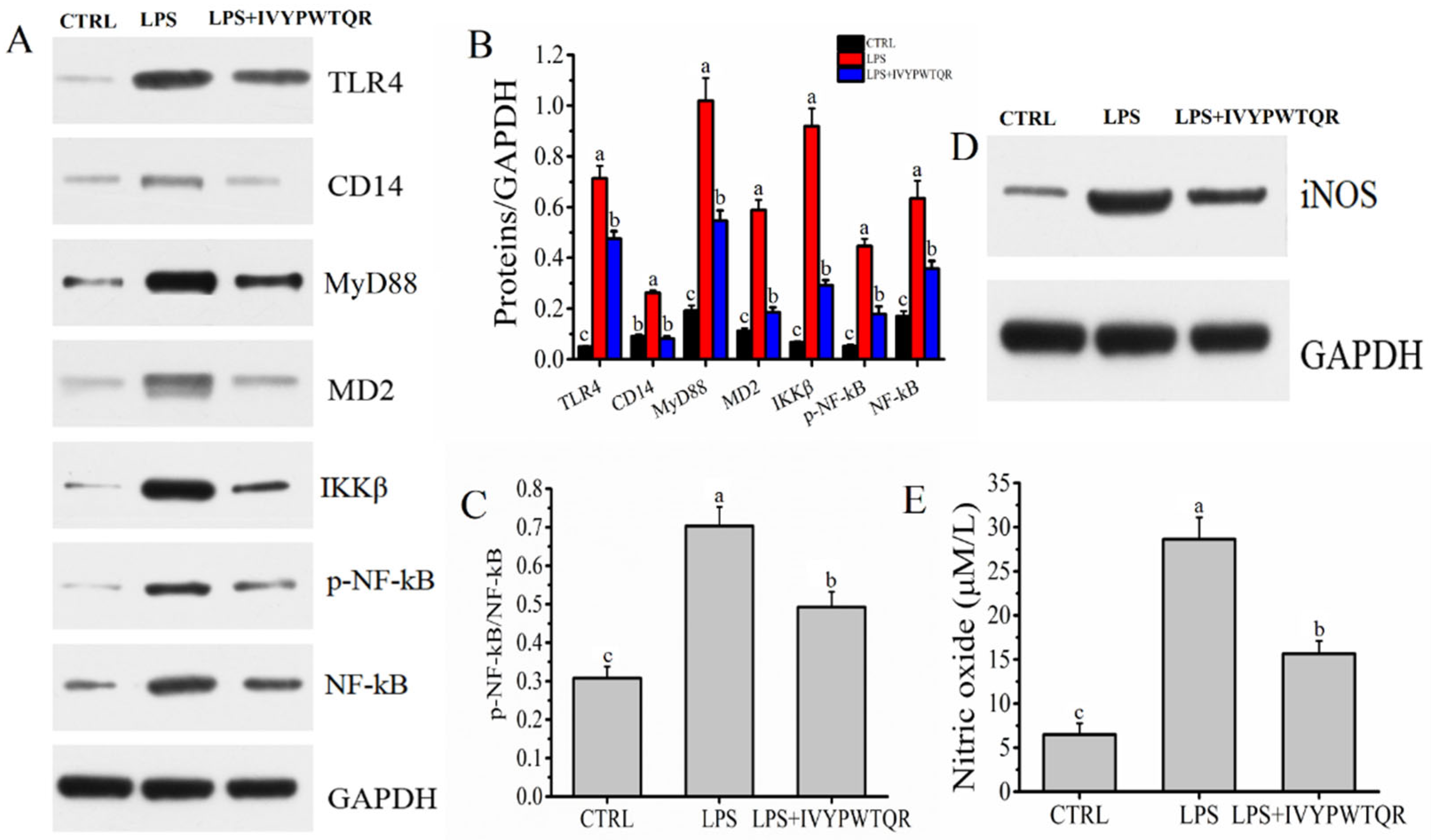
2.7. The Mechanism by Which IVYPWTQR Ameliorated LPS-Mediated Inflammation in RAW264.7 Macrophages
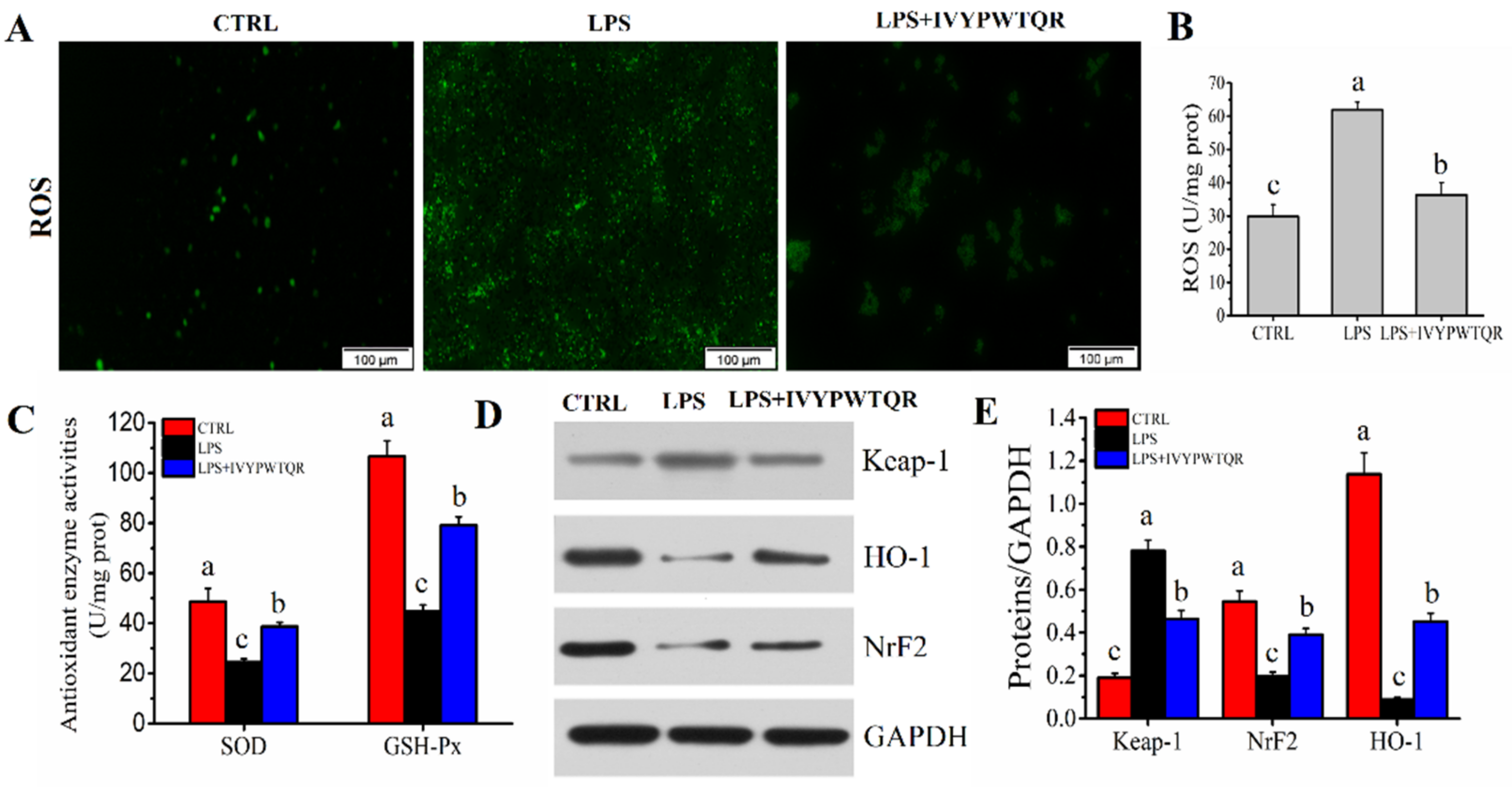
2.8. IVYPWTQR Ameliorated LPS-Mediated Oxidative Stress in RAW264.7 Macrophages
3. Materials and Methods
3.1. Preparation of GBP
3.2. Isolation and Identification of Oligopeptide with Anti-Inflammatory from GBP
3.3. Cell Culture
3.4. Simulated Gastrointestinal (GI) Digestion
3.5. Determination of Cytokines and Oxidative Stress Indicators
3.6. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.H.; Zhao, B.T.; Lee, J.H.; Kim, Y.H.; Min, B.S.; Woo, M.H. Isolation of benzoic and cinnamic acid derivatives from the grains of Sorghum bicolor and their inhibition of lipopolysaccharide-induced nitric oxide production in RAW264.7 cells. Food Chem. 2015, 168, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Dereje, N. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Himoto, T. Diet and Nutrition for Hepatitis. Nutrients 2021, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Gottardi, A.D.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Dupuy, R.; Dessertennes, F.; Vallin, J. High-fat diet in therapy of alcoholic cirrhosis and hepatitis. Rev. Int. Hepatol. 2018, 7, 591–597. [Google Scholar]
- Nie, W.; Du, Y.; Xu, F.R.; Zhou, K.; Wang, Z.M.; Aldalali, S.; Wang, Y.; Li, X.; Ma, Y.; Xie, Y. Oligopeptides from Jinhua ham prevent alcohol-induced liver damage by regulating intestinal homeostasis and oxidative stress in mice. Food Funct. 2021, 12, 10053–10070. [Google Scholar] [CrossRef]
- Moon, S.W.; Ahn, C.B.; Oh, Y.; Je, J.Y. Lotus (Nelumbo nucifera) seed protein isolate exerts anti-inflammatory and antioxidant effects in LPS-stimulated RAW264.7 macrophages via inhibiting NF-κB and MAPK pathways, and upregulating catalase activity. Int. J. Biol. Macromol. 2019, 134, 791–797. [Google Scholar] [CrossRef]
- Nie, W.; Zhou, K.; Wang, Y.; Wang, Z.M.; Xie, Y.; Zhou, H.; Xu, B.C. Isolation and identification of bioactive peptides from xuanwei ham that rescue oxidative stress damage induced by alcohol in HHL-5 hepatocytes. Food Funct. 2020, 11, 9710–9720. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Sung, J.; Ho, C.T.; Wang, Y. Preventive mechanism of bioactive dietary foods on obesity-related inflammation and diseases. Food Funct. 2018, 9, 6081–6095. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Li, M.; Dong, L.; Du, H.; Bao, Z.; Lin, S. Potential mechanisms underlying the protective effects of tricholoma matsutake singer peptides against LPS-induced inflammation in raw264.7 macrophages. Food Chem. 2021, 353, 129452. [Google Scholar] [CrossRef]
- Chen, P.J.; Tseng, J.K.; Lin, Y.L.; Wu, Y.H.; Hsiao, Y.T.; Chen, J.W.; Chen, Y.C. Protective effects of functional chicken liver hydrolysates against liver fibrogenesis: Antioxidation, anti-inflammation, and antifibrosis. J. Agric. Food Chem. 2017, 65, 4961–4969. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, F.; Yu, Y.C.; Jiang, Q.G.; Zhang, Z.F.; Wang, J.B.; Li, Y. Marine collagen peptides protect against early alcoholic liver injury in rats. Br. J. Nutr. 2012, 107, 1160–1166. [Google Scholar] [CrossRef][Green Version]
- Chen, H.L.; Tsai, T.C.; Tsai, Y.C. Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Nutr. Diabetes 2016, 6, e237. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The physiological activity of bioactive peptides obtained from meat and meat by-products. Adv. Food Nutr. Res. 2021, 97, 147–185. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.Y.; Xu, W.M.; Zou, Y.; Fang, W.M.; Tian, H.W.; Zhou, F.Y. Research on characteristics and flavor of three kinds of poultry blood tofu gel. Sci. Technol. Food Ind. 2021, 42, 76–82. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Feng, C.; Luo, Y.M.; Nian, Y.Y.; Liu, D.; Yin, X.R.; Wu, J.; Di, J.; Zhang, R.; Zhang, J. Diallyl disulfide suppresses the inflammation and apoptosis resistance induced by DCA through ROS and the NF-κB signaling pathway in Human Barrett’s Epithelial Cells. Inflammation 2017, 40, 818–831. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Schumacher, M.; Juncker, T.; Yan, C.C.; Inayat-Hussain, S.H.; Hajjouli, S.; Cerella, C.; Dicato, M.; Diederich, M. Styryl-lactone goniothalamin inhibits TNF-α-induced NF-κB activation. Food Chem. Toxicol. 2013, 59, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003, 10, 864–869. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.P.; Li, W.J.; Zheng, W.Y.; Yin, P.F.; Gong, D.M. Macrophage immunomodulatory activity of a purified polysaccharide isolated from Ganoderma atrum. Phytother. Res. 2013, 27, 186–191. [Google Scholar] [CrossRef]
- Li, S.X.; Chi, Z.X.; Li, W.G. In vitro toxicity of dimethyl phthalate to human erythrocytes: From the aspects of antioxidant and immune functions. Environ. Pollut. 2019, 253, 239–245. [Google Scholar] [CrossRef]
- Lee, I.T.; Yang, C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, B.; Park, K.E.; Utsuki, T.; Shin, T.; Oh, C.W.; Kim, H.R. Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2−MAPK signalling pathway in HepG2 cells. Food Chem. 2015, 174, 538–546. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Kati, P.P.; Anthony, M.H.; Reidar, A.G.; Juha, T.P.; Sirkku, A.P. Tight junction proteins zo-1, occludin, and claudins in developing and adult human perineurium. J. Histochem. Cytochem. 2004, 52, 1037–1046. [Google Scholar] [CrossRef]
- Chou, C.H.; Wang, S.Y.; Lin, Y.T.; Chen, Y.C. Antioxidant activities of chicken liver hydrolysates by pepsin treatment. Int. J. Food Sci. Technol. 2014, 49, 1654–1662. [Google Scholar] [CrossRef]
- Xie, N.N.; Liu, S.S.; Wang, C.; Li, B. Stability of casein antioxidant peptide fractions during in vitro digestion/Caco-2 cell model: Characteristics of the resistant peptides. Eur. Food Res. Technol. 2014, 239, 577–586. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhu, S.; Wang, R.; Chen, X.; Cai, K. Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages. Molecules 2022, 27, 8816. https://doi.org/10.3390/molecules27248816
Du Y, Zhu S, Wang R, Chen X, Cai K. Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages. Molecules. 2022; 27(24):8816. https://doi.org/10.3390/molecules27248816
Chicago/Turabian StyleDu, Yeye, Shuangjie Zhu, Ran Wang, Xingyong Chen, and Kezhou Cai. 2022. "Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages" Molecules 27, no. 24: 8816. https://doi.org/10.3390/molecules27248816
APA StyleDu, Y., Zhu, S., Wang, R., Chen, X., & Cai, K. (2022). Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages. Molecules, 27(24), 8816. https://doi.org/10.3390/molecules27248816





