Stability of Dibromo-Dipyrromethene Complexes Coordinated with B, Zn, and Cd in Solutions of Various Acidities
Abstract
1. Introduction
2. Results
2.1. Experimental Stability Study
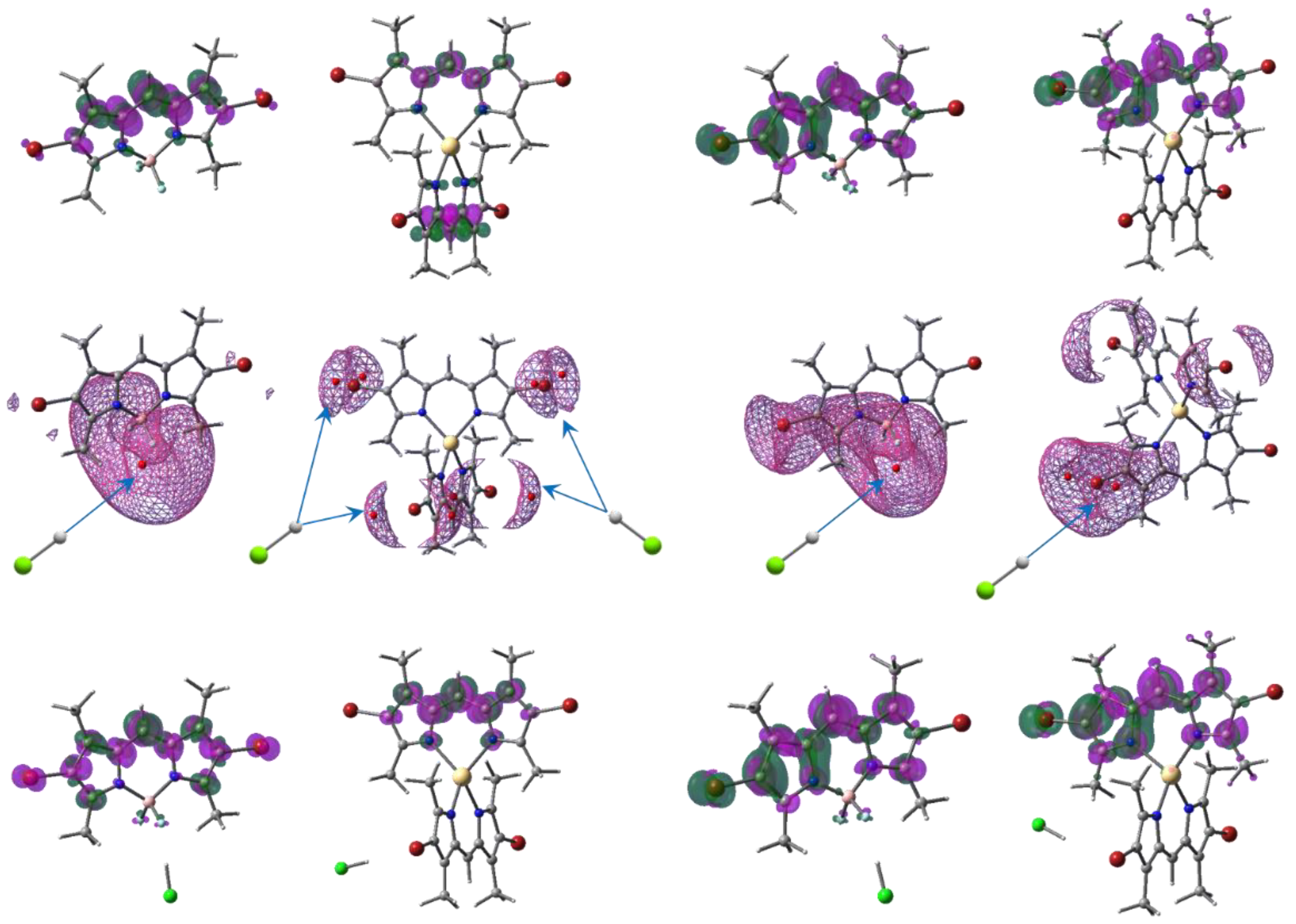
2.2. Quantum Chemical Calculations and Theoretical Analysis
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Burgess, K.; Loudet, A. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Khan, T.K.; Shaikh, M.S.; Ravikanth, M. Synthesis and photophysical properties of covalently linked boron dipyrromethene dyads. Dye. Pigm. 2012, 94, 66–73. [Google Scholar] [CrossRef]
- Radunz, S.; Kraus, W.; Bischoff, F.A.; Emmerling, F.; Tschiche, H.; Resch-Genger, U. Temperature- and structural-dependent optical properties and photophysics of BODIPY dyes. J. Phys. Chem. A 2020, 124, 1787–1797. [Google Scholar] [CrossRef]
- Cohen, S.M.; Halper, S.R. Dipyrromethene complexes of iron. Inorg. Chim. Acta 2002, 341, 12–16. [Google Scholar] [CrossRef]
- Baudron, S.A. Luminescent dipyrrin based metal complexes. Dalton Trans. 2013, 42, 7498–7509. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Iwashima, T.; Tsuchiya, M.; Toyoda, R.; Matsuoka, R.; Kogel, J.F.; Kusaka, S.; Hoshiko, K.; Yagi, T.; Nagayama, T.; et al. New aspects in bis and tris(dipyrrinato)metal complexes: Bright luminescence, self-assembled nanoarchitectures, and materials applications. J. Mater. Chem. A 2015, 3, 15357–15371. [Google Scholar] [CrossRef]
- Baudron, S.A. Luminescent metal–organic frameworks based on dipyrromethene metal complexes and BODIPYs. CrystEngComm. 2016, 18, 4671–4680. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-based fluorescent probes for biothiols. Chem. Eur. J. 2020, 226, 4172–4192. [Google Scholar] [CrossRef]
- Yan, M.; He, D.; Zhang, L.; Sun, P.; Sun, Y.; Qu, L.; Li, Z. Explorations into the meso-substituted BODIPY-based fluorescent probes for biomedical sensing and imaging. TrAC Trend Anal Chem. 2022, 157, 116771. [Google Scholar] [CrossRef]
- Xia, H.C.; Xu, X.H.; Song, Q.H. BODIPY-based fluorescent sensor for the recognization of phosgene in solutions and in gas phase. Anal. Chem. 2017, 89, 4192–4197. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.K.; Zhu, J.; Kong, F.K.W.; Ng, M.; Bian, Q.; Yam, V.W.W.; Tse, A.K.W.; Tse, Y.C.; Leung, K.C.F. A BODIPY-based fluorescent sensor for the detection of Pt2+ and Pt drugs. Chem. Commun. 2020, 56, 2695–2698. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Sankeerthana, P.A.; Jayaraj, A.; Swamy, P.C.A. Recent developments on BODIPY based chemosensors for the detection of group IIB metal ions. Results Chem. 2022, 4, 100297. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castaneda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; Garcıa-Fresnadillo, D.; Lopez-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; de la Moya, S.; Martınez, V. Exploring BODIPY derivatives as singlet oxygen photosensitizers for PDT photochemistry and photobiology. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.X.; Chen, W.K.; Niu, L.Y.; Fang, W.H.; Cui, G.; Yang, Q.Z. BODIPY-based photodynamic agents for exclusively generating superoxide radical over singlet oxygen. Angew. Chem. Int. Ed. 2021, 60, 19912–19920. [Google Scholar] [CrossRef]
- Deckers, J.; Cardeynaels, T.; Doria, S.; Tumanov, N.; Lapini, A.; Ethirajan, A.; Ameloot, M.; Wouters, J.; Di Donato, M.; Champagne, B.; et al. Balancing fluorescence and singlet oxygen formation in push–pull type near-infrared BODIPY photosensitizers. Mater. J. Chem. C 2022, 10, 9344–9355. [Google Scholar] [CrossRef]
- Prasannan, D.; Arunkumar, C. A “turn-on-and-off” pH sensitive BODIPY fluorescent probe for imaging E. coli cells. New J. Chem. 2018, 42, 3473–3482. [Google Scholar] [CrossRef]
- Radunz, S.; Andresen, E.; Würth, C.; Koerdt, A.; Tschiche, H.R.; Resch-Genger, U. Simple self-referenced luminescent pH sensors based on upconversion nanocrystals and pH-sensitive fluorescent bodipy dyes. Anal. Chem. 2019, 91, 7756–7764. [Google Scholar] [CrossRef]
- Öztürk, D.; Ömeroğlu, İ.; Köksoy, B.; Göl, C.; Durmuş, M. A BODIPY decorated multiple mode reusable paper-based colorimetric and fluorometric pH sensor. Dye. Pigm. 2022, 205, 110510. [Google Scholar] [CrossRef]
- Baudron, S.A. Dipyrrin based metal complexes: Reactivity and catalysis. Dalton Trans. 2020, 49, 6161–6175. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, R.T.; Aksenova, I.V.; Bashkirtsev, D.E.; Prokopenko, A.A.; Pomogaev, V.A.; Antina, E.V.; Berezin, M.B.; Bumagina, N.A. Photonics of coordination complexes of dipyrrins with p- and d-block elements for application in optical devices. J. Photochem. Photobiol. A 2018, 354, 147–154. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Aksenova, I.V.; Prokopenko, A.A.; Pomogaev, V.A.; Antina, E.V.; Berezin, M.B.; Antina, L.A.; Bumagina, N.A. Photonics of boron(III) and zinc(II) dipyrromethenates as active media for modern optical devices. J. Mol. Liq. 2019, 278, 5–11. [Google Scholar] [CrossRef]
- Aksenova, I.V.; Pomogaev, V.; Prokopenko, A.A.; Antina, E.V.; Berezin, M.B.; Guseva, G.B.; Nuraneeva, E.N.; Kuznetsova, R.T. Design and photophysical investigation of dipyrromethenates coordinated with the boron(III), zinc(II) and cadmium(II) as optical elements. Opt. Mater. 2021, 119, 111321. [Google Scholar] [CrossRef]
- Bañuelos, J.; López Arbeloa, F.; Arbeloa, T.; Salleres, S.; Vilas, J.L.; Amat-Guerri, F.; Liras, M.; López Arbeloa, I. Photophysical characterization of new 3-amino and 3-acetamido BODIPY dyes with solvent sensitive properties. J. Fluoresc. 2008, 18, 899–907. [Google Scholar] [CrossRef]
- Prieto, J.B.; Arbeloa, F.L.; López, T.A.; Martínez, V.M.; Arbeloa, I.L. BODIPY laser dyes applied in sensing and monitoring environmental properties. In Chromic Materials Phenomena and Their Technological Applications; Somani, P.R., Ed.; Applied Science Innovations Pvt. Ltd.: Pune, India, 2010; pp. 641–677. [Google Scholar]
- Sevinc, G.; Kücüköz, B.; Yılmaz, H.; Sirikci, G.; Yaglioglu, H.G.; Hayvalı, M.; Elmali, A. Explanation of pH probe mechanism inborondipyrromethene-benzimidazole compound using ultrafastspectroscopy technique. Sens. Actuators B Chem. 2014, 193, 737–744. [Google Scholar] [CrossRef]
- Solov’ev, K.N.; Borisevich, E.A. Intramolecular heavy-atom effect in the photophysics of organic molecules. Phys.—Usp. 2005, 48, 231–253. [Google Scholar] [CrossRef]
- Pomogaev, V.; Chiodo, S.; Ruud, K.; Kuznetsova, R.; Avramov, P. Computational investigation on the photophysical properties of halogenated tetraphenyl BODIPY. J. Phys. Chem. C 2020, 124, 11100–11109. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Guseva, G.B.; Antina, E.V.; Kuznetsova, R.T.; Berezin, M.B.; V’yugin, A.I. Synthesis, spectral luminescent properties, and photostability of monoiodo- and dibromo-substituted BF2-dipyrrinates. Russ. J. Gen. Chem. 2016, 86, 840–847. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Guseva, G.B.; Antina, E.V.; Berezin, M.B.; V’yugin, A.I. Synthesis and luminescent properties of zinc(II) complexes with iodo- and bromosubstituted 2,2′ -dipyrrines. J. Lumin. 2016, 17, 248–254. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Antina, E.V.; Guseva, G.B.; Berezin, M.B.; V’yugin, A.I. Effect of structure and medium on photostability of halogenated boron(III), zinc(II), and cadmium(II) dipyrromethenates. Russ. J. Gen. Chem. 2018, 88, 1172–1179. [Google Scholar] [CrossRef]
- Aksenova, I.V.; Kuznetsova, R.T.; Tel’minov, E.N.; Mayer, G.V.; Antina, E.V.; Berezin, M.B. Stabilities of a series of dipyrrin difluoroborates in protic solvents in the ground and electron-excited states. Rus. J. Phys. Chem. A 2016, 90, 349–355. [Google Scholar] [CrossRef]
- Prokopenko, A.A.; Kuznetsova, R.T.; Aksenova, I.V.; Telminov, E.N.; Berezin, M.B.; Antina, E.V. Spectral luminescence properties and stability of zinc(II) dipyrromethenates with different structures in proton-donor media in the ground and excited electronic states. Rus. J. Phys. Chem. A 2019, 93, 301–307. [Google Scholar] [CrossRef]
- Reijenga, J.; van Hoof, A.; van Loon, A.; Teunissen, B. Development of methods for the determination of pKa values. Anal. Chem. Insights 2013, 8, 53–71. [Google Scholar] [CrossRef]
- Christian, G.D.; Dasgupta, P.K.; Schug, K.A. Analytical Chemistry, 7th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Busch, M.; Ahlberg, E.; Ahlberg, E.; Laasonen, K. How to predict the pKa of any compound in any solvent. ACS Omega 2022, 7, 17369–17383. [Google Scholar] [CrossRef]
- Parker, C.A. Photoluminescence of Solutions; Elsevier: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Ireland, J.F.; Wyatt, P.A.H. Acid-base properties of’ electronically excited states of organic molecules. Adv. Phys. Org. Chem. 1976, 12, 131–221. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rubaszewska, W. Generalised Förster cycle. Thermodynamic and extrathermodynamic relationships between proton transfer, electron transfer and electronic excitation. J. Chem. Soc. Faraday Trans. 1977, 73, 11–28. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
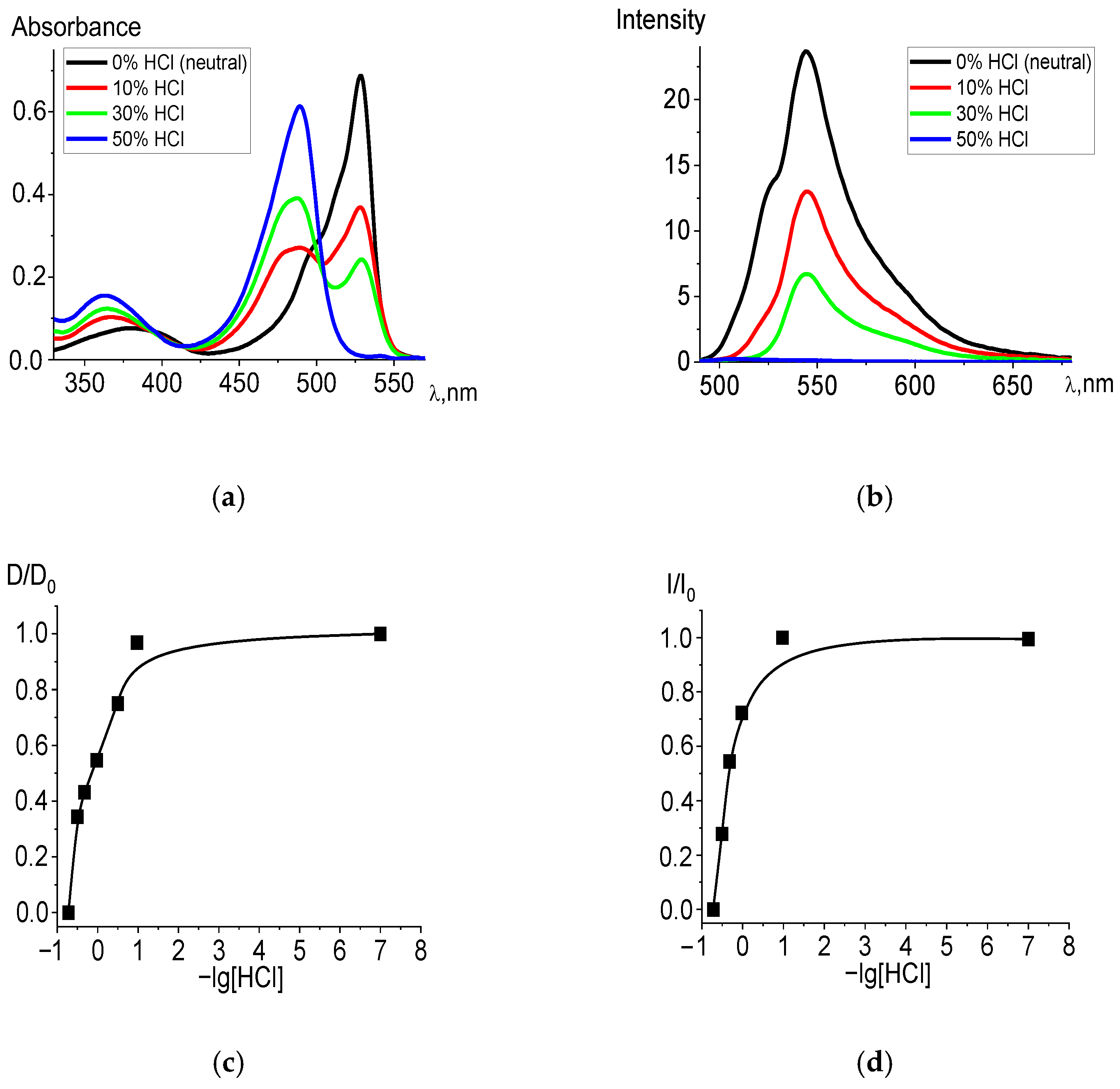



| Compound | λabs, nm (Neutral) | λabs, nm (Acidic) | −lg[HCl]50 (S0) | −lg[HCl]50 (S1)F-C | −lg[HCl]50 (Sfl) |
|---|---|---|---|---|---|
| Br2(CH3)4BODIPY | 528/528 (0.85) | 489/488 (0.95) | −0.2 | −3.2 | −0.4 |
| Zn[Br2(CH3)4dpm]2 | 504/504 (1.12) | 489/485 (1.15) | 4.3 | 3.1 | 4.3 |
| Cd[Br2(CH3)4dpm]2 | 498/502 (1.12) | 488/487 (1.15) | 4.7 | 3.9 | 5.0 |
| Compound | λfl, nm; Neutral | λfl, nm; Acidic | λabs, nm; Neutral | λabs, nm; Acidic |
|---|---|---|---|---|
| Br2(CH3)4BODIPY | 528.3 (0.467) | 528.9 (0.482) | 487.5 (0.854) | 451 (0.405) |
| Zn[Br2(CH3)4dpm]2 | 543.7 (0.001) 508.2 (0.343) | 530.8 (0.002) 507.6 (0.356) | 482.8 (0.001) 464.4 (1.122) | 477.0 (0.001) 455.1 (1.145) |
| Cd[Br2(CH3)4dpm]2 | 532.3 (0.000) 507.4 (0.287) | 521.5 (0.001) 504.6 (0.332) | 472.3 (0.001) 461.8 (1.122) | 473.0 (0.001) 456.5 (1.159) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenova, I.; Pomogaev, V. Stability of Dibromo-Dipyrromethene Complexes Coordinated with B, Zn, and Cd in Solutions of Various Acidities. Molecules 2022, 27, 8815. https://doi.org/10.3390/molecules27248815
Aksenova I, Pomogaev V. Stability of Dibromo-Dipyrromethene Complexes Coordinated with B, Zn, and Cd in Solutions of Various Acidities. Molecules. 2022; 27(24):8815. https://doi.org/10.3390/molecules27248815
Chicago/Turabian StyleAksenova, Iuliia, and Vladimir Pomogaev. 2022. "Stability of Dibromo-Dipyrromethene Complexes Coordinated with B, Zn, and Cd in Solutions of Various Acidities" Molecules 27, no. 24: 8815. https://doi.org/10.3390/molecules27248815
APA StyleAksenova, I., & Pomogaev, V. (2022). Stability of Dibromo-Dipyrromethene Complexes Coordinated with B, Zn, and Cd in Solutions of Various Acidities. Molecules, 27(24), 8815. https://doi.org/10.3390/molecules27248815







