Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry
Abstract
1. Introduction
2. Results
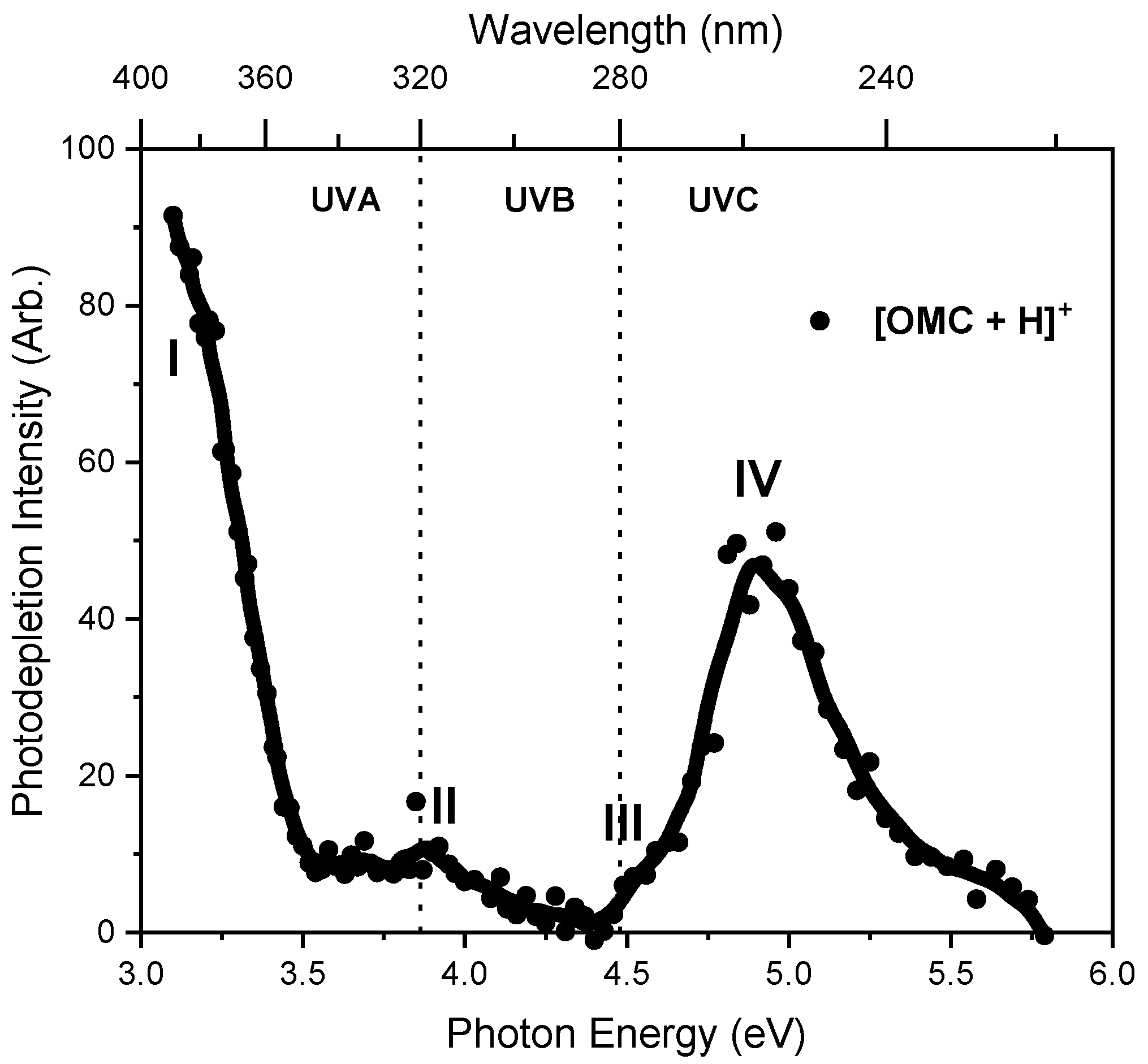
2.1. Gas-Phase Absorption Spectrum of Protonated OMC
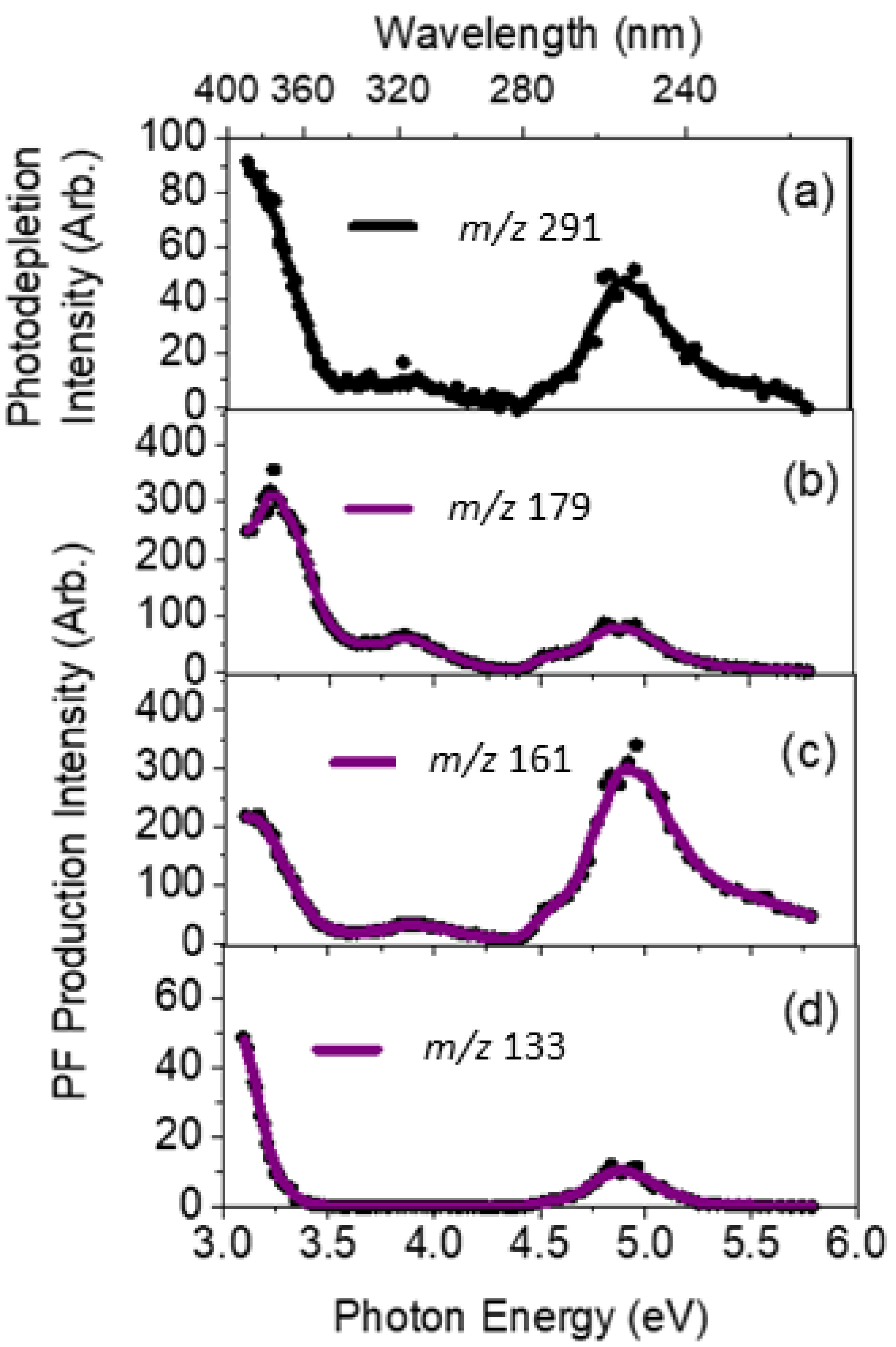
2.2. Gas-Phase Absorption Spectrum of Photofragment Ions of [OMC·H]+
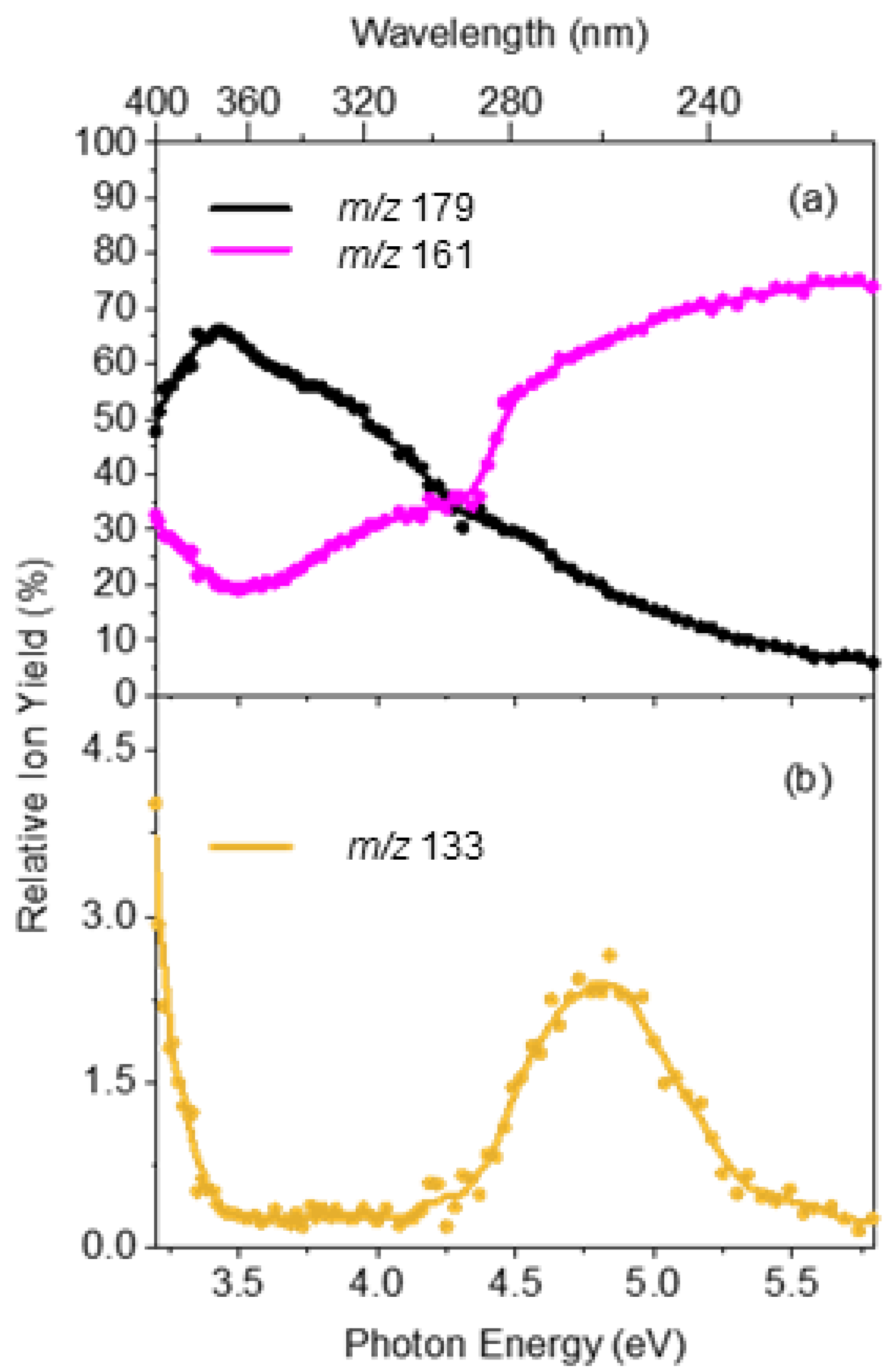
2.3. Comparison of Photofragmentation and HCD Fragmentation of [OMC·H]+
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forestier, S. Rationale for Sunscreen Development. J. Am. Acad. Dermatol. 2008, 58, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Setlow, R.B.; Setlow, J.K. Evidence that ultraviolet-induced thymine dimers in dna cause biological damage. Proc. Natl. Acad. Sci. USA 1962, 48, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Mahler, V. The Epidemiology of Skin Cancer. Br. J. Dermatol. 2002, 146, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shaath, N. (Ed.) Sunscreens: Regulations and Commercial Development, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-8493-9859-9. [Google Scholar]
- Baker, L.A.; Marchetti, B.; Karsili, T.N.V.; Stavros, V.G.; Ashfold, M.N.R. Photoprotection: Extending Lessons Learned from Studying Natural Sunscreens to the Design of Artificial Sunscreen Constituents. Chem. Soc. Rev. 2017, 46, 3770–3791. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Gratton, E.; Bardeen, C.J. Sunscreen Enhancement of UV-Induced Reactive Oxygen Species in the Skin. Free Radic. Biol. Med. 2006, 41, 1205–1212. [Google Scholar] [CrossRef]
- Giokas, D.L.; Sakkas, V.A.; Albanis, T.A.; Lampropoulou, D.A. Determination of UV-Filter Residues in Bathing Waters by Liquid Chromatography UV-Diode Array and Gas Chromatography–Mass Spectrometry after Micelle Mediated Extraction-Solvent Back Extraction. J. Chromatogr. A 2005, 1077, 19–27. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Organic UV Filters in Marine Environments: An Update of Analytical Methodologies, Occurrence and Distribution. Trends Environ. Anal. Chem. 2020, 25, e00079. [Google Scholar] [CrossRef]
- Wong, N.G.K.; Dessent, C.E.H. Illuminating the Effect of the Local Environment on the Performance of Organic Sunscreens: Insights from Laser Spectroscopy of Isolated Molecules and Complexes. Front. Chem. 2022, 9, 812098. [Google Scholar] [CrossRef]
- Tan, E.M.M.; Hilbers, M.; Buma, W.J. Excited-State Dynamics of Isolated and Microsolvated Cinnamate-Based UV-B Sunscreens. J. Phys. Chem. Lett. 2014, 5, 2464–2468. [Google Scholar] [CrossRef]
- Pattanaargson, S.; Munhapol, T.; Hirunsupachot, P.; Luangthongaram, P. Photoisomerization of Octyl Methoxycinnamate. J. Photochem. Photobiol. A Chem. 2004, 161, 269–274. [Google Scholar] [CrossRef]
- Krishnan, R.; Nordlund, T.M. Fluorescence Dynamics of Three UV-B Sunscreens. J. Fluoresc. 2008, 18, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Karpkird, T.M.; Wanichweacharungruang, S.; Albinsson, B. Photophysical Characterization of Cinnamates. Photochem. Photobiol. Sci. 2009, 8, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Beyere, L.; Yarasi, S.; Loppnow, G.R. Solvent Effects on Sunscreen Active Ingredients Using Raman Spectroscopy. J. Raman Spectrosc. 2003, 34, 743–750. [Google Scholar] [CrossRef]
- Gackowska, A.; Przybyłek, M.; Studziński, W.; Gaca, J. Formation of Chlorinated Breakdown Products during Degradation of Sunscreen Agent, 2-Ethylhexyl-4-Methoxycinnamate in the Presence of Sodium Hypochlorite. Environ. Sci. Pollut. Res. 2016, 23, 1886–1897. [Google Scholar] [CrossRef]
- Stein, H.V.; Berg, C.J.; Maung, J.N.; O’Connor, L.E.; Pagano, A.E.; MacManus-Spencer, L.A.; Paulick, M.G. Photolysis and Cellular Toxicities of the Organic Ultraviolet Filter Chemical Octyl Methoxycinnamate and Its Photoproducts. Environ. Sci. Processes Impacts 2017, 19, 851–860. [Google Scholar] [CrossRef]
- Herzog, B.; Amorós-Galicia, L.; Sohn, M.; Hofer, M.; Quass, K.; Giesinger, J. Analysis of Photokinetics of 2′-Ethylhexyl-4-Methoxycinnamate in Sunscreens. Photochem. Photobiol. Sci. 2019, 18, 1773–1781. [Google Scholar] [CrossRef]
- Fois, E.; Oriani, M.; Tabacchi, G. A Post-HF Approach to the Sunscreen Octyl Methoxycinnamate. J. Chem. Phys. 2021, 154, 144304. [Google Scholar] [CrossRef]
- Chang, X.-P.; Li, C.-X.; Xie, B.-B.; Cui, G. Photoprotection Mechanism of p -Methoxy Methylcinnamate: A CASPT2 Study. J. Phys. Chem. A 2015, 119, 11488–11497. [Google Scholar] [CrossRef]
- Garcia, R.D.; Maltarollo, V.G.; Honório, K.M.; Trossini, G.H.G. Benchmark Studies of UV–Vis Spectra Simulation for Cinnamates with UV Filter Profile. J. Mol. Model 2015, 21, 150. [Google Scholar] [CrossRef]
- Serpone, N.; Salinaro, A.; Emeline, A.V.; Horikoshi, S.; Hidaka, H.; Zhao, J. An in Vitro Systematic Spectroscopic Examination of the Photostabilities of a Random Set of Commercial Sunscreen Lotions and Their Chemical UVB/UVA Active Agents. Photochem. Photobiol. Sci. 2002, 1, 970–981. [Google Scholar] [CrossRef]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen, C.J. Photochemical Degradation of the UV Filter Octyl Methoxycinnamate in Solution and in Aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter-Filter Interactions. Photostabilization, Triplet Quenching and Reactivity with Singlet Oxygen. Photochem. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Berenbeim, J.A.; Hawkridge, M.; Matthews, E.; Dessent, C.E.H. Mapping the Intrinsic Absorption Properties and Photodegradation Pathways of the Protonated and Deprotonated Forms of the Sunscreen Oxybenzone. Phys. Chem. Chem. Phys. 2019, 21, 14311–14321. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Rankine, C.D.; Dessent, C.E.H. Linking Electronic Relaxation Dynamics and Ionic Photofragmentation Patterns for the Deprotonated UV Filter Benzophenone-4. J. Phys. Chem. Lett. 2021, 12, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Berenbeim, J.A.; Wong, N.G.K.; Cockett, M.C.R.; Berden, G.; Oomens, J.; Rijs, A.M.; Dessent, C.E.H. Unravelling the Keto–Enol Tautomer Dependent Photochemistry and Degradation Pathways of the Protonated UVA Filter Avobenzone. J. Phys. Chem. A 2020, 124, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Rankine, C.D.; Anstöter, C.S.; Dessent, C.E.H. Photostability of the deprotonated forms of the UV filters homosalate and octyl salicylate: Molecular dissociation versus electron detachment following UV excitation. Phys. Chem. Chem. Phys. 2022, 24, 17068–17076. [Google Scholar] [CrossRef]
- MacManus-Spencer, L.A.; Tse, M.L.; Klein, J.L.; Kracunas, A.E. Aqueous Photolysis of the Organic Ultraviolet Filter Chemical Octyl Methoxycinnamate. Environ. Sci. Technol. 2011, 45, 3931–3937. [Google Scholar] [CrossRef]
- Uleanya, K.O.; Cercola, R.; Nikolova, M.; Matthews, E.; Wong, N.G.K.; Dessent, C.E.H. Observation of Enhanced Dissociative Photochemistry in the Non-Native Nucleobase 2-Thiouracil. Molecules 2020, 25, 3157. [Google Scholar] [CrossRef]
- Lucas, B.; Barat, M.; Fayeton, J.A.; Jouvet, C.; Çarçabal, P.; Grégoire, G. Statistical versus Non-Statistical Photo-Fragmentation of Protonated GWG Tri-Peptide Induced by UV Excitation. Chem. Phys. 2008, 347, 324–330. [Google Scholar] [CrossRef]
- Wong, N.G.K.; Berenbeim, J.A.; Dessent, C.E.H. Direct Observation of Photochemical Free Radical Production from the Sunscreen 2-Phenylbenzimidazole-5-Sulfonic Acid via Laser-Interfaced Mass Spectrometry. ChemPhotoChem 2019, 3, 1231–1237. [Google Scholar] [CrossRef]
- Muramatsu, S.; Nakayama, S.; Kinoshita, S.; Onitsuka, Y.; Kohguchi, H.; Inokuchi, Y.; Zhu, C.; Ebata, T. Electronic State and Photophysics of 2-Ethylhexyl-4-Methoxycinnamate as UV-B Sunscreen under Jet-Cooled Condition. J. Phys. Chem. A 2020, 124, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E. Photodissociation Spectroscopy of Gaseous Bio-Ions in a Commercial Quadrupole Ion Trap Mass Spectrometer. Ph.D. Thesis, University of York, York, UK, 2018. [Google Scholar]
- Matthews, E.; Sen, A.; Yoshikawa, N.; Bergström, E.; Dessent, C.E.H. UV Laser Photoactivation of Hexachloroplatinate Bound to Individual Nucleobases: In Vacuo as Molecular Level Probes of a Model Photopharmaceutical. Phys. Chem. Chem. Phys. 2016, 18, 15143–15152. [Google Scholar] [CrossRef]
- Yao, H.; Jockusch, R.A. Fluorescence and Electronic Action Spectroscopy of Mass-Selected Gas-Phase Fluorescein, 2′,7′-Dichlorofluorescein, and 2′,7′-Difluorofluorescein Ions. J. Phys. Chem. A 2013, 117, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Antoine, R.; Dugourd, P. Visible and Ultraviolet Spectroscopy of Gas Phase Protein Ions. Phys. Chem. Chem. Phys. 2011, 13, 16494–16509. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.G.K.; Rhodes, C.; Dessent, C.E.H. Photodegradation of Riboflavin under Alkaline Conditions: What Can Gas-Phase Photolysis Tell Us about What Happens in Solution? Molecules 2021, 26, 6009. [Google Scholar] [CrossRef]
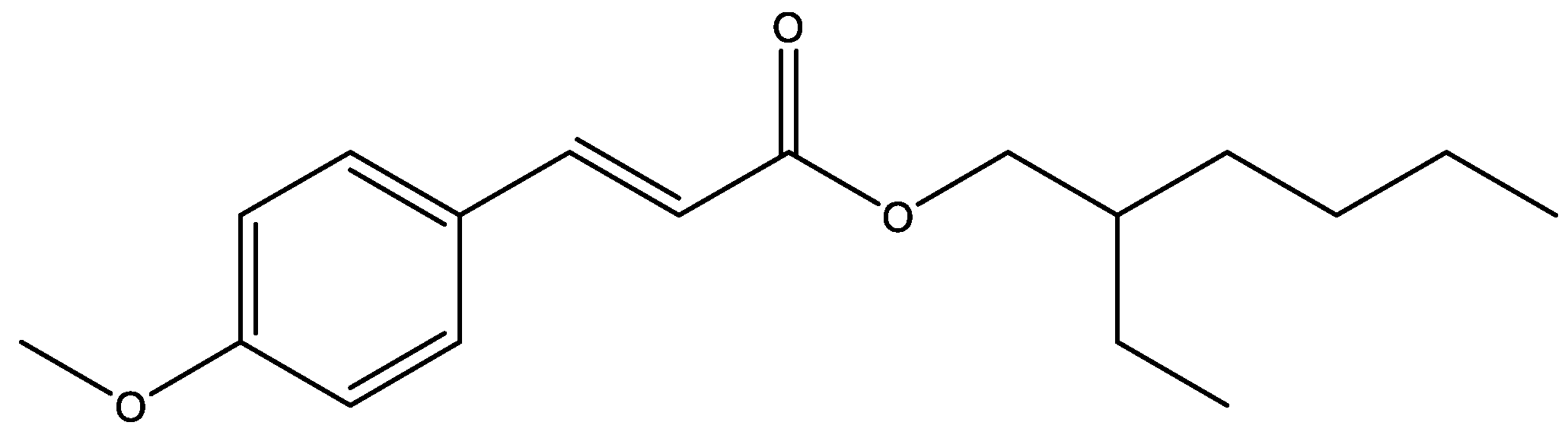
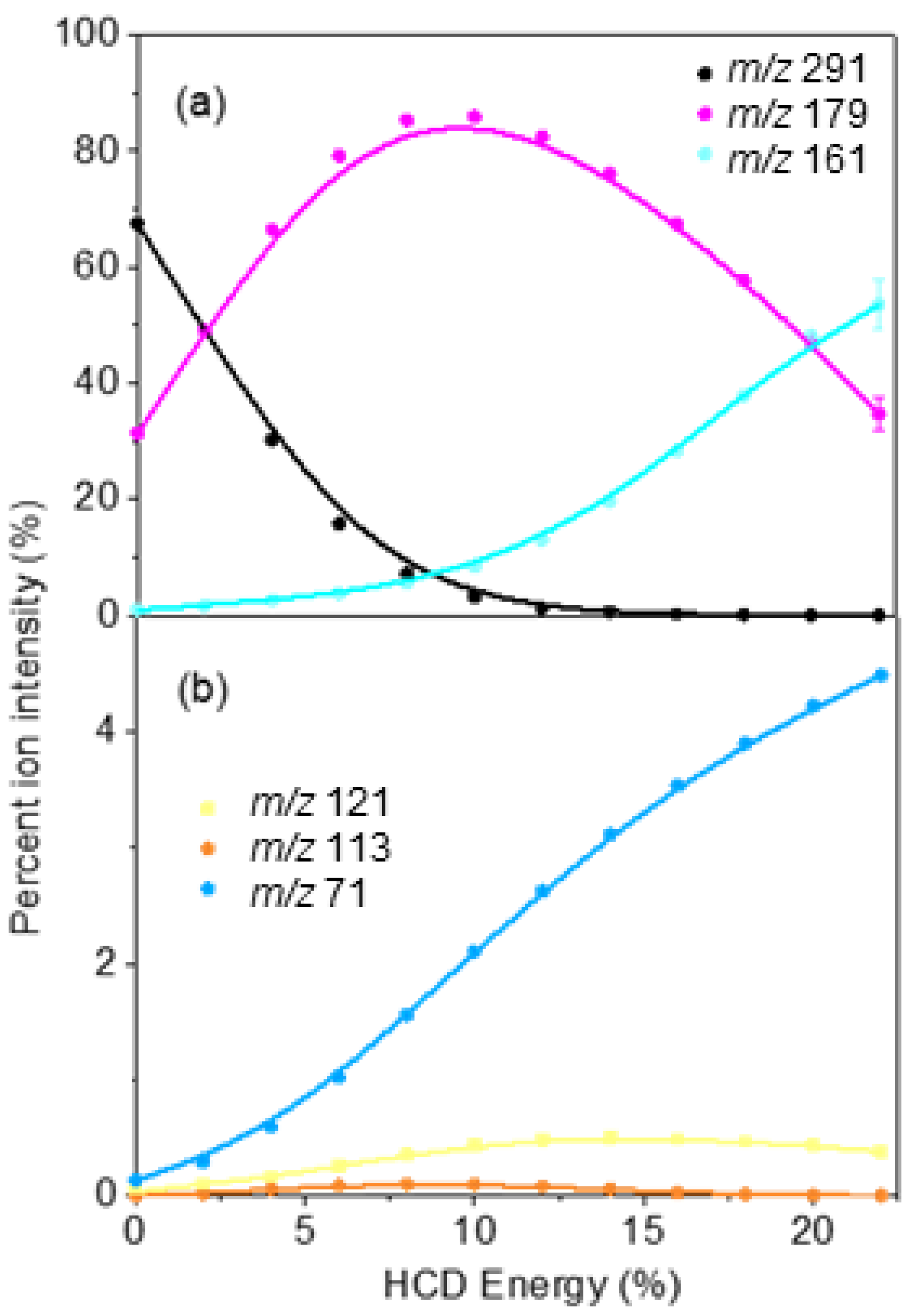
| Fragment Mass (m/z) | Proposed Fragment Structure | ∆m/z 1 | Observed HCD Fragment (Y/N) 2 | Observed Photofragment (Y/N) 2 |
|---|---|---|---|---|
| 179 | 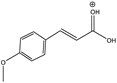 | 112 | Y (s) | Y (vs) |
| 161 | 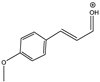 | 130 | Y (s) | Y (vs) |
| 133 | 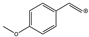 | 158 | N | Y (vw) |
| 121 | - | 170 | Y (w) | Y (vw) |
| 113 | - | 178 | Y (vw) | N |
| 71 | - | 220 | Y (vw) | Y (vw) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, N.G.K.; Sereli, M.; Anstöter, C.S.; Dessent, C.E.H. Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry. Molecules 2022, 27, 8796. https://doi.org/10.3390/molecules27248796
Wong NGK, Sereli M, Anstöter CS, Dessent CEH. Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry. Molecules. 2022; 27(24):8796. https://doi.org/10.3390/molecules27248796
Chicago/Turabian StyleWong, Natalie G. K., Maria Sereli, Cate S. Anstöter, and Caroline E. H. Dessent. 2022. "Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry" Molecules 27, no. 24: 8796. https://doi.org/10.3390/molecules27248796
APA StyleWong, N. G. K., Sereli, M., Anstöter, C. S., & Dessent, C. E. H. (2022). Photochemical Degradation of the UV Filter Octyl Methoxy Cinnamate Probed via Laser-Interfaced Mass Spectrometry. Molecules, 27(24), 8796. https://doi.org/10.3390/molecules27248796







