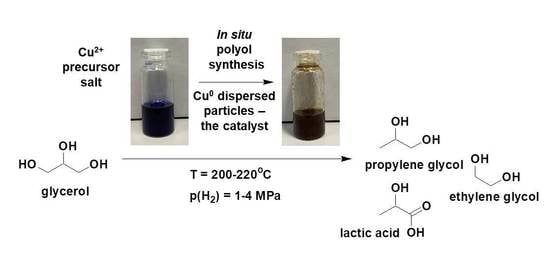
In Situ-Generated, Dispersed Cu Catalysts for the Catalytic Hydrogenolysis of Glycerol
Abstract
:1. Introduction
- The dispersion of catalyst particles affects the reaction rate;
- The thermal effect of the reaction complicates the use of a fixed-bed reactor;
- The catalytic dispersed phase can be formed in situ;
- The reaction medium is able to inhibit the coagulation of the particles;
- The reaction products have a boiling point lower than the raw material and can be separated from the reaction mass by distillation.
- A description of the phenomenon of dispersed catalyst formation in situ in the reaction medium;
- Characterization of the catalytic activity of the resulting catalyst, including the dependence of product yields on the reaction conditions;
- Description of the structure and morphology of in situ-generated catalysts.
2. Results and Discussion
2.1. Phenomenon of Catalytic Activity in the Glycerol Hydrogenolysis Reaction
- A heterogeneous catalyst must be present in the reaction mixture;
- The resulting reaction mixture must contain reaction products—in particular, the target PG;
- The conversion of glycerol and the yield of the desired products should increase with increasing temperature and reaction time;
- The conversion of glycerol and the yield of the desired products should change when the Gly/Cu ratio changes;
- Propylene glycol should be formed only in a hydrogen medium and only in the presence of a copper precursor salt.
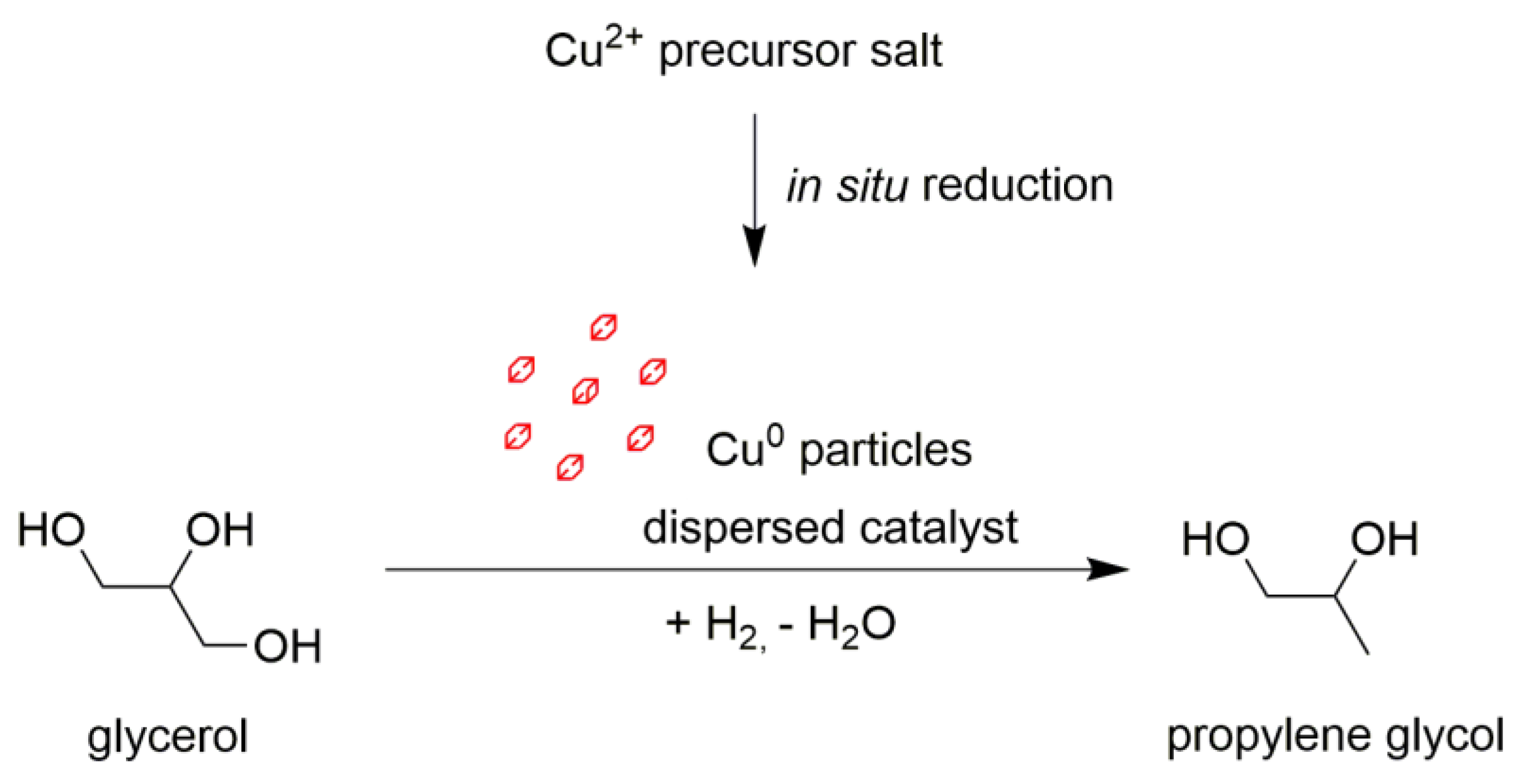
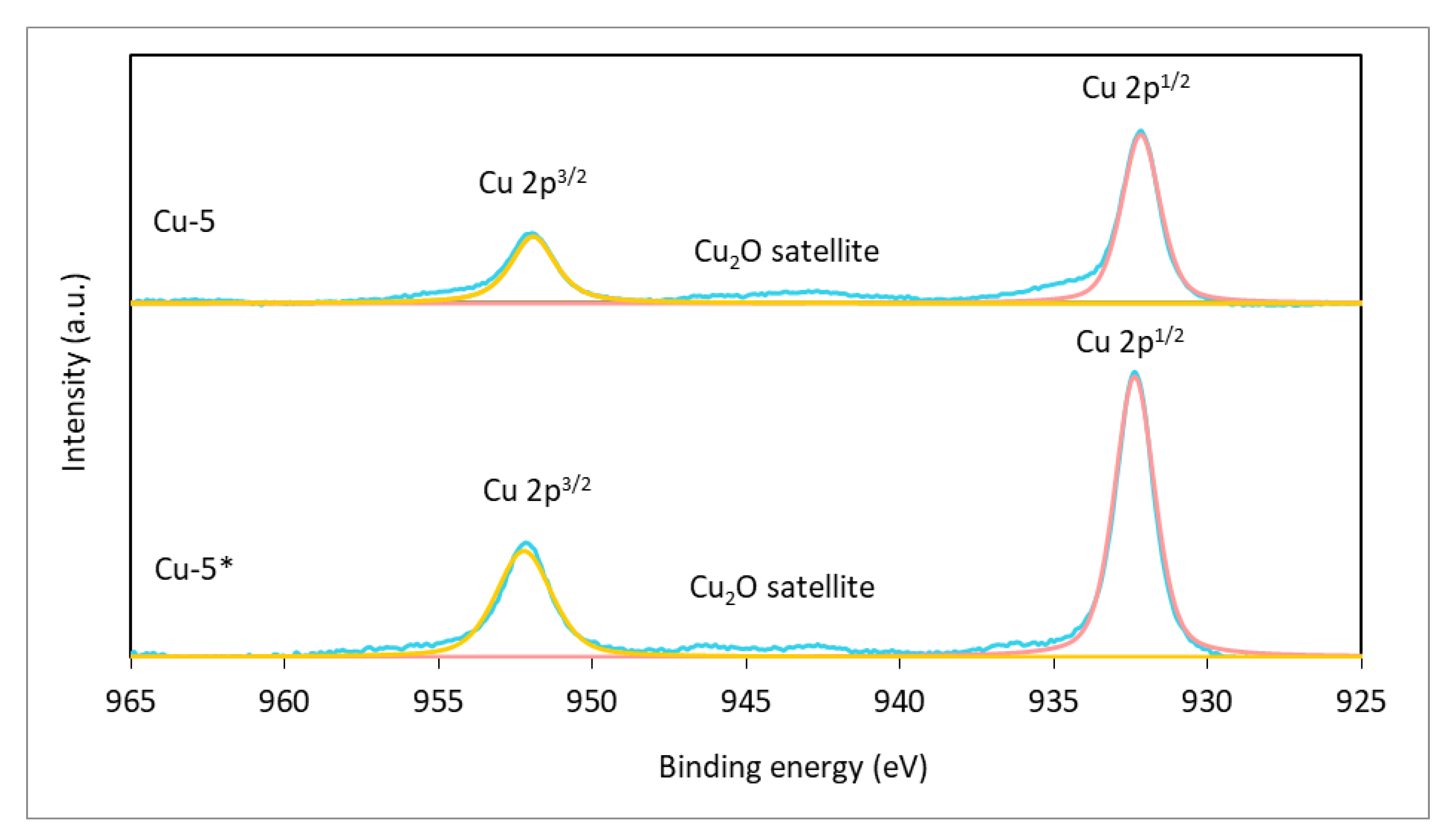
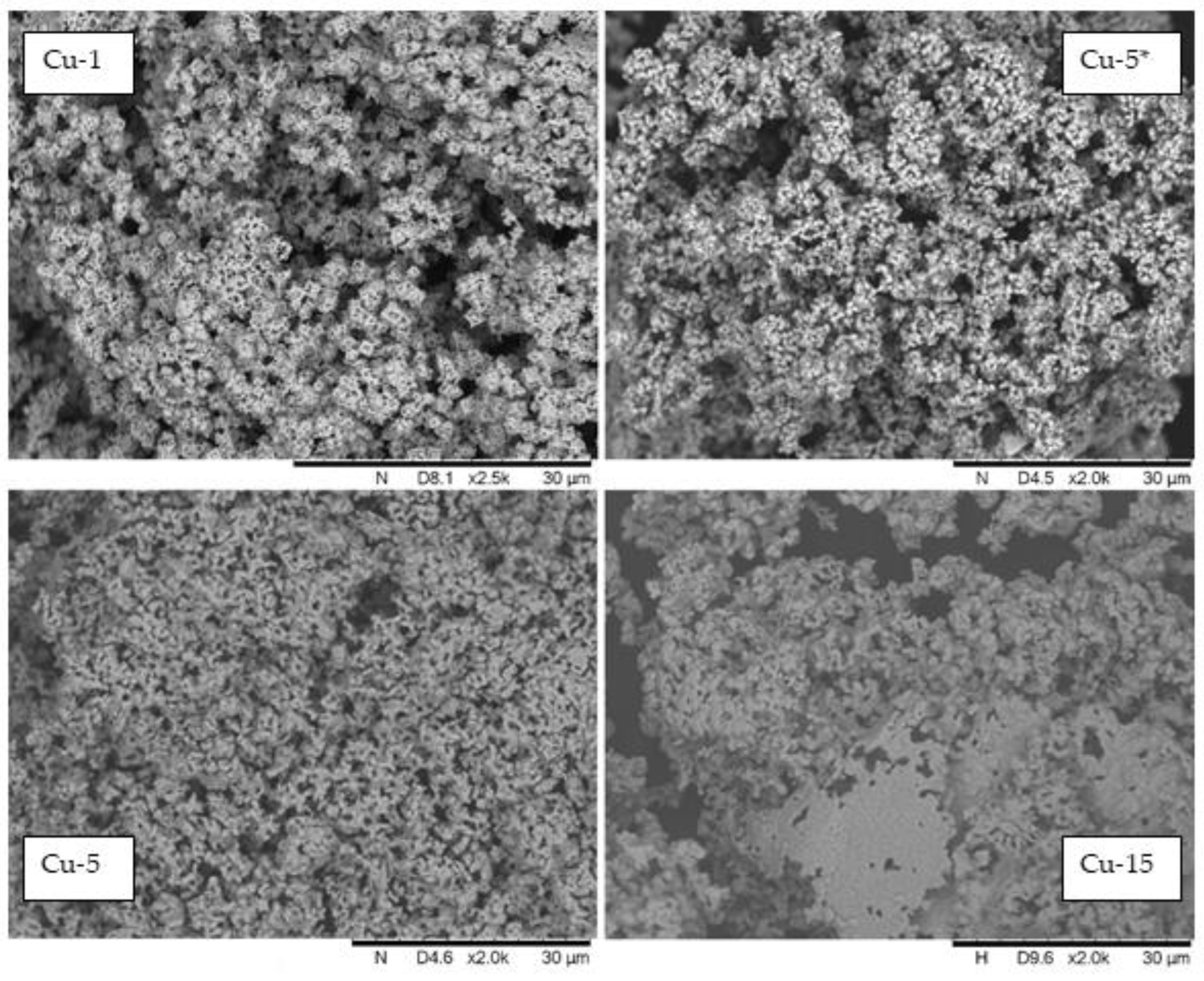
2.2. In Situ-Generated Catalyst Characterization
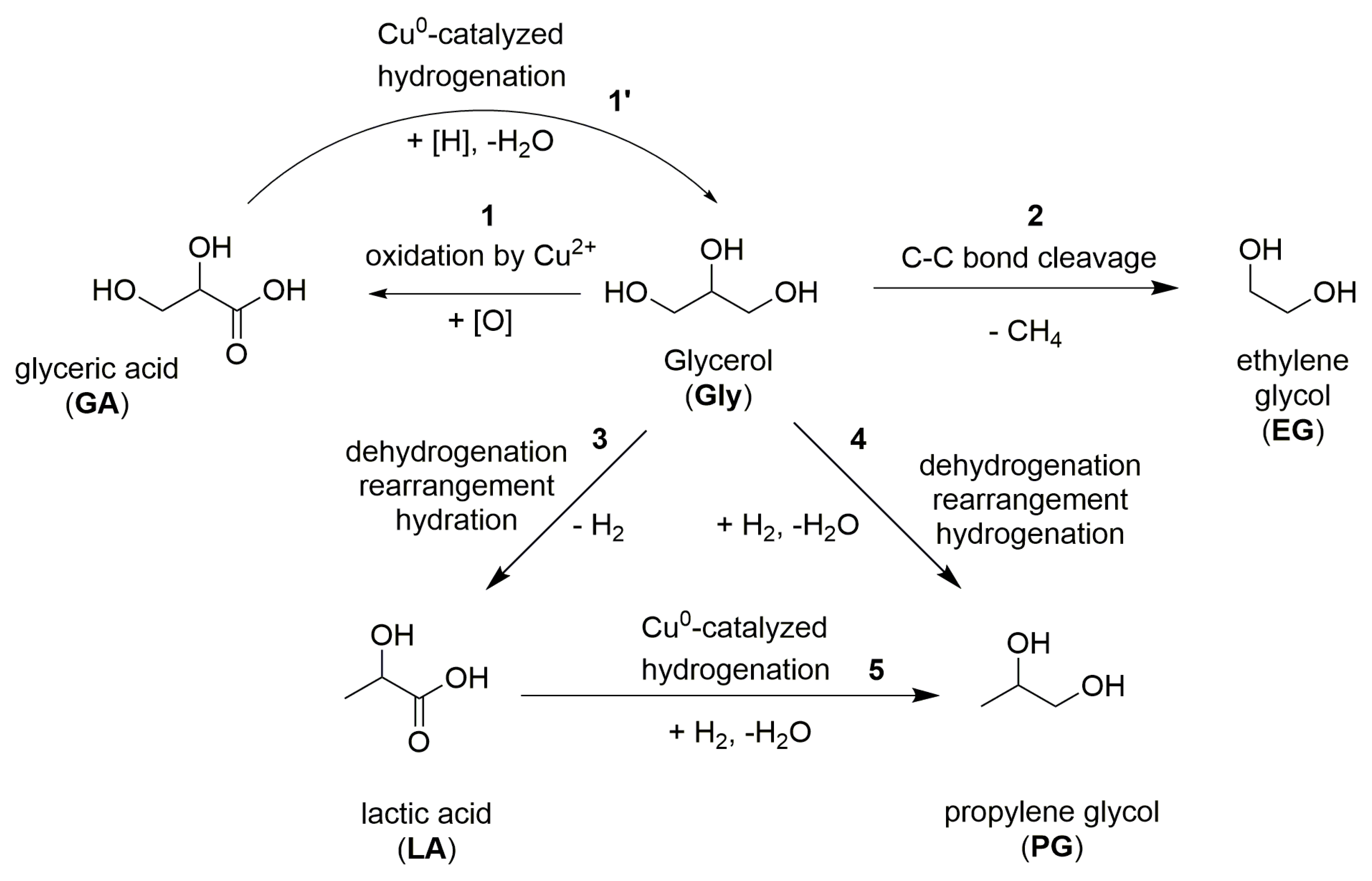
2.3. The Discussion Regarding the Reaction Routes and the Specific Activity
3. Materials and Methods
3.1. Materials
3.2. Catalytic Test
3.3. Analysis of Products
3.4. Silylation Protocol
3.5. Characterization of Copper Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sullivan, C.J.; Kuenz, A.; Vorlop, K.-D. Propanediols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co., Ltd.: Weinheim, Germany, 2018; pp. 1–15. [Google Scholar]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- BASF and Oleon Celebrate Grand Opening of Propylene Glycol Production Plant. Available online: https://www.chemeurope.com/en/news/138616/basf-and-oleon-celebrate-grand-opening-of-propylene-glycol-production-plant.html (accessed on 10 November 2022).
- Nanda, M.R.; Yuan, Z.; Shui, H.; Charles Xu, C. Selective Hydrogenolysis of Glycerol and Crude Glycerol (A by-Product Orwaste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 promoted Cu/Al2O3 catalysts. Catalysts 2017, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda, J.; Manuale, D.; Santiago, L.; Carrara, N.; Torres, G.; Vera, C.; Goncalves, M.; Carvalho, W.; Mandelli, D. Artigo. Selective hydrogenolysis of glycerol to propylene glycol in a continuous flow trickle bed reactor using copper chromite and Cu/Al2O3 catalysts. Quim. Nova 2018, 41, 926–932. [Google Scholar]
- Vasiliadou, E.S.; Lemonidou, A.A. Kinetic Study of Liquid-Phase Glycerol Hydrogenolysis over Cu/SiO2 Catalyst. Chem. Eng. J. 2013, 231, 103–112. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Dmitriev, G.S.; Zanaveskin, K.L.; Egorova, T.B.; Khadzhiev, S.N. Selective Hydrogenolysis of Glycerol to 1,2-Propylene Glycol on Ultrafine Copper Particles. Pet. Chem. 2017, 57, 1074–1080. [Google Scholar] [CrossRef]
- Dmitriev, G.S.; Khadzhiev, V.I.; Nikolaev, S.A.; Ezzhelenko, D.I.; Mel’chakov, I.S.; Zanaveskin, L.N. Copper-Containing Catalysts in the Liquid-Phase Hydrogenolysis of Glycerol. Pet. Chem. 2020, 60, 1066–1072. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Güemez, M.B.; Fierro, J.L.G. Hydrogenolysis of Glycerol to Propanediols over a Pt/ASA Catalyst: The Role of Acid and Metal Sites on Product Selectivity and the Reaction Mechanism. Appl. Catal. B Environ. 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.A.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol Hydrogenolysis on Heterogeneous Catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Feng, J.; Fu, H.; Wang, J.; Li, R.; Chen, H.; Li, X. Hydrogenolysis of Glycerol to Glycols over Ruthenium Catalysts: Effect of Support and Catalyst Reduction Temperature. Catal. Commun. 2008, 9, 1458–1464. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous Phase Reforming of Glycerol to 1,2-Propanediol over Pt-Nanoparticles Supported on Hydrotalcite in the Absence of Hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Shi, R.; Liu, Q.; Zhan, E.; Shen, W. Co/MgO Catalysts for Hydrogenolysis of Glycerol to 1, 2-Propanediol. Appl. Catal. A Gen. 2009, 371, 108–113. [Google Scholar] [CrossRef]
- Greish, A.A.; Finashina, E.D.; Tkachenko, O.P.; Kustov, L.M. Preparation of Propanols by Glycerol Hydrogenolysis over Bifunctional Nickel-Containing Catalysts. Molecules 2021, 26, 1565. [Google Scholar] [CrossRef] [PubMed]
- van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Green, M.J.; Friedrich, H.B. A Catalytic Route to Lower Alcohols from Glycerol Using Ni-Supported Catalysts. Green Chem. 2011, 13, 1819–1827. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective Hydrogenolysis of Glycerol to Propylene Glycol on Cu-ZnO Catalysts. Catal. Lett. 2007, 117, 62–67. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-Pressure Hydrogenolysis of Glycerol to Propylene Glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Zeng, T.; Hong, W.; Cheng, Z.; Yuan, W. Kinetics of Hydrogenolysis of Glycerol to Propylene Glycol over Cu-ZnO-Al2O3 Catalysts. Chin. J. Chem. Eng. 2010, 18, 384–390. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, J.; Mao, J.; Guo, X.; Zhang, S. Supported Cu Catalysts for the Selective Hydrogenolysis of Glycerol to Propanediols. Appl. Catal. A Gen. 2009, 367, 93–98. [Google Scholar] [CrossRef]
- Casale, B.; Gomez, A.M. A method of hydrogenation glycerol. Eur. Pat. Appl. 1992, 0523015A2. [Google Scholar]
- Kumar, P.; Shah, A.K.; Lee, J.H.; Park, Y.H.; Štangar, U.L. Selective Hydrogenolysis of Glycerol over Bifunctional Copper-Magnesium-Supported Catalysts for Propanediol Synthesis. Ind. Eng. Chem. Res. 2020, 59, 6506–6516. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous Catalysis of the Glycerol Hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Sai Prasad, P.S.; Prasad, R.B.N.; Lingaiah, N. Selective Hydrogenolysis of Glycerol to 1, 2 Propanediol over Cu-ZnO Catalysts. Catal. Lett. 2008, 126, 119–124. [Google Scholar] [CrossRef]
- Omar, L.; Perret, N.; Daniele, S. Self-Assembled Hybrid ZnO Nanostructures as Supports for Copper-Based Catalysts in the Hydrogenolysis of Glycerol. Catalysts 2021, 11, 516. [Google Scholar] [CrossRef]
- Dmitriev, G.S.; Melchakov, I.S.; Samoilov, V.O.; Ramazanov, D.N.; Zanaveskin, L.N. Synthesis of 1,2-Propylene Glycol in a Continuous Down-Flow Fixed-Bed Reactor With Cu/Al2O3 Catalyst. ChemistrySelect 2022, 7, e202104257. [Google Scholar] [CrossRef]
- Schmidt, S.R.; Tanielyan, S.K.; Marin, N.; Alvez, G.; Augustine, R.L. Selective Conversion of Glycerol to Propylene Glycol over Fixed Bed Raney® Cu Catalysts. In Topics in Catalysis; Springer: New York, NY, USA, 2010; Volume 53, pp. 1214–1216. [Google Scholar]
- Gao, Q.; Xu, B.; Tong, Q.; Fan, Y. Selective Hydrogenolysis of Raw Glycerol to 1,2-Propanediol over Cu-ZnO Catalysts in Fixed-Bed Reactor. Biosci. Biotechnol. Biochem. 2015, 80, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meher, L.C.; Gopinath, R.; Naik, S.N.; Dalai, A.K. Catalytic Hydrogenolysis of Glycerol to Propylene Glycol over Mixed Oxides Derived from a Hydrotalcite-Type Precursor. Ind. Eng. Chem. Res. 2009, 48, 1840–1846. [Google Scholar] [CrossRef]
- Shesterkina, A.; Vikanova, K.; Kostyukhin, E.; Strekalova, A.; Shuvalova, E.; Kapustin, G.; Salmi, T. Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds. Molecules 2022, 27, 988. [Google Scholar] [CrossRef]
- Bienholz, A.; Hofmann, H.; Claus, P. Selective Hydrogenolysis of Glycerol over Copper Catalysts Both in Liquid and Vapour Phase: Correlation between the Copper Surface Area and the Catalyst’s Activity. Appl. Catal. A Gen. 2011, 391, 153–157. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gómez, M. Metal-Based Nanoparticles Dispersed in Glycerol: An Efficient Approach for Catalysis. Catal. Today 2018, 310, 98–106. [Google Scholar] [CrossRef]
- Carroll, K.J.; Reveles, J.U.; Shultz, M.D.; Khanna, S.N.; Carpenter, E.E. Preparation of Elemental Cu and Ni Nanoparticles by the Polyol Method: An Experimental and Theoretical Approach. J. Phys. Chem. C 2011, 115, 2656–2664. [Google Scholar] [CrossRef]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and Size Control of Monodisperse Copper Nanoparticles by Polyol Method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- Sun, J.; Jing, Y.; Jia, Y.; Tillard, M.; Belin, C. Mechanism of Preparing Ultrafine Copper Powder by Polyol Process. Mater. Lett. 2005, 59, 3933–3936. [Google Scholar] [CrossRef]
- Chokratanasombat, P.; Nisaratanaporn, E. Preparation of Ultrafine Copper Powders with Controllable Size via Polyol Process with Sodium Hydroxide Addition. Eng. J. 2012, 16, 39–46. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The Polyol Process: A Unique Method for Easy Access to Metal Nanoparticles with Tailored Sizes, Shapes and Compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zeng, P.; He, M.; He, X.; Xie, X. Morphological Effect of Non-Supported Copper Nanocrystals on Furfural Hydrogenation. Catal. Commun. 2016, 86, 5–8. [Google Scholar] [CrossRef]
- Copper X-ray Photoelectron Spectra, Copper Electron Configuration, and Other Elemental Information. Available online: https://www.thermofisher.com/ru/ru/home/materials-science/learning-center/periodic-table/transition-metal/copper.html (accessed on 1 November 2022).
- Montassier, C.; Ménézo, J.C.; Hoang, L.C.; Renaud, C.; Barbier, J. Aqueous Polyol Conversions on Ruthenium and on Sulfur-Modified Ruthenium. J. Mol. Catal. 1991, 70, 99–110. [Google Scholar] [CrossRef]
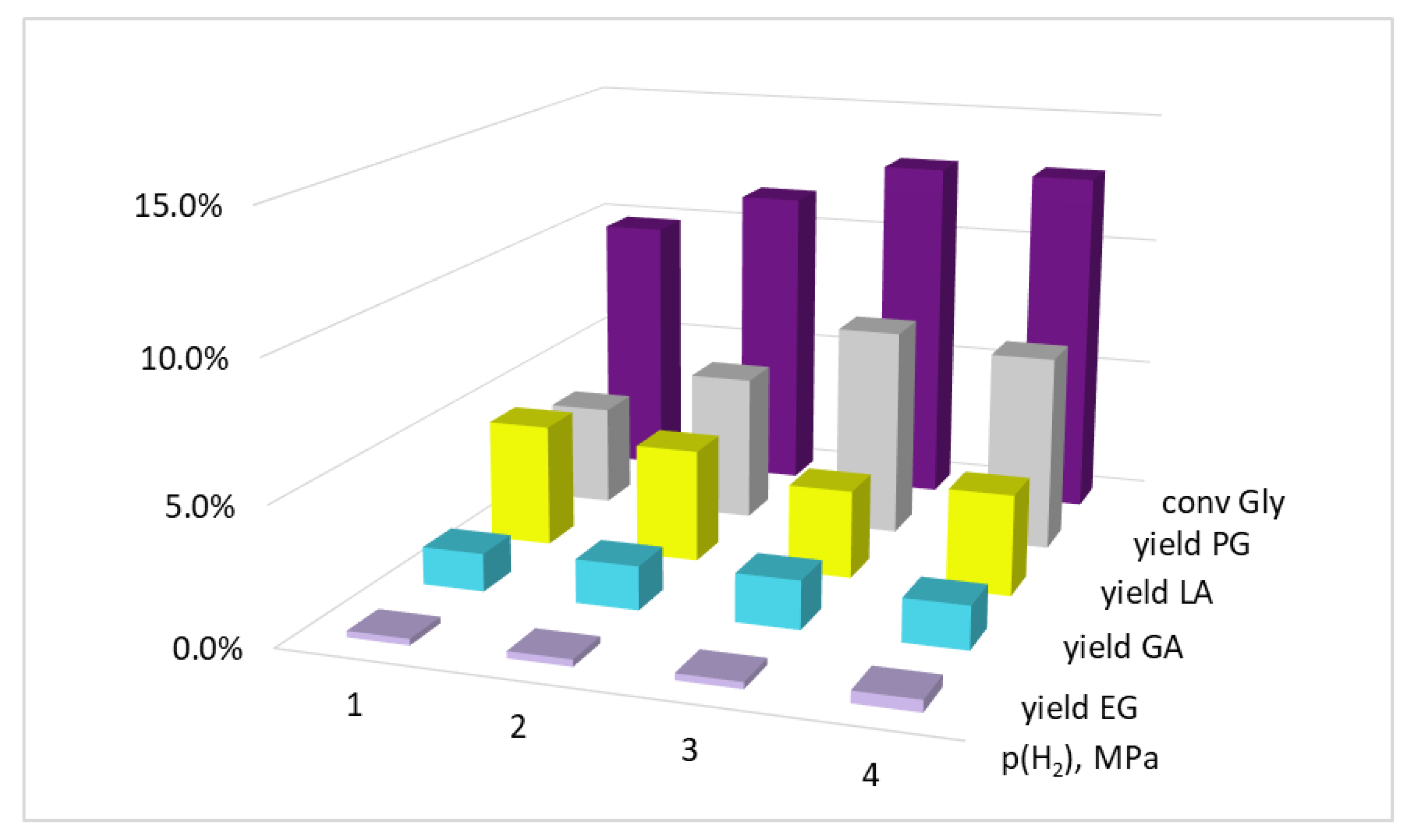

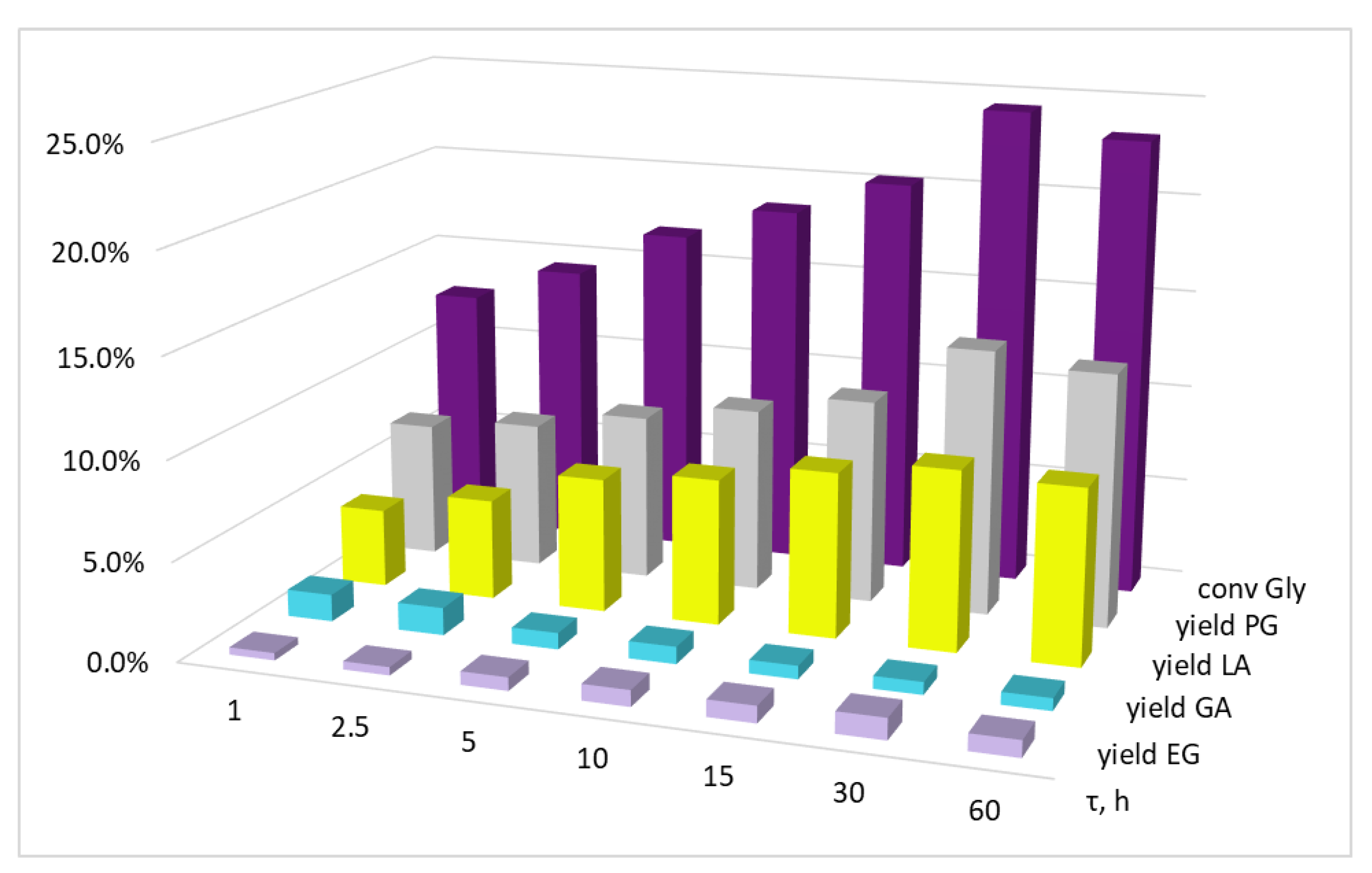

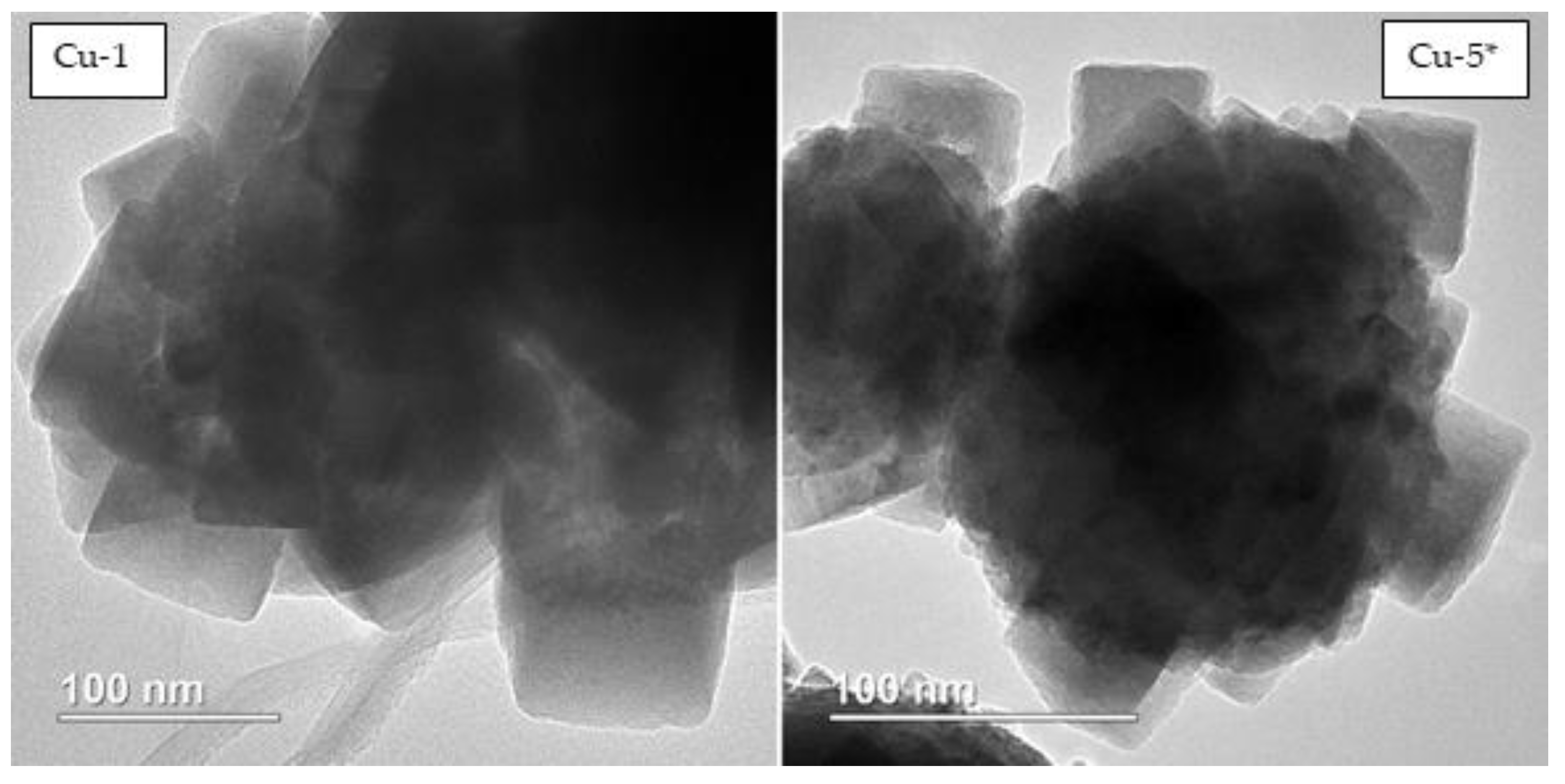
| Entry | Precursor Salt | T, °C | Gly/Cu, mol | τ, h | XGly, % | YEG, % | YPG, % | YLA, % | YGA, % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 220 | - | 5 | 2.2 | 0.1 | 0.2 | 2.0 | - |
| 2 | CuSO4·5H2O | 200 | 50 | 5 | 15.0 | 0.4 | 7.0 | 7.0 | 0.6 |
| 3 | 10 | 15.2 | 0.5 | 8.9 | 5.0 | 0.8 | |||
| 4 | 100 | 5 | 7.0 | 0.2 | 4.0 | 2.5 | 0.3 | ||
| 5 | 10 | 8.1 | 0.4 | 4.7 | 2.9 | 0.1 | |||
| 6 | 220 | 50 | 5 | 16.6 | 0.8 | 10.4 | 5.1 | 0.3 | |
| 7 | 10 | 18.4 | 0.9 | 10.7 | 6.8 | - | |||
| 8 | 100 | 5 | 8.5 | 0.4 | 4.7 | 3.3 | 0.1 | ||
| 9 | 10 | 8.5 | 0.3 | 4.8 | 3.4 | - | |||
| 10 | Cu(OAc)2·H2O | 200 | 50 | 5 | 14.8 | 0.3 | 8.9 | 3.7 | 1.9 |
| 11 | 10 | 15.5 | 0.6 | 8.0 | 5.7 | 1.2 | |||
| 12 | 100 | 5 | 7.0 | 0.1 | 3.6 | 2.6 | 0.7 | ||
| 13 | 10 | 7.1 | 0.2 | 3.7 | 2.5 | 0.7 | |||
| 14 | 220 | 50 | 5 | 16.8 | 0.7 | 8.5 | 6.8 | 0.8 | |
| 15 | 10 | 18.5 | 0.8 | 9.4 | 7.4 | 0.9 | |||
| 16 | 100 | 5 | 7.8 | 0.2 | 4.0 | 3.2 | 0.4 | ||
| 17 | 10 | 10.6 | 0.5 | 5.3 | 4.2 | 0.6 | |||
| 18 | Nitrogen | 200 | 100 | 5 | 4.6 | 0.1 | 0.7 | 3.1 | 0.7 |
| 19 | 220 | 5 | 4.4 | 0.1 | 0.7 | 2.8 | 0.8 | ||
| 20 | 10 | 4.8 | 0.1 | 0.8 | 3.3 | 0.6 | |||
| 21 | Cu-Cr2O3 1 | 200 | 50 | 5 | 7.4 | 0.1 | 6.0 | 1.3 | - |
| 22 | 10 | 10.6 | 0.1 | 10.1 | 0.4 | - | |||
| 23 | 100 | 5 | 3.6 | - | 3.6 | - | - | ||
| 24 | 10 | 9.3 | 0.3 | 8.3 | 0.7 | - | |||
| 25 | 220 | 50 | 5 | 19.0 | 0.2 | 17.3 | 1.5 | - | |
| 26 | 10 | 26.1 | 0.2 | 25.4 | 0.5 | - | |||
| 27 | 100 | 5 | 11.9 | 0.1 | 8.6 | 3.1 | - | ||
| 28 | 10 | 19.4 | 0.1 | 19.0 | 0.4 | - | |||
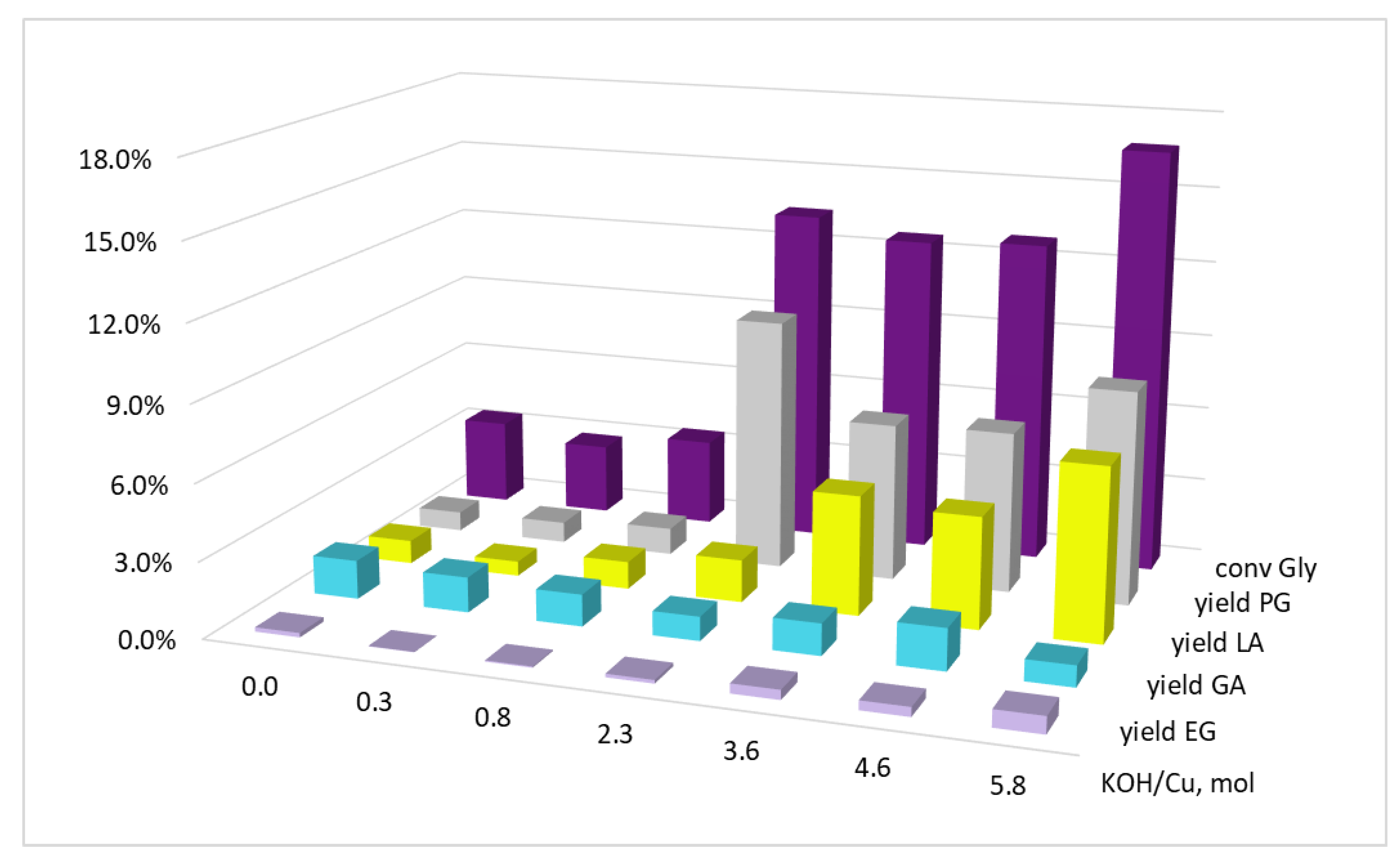
| 29 | Cu-Cr2O3 + KOH | 220 | 50 | 5 | 34.5 | 1.5 | 23.6 | 9.4 | - |
| 30 | CuCl2·2H2O | 200 | 50 | 5 | 11.0 | 0.3 | 5.8 | 4.3 | 0.6 |
| 31 | 220 | 50 | 5 | 16.2 | 0.5 | 6.7 | 8.3 | 0.7 |
| Reaction Conditions | ωCu2+ at the Beginning of the Reaction, % | ∑Cu after Hydrogenolysis, 10−4% |
|---|---|---|
| τ = 5 h, Without alkali | 2.93 | 11 |
| τ = 5 h, nKOH/nCu = 5.8 | 1 | |
| τ = 1 h, nKOH/nCu = 5.8 | 7 |
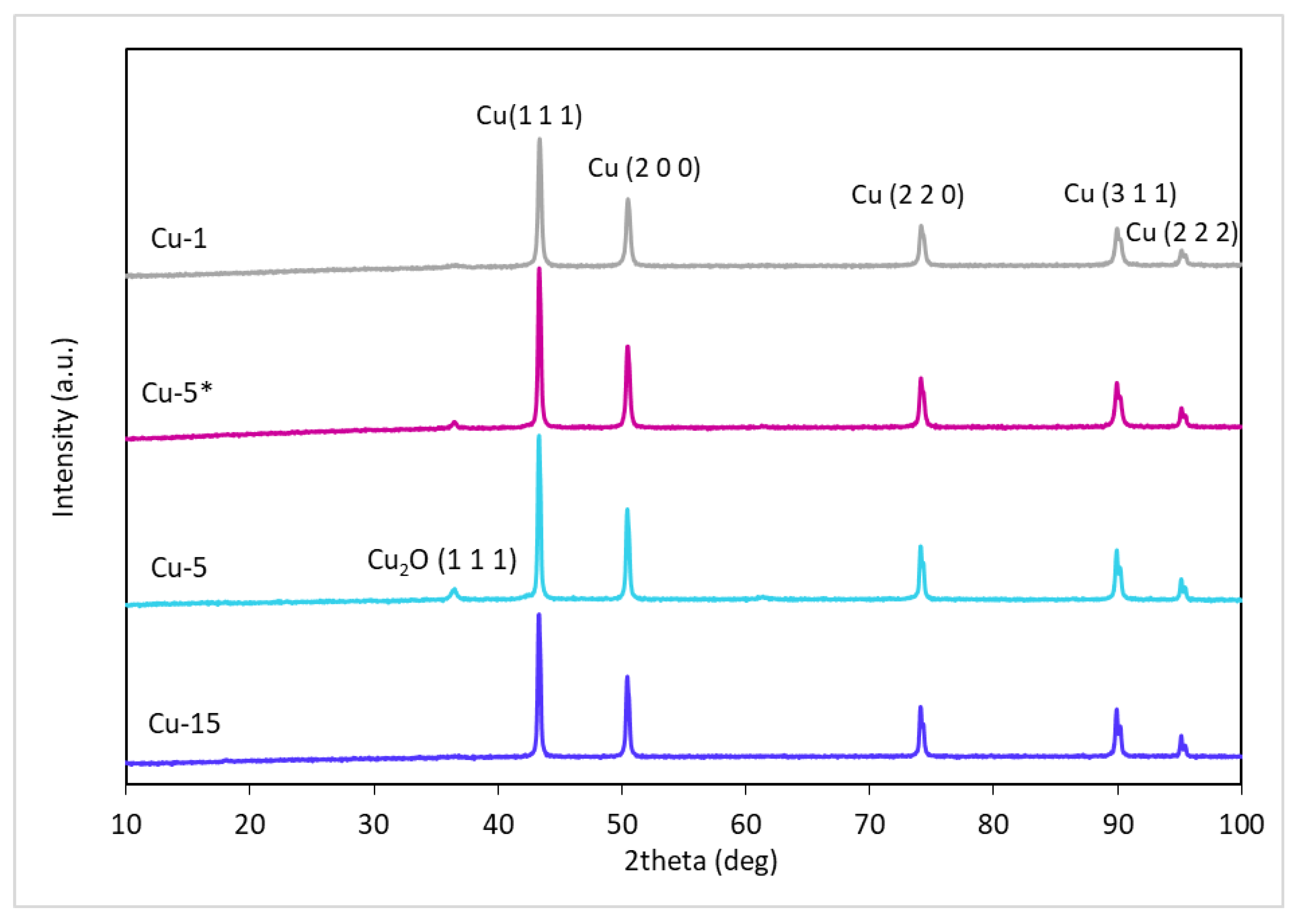
| Catalyst | Phase Composition | Average Crystallite Size, nm |
|---|---|---|
| Cu-1 | Cu | 35 |
| Cu-5 * | 42 | |
| Cu-5 | 44 | |
| Cu-15 | 49 |
| Precursor Salt | SPGly, mmol Gly gcatalyst−1 h−1 | SPPG, mmol PG gcatalyst−1 h−1 |
|---|---|---|
| CuSO4·5H2O | 23.6 | 10.7 |
| Cu(OAc)2·H2O | 18.6 | 9.4 |
| Cu-Cr2O3 1 | 6.5 | 5.3 |
| CuCl2 | 17.3 | 9.1 |
| Precursor Salt | τ, h | SPGly, mmol Gly gcatalyst−1 h−1 | SPPG, mmol PG gcatalyst−1 h−1 |
|---|---|---|---|
| Cu-Cr2O3 | 5 | 16.7 | 15.2 |
| Cu(OAc)2 | 1 | 22.8 | 15.0 |
| 2.5 | 25.2 | 19.2 | |
| 5 | 21.1 | 15.7 |
| Precursor Salt | τ, h | SPGly, mmol Gly gcatalyst−1 h−1 | SPPG, mmol PG gcatalyst−1 h−1 |
|---|---|---|---|
| CuSO4·5H2O | 5 | 26.1 | 16.4 |
| Cu-Cr2O3 | 5 | 30.4 | 20.8 |
| Cu(OAc)2·H2O | 1 | 99.2 | 54.3 |
| 2.5 | 45.4 | 23.6 | |
| 5 | 26.6 | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porukova, I.; Samoilov, V.; Ramazanov, D.; Kniazeva, M.; Maximov, A. In Situ-Generated, Dispersed Cu Catalysts for the Catalytic Hydrogenolysis of Glycerol. Molecules 2022, 27, 8778. https://doi.org/10.3390/molecules27248778
Porukova I, Samoilov V, Ramazanov D, Kniazeva M, Maximov A. In Situ-Generated, Dispersed Cu Catalysts for the Catalytic Hydrogenolysis of Glycerol. Molecules. 2022; 27(24):8778. https://doi.org/10.3390/molecules27248778
Chicago/Turabian StylePorukova, Iuliana, Vadim Samoilov, Dzhamalutdin Ramazanov, Mariia Kniazeva, and Anton Maximov. 2022. "In Situ-Generated, Dispersed Cu Catalysts for the Catalytic Hydrogenolysis of Glycerol" Molecules 27, no. 24: 8778. https://doi.org/10.3390/molecules27248778
APA StylePorukova, I., Samoilov, V., Ramazanov, D., Kniazeva, M., & Maximov, A. (2022). In Situ-Generated, Dispersed Cu Catalysts for the Catalytic Hydrogenolysis of Glycerol. Molecules, 27(24), 8778. https://doi.org/10.3390/molecules27248778