Sprouts of Moringa oleifera Lam.: Germination, Polyphenol Content and Antioxidant Activity
Abstract
1. Introduction
2. Results
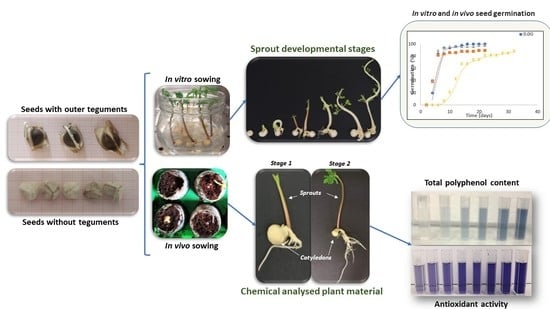
2.1. Moringa Seed Germination
2.1.1. In Vivo Seed Sowing
2.1.2. In Vitro Seed Germination
2.1.3. Effect of Culture Medium Composition on In Vitro Moringa Seed Germination
2.2. Chemical Characterization of Moringa sprouts
2.2.1. Effect of Developmental Stage and Type of Sowing Substrate on the Total Polyphenol Content and Antioxidant Activity of Moringa sprout Extracts
2.2.2. Effect of Developmental Stage and Type of Sowing Substrate on the Total Polyphenol Content and Antioxidant Activity of Moringa Cotyledon Extracts
2.2.3. Effect of Developmental Stage and Culture Medium Composition on Total Polyphenol Content and Antioxidant Activity of Moringa sprout Extracts
2.2.4. Effect of Developmental Stage and Culture Medium Composition on the Polyphenol Content and Antioxidant Activity of Moringa cotyledon Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Moringa Seed Germination
4.2.1. In Vivo Seed Sowing
4.2.2. In Vitro Establishment of Culture
4.2.3. Effect of Culture Medium Composition on In Vitro Moringa Seed Germination
4.2.4. Analysis of Data
4.3. Chemical Characterization of Moringa sprouts and Cotyledons
4.3.1. Plant Material
4.3.2. Chemical Materials
4.3.3. Extraction of Antioxidant Components
4.3.4. Determination of Total Polyphenolic Content
4.3.5. Evaluation of Antioxidant Activity
- DPPH radical scavenging activity test
- ABTS radical scavenging activity test
- Ferric ion reducing power (FRAP)
4.4. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Makkar, H.P.S.; Becker, K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. Cambr. 1997, 128, 311–332. [Google Scholar] [CrossRef]
- Nouman, W.; Siddiqui, M.T.; Basra, S.M.A.; Afzal, I.; Rehman, H. Enhancement of emergence potential and stand establishment of Moringa oleifera Lam. by seed priming. Turk. J. Agric. For. 2012, 36, 227–235. [Google Scholar] [CrossRef]
- Olson, M.E.; Carlquist, S. Stem and root anatomical correlations with life form diversity, ecology, and systematics in Moringa (Moringaceae). Bot. J. Linn. Soc. 2001, 135, 315–348. [Google Scholar] [CrossRef]
- Morton, J.F. The horseradish tree, Moringa pterygosperma (Moringaceae)—A boon to arid lands? Econ. Bot. 1991, 45, 318–333. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M.I. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Ashraf, M.; Bhanger, M.I. Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J. Am. Oil Chem. Soc. 2005, 82, 45–51. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- D’souza, J.; Kulkarni, A.R. Comparative studies on nutritive values of tender foliage of seedlings and mature plants of Moringa oleifera Lam. J. Econ. Taxon Bot. 1993, 17, 479–485. [Google Scholar]
- Fuglie, L.J. The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics; Church World Service: Dakar, Senegal, 1999. [Google Scholar]
- Berger, M.R.; Habs, M.; John, S.A.A.; Schmahi, D. Toxicological assessment of seeds from Moringa oleifera and M. stenopetala two efficient primary coagulants for domestic water treatment of tropical waters. East Afr. Med. J. 1984, 61, 712–716. [Google Scholar]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health: A review. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Förster, N.; Mewis, I.; Ulrichs, C. Moringa oleifera—Establishment and multiplication of different ecotypes in vitro. Gesunde Pflanz. 2013, 65, 21–31. [Google Scholar] [CrossRef]
- Luqman, S.; Srivastava, A.; Kumar, R.; Kumar, A.; Chanda, D. Experimental assessment of i leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays Evid. Evid. Based Complement. Altern. Med. 2012, 2012, 519084. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Cheenpracha, S.; Chang, C.L.; Kondratyuk, P.T.; Pezzuto, M.J. Inhibition of lipopolysaccharide-induced cyclooxygenase2 expression and inducible nitric oxide synthase by 4-[(2′ -oacetyl-α-Lrhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera. Nutr. Cancer 2011, 63, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). J. Agric. Food Chem. 2003, 15, 2144–2155. [Google Scholar] [CrossRef]
- Estrella, M.C.P.; Mantaring, J.B.V.; David, G.Z. A double blind, randomised controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breastmilk among non-nursing mothers of preterm infants. Philipp. J. Pediatr. 2000, 49, 3–6. [Google Scholar]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Murakami, T.; Ise, K.; Hayakawa, M.; Kamei, S.; Takagi, S.I. Stabilities of metal complexes of mugineic acids and their specific affinities for iron (III). Chem. Lett. 1989, 18, 2137–2140. [Google Scholar] [CrossRef]
- Pari, L.; Kumar, N.A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J. Med. Food. 2002, 5, 171–177. [Google Scholar] [CrossRef]
- Coello, K.E.; Frias, J.; Martínez-Villaluenga, C.; Cartea, M.E.; Abilleira, R.; Peñas, E. Potential of germination in selected conditions to improve the nutritional and bioactive properties of Moringa (Moringa oleifera L.). Foods 2020, 9, 1639. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Adeoti, O.A.; Ariyo, O. Comparative study on nutrient composition, phytochemical, and functional characteristics of raw, germinated, and fermented Moringa oleifera seed flour. Food Sci. Nutr. 2013, 1, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Jahn, S.A.; Musnad, H.A.; Burgstaller, H. The tree that purifies water: Cultivating multipurpose Moringaceae in the Sudan. Unasylva 1986, 38, 23–28. [Google Scholar]
- Sharma, G.K.; Raina, V. Propagation techniques of Moringa oleifera Lam. In Improvement of Forest Biomass, Proceedings of a Symposium; Khosla, P.K., Ed.; Indian Society of Tree Scientists (ISTS): Solan, India, 1982; pp. 175–181. [Google Scholar]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.M. In vitro propagation of Moringa oleifera L. under salinity and ventilation conditions. Genet. Plant Physiol. 2016, 6, 54–64. [Google Scholar]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutical active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Maheshwar, P.S.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tiss. Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.; Cui, G.; Mao, Y.; He, X. Effect of Gibberellins and Its Synthetic Inhibitor on Metabolism of Tanshinones. Chin. J. Exp. Tradit. Med. Formulae 2008, 14, 1–3. [Google Scholar]
- Liang, Z.; Ma, Y.; Xu, T.; Cui, B.; Liu, Y.; Guo, Z.; Dongfeng, Y. Effects of Abscisic Acid, Gibberellin, Ethylene and Their Interactions on Production of Phenolic Acids in Salvia miltiorrhiza Bunge Hairy Roots. PLoS ONE 2013, 8, e72806. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef]
- Bais, H.P.; Gokare, S.G.; Ravishankar, G.A. Enhancement of growth and coumarin production in hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow local) under the influence of fungal elicitors. J. Biosci. Bioeng. 2000, 90, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.; Sudha, G.; George, J.; Ravishankar, G.A. Influence of exogenous hormones on growth and secondary metabolite production in hairy root cultures of Cichorium intybus L. CV. Lucknow local. Vitr. Cell Dev. Biol-Plant 2001, 37, 293–299. [Google Scholar] [CrossRef]
- Smith, T.C.; Weathers, P.J.; Cheetham, R.D. Effects of Gibberellic Acid on Hairy Root Cultures of Artemisia annua: Growth and Artemisinin Production. Vitr. Cell. Dev. Biol. Plant 1997, 33, 75–79. [Google Scholar] [CrossRef]
- De Vogel, E.F. Seedlings of Dicotyledons; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1980. [Google Scholar]
- Boukandoul, S.; Casal, S.; Zaidi, F. The potential of some moringa species for seed oil production. Agriculture 2018, 8, 150. [Google Scholar] [CrossRef]
- Steinitz, B.; Tabib, Y.; Gaba, V.; Gefen, T.; Vaknin, Y. Vegetative micro-cloning to sustain biodiversity of threatened Moringa species. Vitr. Cell. Dev. Biol. Plant 2009, 45, 65. [Google Scholar] [CrossRef]
- Amabye, T.G.; Tadesse, F.M. Phytochemical and antibacterial activity of Moringa oleifera available in the market of Mekelle. J. Anal. Pharm. Res. 2016, 2, 00011. [Google Scholar] [CrossRef]
- Ma, Z.F.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Radovich, T. Farm and Forestry Production and Marketing Profile for Moringa (Moringa oleifera). Speciality Crops for Pacific Island Agroforestry. 2012. Available online: http://agroforestry.net/scps (accessed on 15 February 2016).
- Bezerra, A.M.E.; Filho, S.M.; Freitas, J.B.S.; Teófilo, E.M. Evaluation of quality of the drumstick seeds during the storage. Ciencia e Agrotecnologia 2004, 28, 1240–1246. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Ribeiro, M.C.C.; Maracaja, P.B.; Carvalho, G.S. Qualidade fisiologica de sementes de Moringa emfunção do tipo de embalagem, ambiente e tempo de armazenamento 1 (In Portuguese with English Abstract). Rev. Caatinga 2009, 22, 70–75. [Google Scholar]
- Fotouo, M.H.; du Toit, E.S.; Robbertse, P.J. Germination and ultrastructural studies of seeds produced by a fast-growing, drought-resistant tree: Implications for its domestication and seed storage. AoB Plants 2015, 7, plv016. [Google Scholar] [CrossRef] [PubMed]
- Madinur, N.I. Seed Viability in Drumstick (Moringa oleifera Lamk.). Master Thesis, University of Agricultural Sciences, Dwarwad, India, 2007. [Google Scholar]
- Stephenson, K.K.; Fahey, J.W. Development of tissue culture methods for the rescue and propagation of endangered Moringa spp. germplasm. Econ. Bot. 2004, 58, S116–S124. [Google Scholar] [CrossRef]
- Mathur, M.; Yadav, S.; Katariya, P.K.; Kamal, R. In vitro propagation and biosynthesis of steroidal sapogenins from various morphogenetic stages of Moringa oleifera Lam., and their antioxidant potential. Acta Physiol. Plant. 2014, 36, 1749–1762. [Google Scholar] [CrossRef]
- Afolabi, J.O.; Olomola, D.B.; Osunlaja, O.A.; Oloyede, E.O.; Bolanle-Ojo, I.O. Effect of different media on seed germination and in vitro propagation of Moringa oleifera L. J. For. Res. 2018, 15, 13–21. [Google Scholar] [CrossRef]
- Materechera, S.A. Influence of pre-sowing seed treatments on the germination of Moringa (Moringa oleifera Lam.). Acta Hortic. 2017, 1158, 149–158. [Google Scholar] [CrossRef]
- Muhl, Q.E.; Du Toit, E.S.; Robbertse, P.J. Temperature effect on seed germination and seedling growth of Moringa oleifera Lam. Seed Sci. Technol. 2011, 39, 208–213. [Google Scholar] [CrossRef]
- Katoriya, R.S.; Kureel, M.K.; Mandloi, D.S.; Suryawanshi, K.D.; Lekhi, R.; Rathore, S.S. Seed germination of drumstick Cv. PKM-1 as effected by different concentrations of Gibberellic acid and soaking time. Int. J. Chem. Stud. 2019, 7, 739–744. [Google Scholar]
- Gupta, S.; Jain, R.; Kachhwaha, S.; Kothari, S. Nutritional and medicinal applications of Moringa oleifera Lam.—Review of current status and future possibilities. J. Herb. Med. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Jaja-Chimedz, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.R.; Ribeiro de Souza, R.; Coimbra, M.; Nery, F.; Alvarenga, A.; Paiva, R. Light quality on growth and phenolic compounds accumulation in Moringa oleifera L. grown in vitro. Comun. Sci. 2020, 11, e3313. [Google Scholar] [CrossRef]
- Gómez-Martínez, M.; Ascacio-Valdés, J.A.; Flores-Gallegos, A.C.; González-Domínguez, J.; Gómez-Martínez, S.; Aguilar, C.N.; Morlett-Chávez, J.A.; Rodríguez-Herrera, R. Location and tissue effects on phytochemical composition and in vitro antioxidant activity of Moringa oleifera. Ind. Crops Prod. 2020, 151, 112439. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Santos, A.S.F.; Agrolo, A.C.C.; Paiva, P.M.G.; Coelho, L.C.B.B. Antioxidant activity of Moringa oleifera tissue extracts. Phytother. Res. 2012, 26, 1366–1370. [Google Scholar] [CrossRef]
- De-la-Cruz Chacón, I.; Riley-Saldaña, C.A.; González-Esquinca, A.R. Secondary metabolites during early development in plants. Phytochem. Rev. 2013, 12, 47–64. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Biondi, S.; Lenzi, C.; Baraldi, R.; Bagni, N. Hormonal Effects on growth and morphology of normal and hairy roots of Hyoscyamus muticus. J. Plant Growth Regul. 1997, 16, 159–167. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Schnitzler, W.H.; Nitz, G.; Razin, A.M.; El-Oksh, I.I. The effect of gibberellic acid (GA3) on some phenolic substances inglobe artichoke (Cynara cardunculus var. scolymus (L.) Fiori). Sci. Hortic. 2007, 111, 326–329. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym. Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Banyai, W.; Mii, M.; Supaibulwatana, K. Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA3 treatment. Plant Growth Regul. 2011, 63, 45–54. [Google Scholar] [CrossRef]
- Radić, S.; Vujčić, V.; Glogoški, M.; Radić-Stojković, M. Influence of pH and plant growth regulators on secondary metabolite production and antioxidant activity of Stevia rebaudiana (Bert). Period. Biol. 2016, 118, 9–19. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Thidiazuron-induced changes in biomass parameters, total phenolic content, and antioxidant activity in callus cultures of Artemisia absinthium L. Appl. Biochem. Biotechnol. 2014, 172, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kader, M. Varying temperature regimes affect osmotically primed sorghum seeds and seedlings. Int. Sorghum Millets Newsl. 2005, 42, 39. [Google Scholar]
- Czabator, F.J. Germination value: An index combining speed and completeness of pine seed germination. For. Sci. 1962, 8, 386–396. [Google Scholar]
- Salehzade, H.; Shishvan, M.I.; Ghiyasi, M.; Forouzin, F.; Siyahjani, A.A. Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). Res. J. Biol. Sci. 2009, 4, 629–631. [Google Scholar]
- Sharma, P.; Wichaphon, J.; Klangpetch, W. Antimicrobial and antioxidant activities of defatted Moringa oleifera seed meal extract obtained by ultrasound-assisted extraction and application as a natural antimicrobial coating for raw chicken sausages. Int. J. Food Microbiol. 2020, 332, 108770. [Google Scholar] [CrossRef]






| Developmental Stage | Growing Substrate | TPC | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/g | ±SD | mM TEAC | ±SD | mM TEAC | ±SD | mM TEAC | ±SD | ||
| S1 | Agarized medium | 115.68 | ±6.41 | 190.90 | ±12.47 | 185.99 | ±5.76 | 134.56 | ±14.49 |
| Jiffy® | 58.29 | ±0.66 | 48.37 | ±16.35 | 136.32 | ±11.87 | 7.72 | ±2.02 | |
| S2 | Agarized medium | 123.73 | ±16.43 | 148.89 | ±29.75 | 184.28 | ±23.95 | 72.47 | ±0.51 |
| Jiffy® | 94.41 | ±17.37 | 114.85 | ±12.32 | 109.30 | ±5.98 | 78.25 | ±0.19 | |
| Statistical analysis | p | p | p | p | |||||
| Developmental Stage (DS) | 0.065 | 0.416 | 0.220 | 0.023 | |||||
| Growing Substrate (GS) | 0.008 | 0.003 | 0.003 | 0.000 | |||||
| DS × GS | 0.184 | 0.016 | 0.270 | 0.000 | |||||
| Developmental Stage | Growing Substrate | TPC | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/g | ±SD | mM TEAC | ±SD | mM TEAC | ±SD | mM TEAC | ±SD | ||
| S1 | Agarized medium | 3.27 | ±0.21 | 25.92 | ±2.22 | 25.95 | ±6.58 | 11.61 | ±0.25 |
| Jiffy® | 7.91 | ±0.33 | 7.02 | ±1.60 | 41.28 | ±2.27 | 2.22 | ±0.78 | |
| S2 | Agarized medium | 5.32 | ±0.43 | 23.52 | ±0.82 | 38.06 | ±5.84 | 8.96 | ±0.18 |
| Jiffy® | 12.04 | ±0.01 | 36.12 | ±1.55 | 41.48 | ±2.90 | 14.36 | ±0.36 | |
| Statistical analysis | p | p | p | p | |||||
| Developmental Stage (DS) | 0.065 | 0.416 | 0.220 | 0.023 | |||||
| Growing Substrate (GS) | 0.008 | 0.003 | 0.003 | 0.000 | |||||
| DS × GS | 0.184 | 0.016 | 0.270 | 0.000 | |||||
| Developmental Stage | Culture Medium Composition | TPC | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/g | ±DS | mM TEAC | ±DS | mM TEAC | ±DS | mM TEAC | ±DS | ||
| S1 | 0.0 G | 115.68 | ±6.41 | 190.90 | ±12.47 | 185.99 | ±5.76 | 134.56 | ±2.61 |
| 0.5 G | 71.69 | ±2.03 | 113.19 | ±27.66 | 206.48 | ±3.62 | 63.39 | ±20.27 | |
| 1.0 G | 72.28 | ±0.85 | 124.63 | ±7.31 | 224.98 | ±9.26 | 75.76 | ±2.79 | |
| S2 | 0.0 G | 123.73 | ±16.43 | 148.89 | ±29.75 | 184.28 | ±23.95 | 72.47 | ±0.51 |
| 0.5 G | 226.30 | ±78.60 | 320.19 | ±34.55 | 392.68 | ±5.08 | 305.53 | ±12.68 | |
| 1.0 G | 143.30 | ±45.37 | 275.30 | ±22.35 | 352.18 | ±6.61 | 255.86 | ±6.62 | |
| Statistical analysis | p | p | p | p | |||||
| Developmental Stage (DS) | 0.012 | 0.000 | 0.000 | 0.000 | |||||
| Culture Medium Composition (CMC) | 0.349 | 0.086 | 0.000 | 0.000 | |||||
| DS × CMC | 0.086 | 0.001 | 0.000 | 0.000 | |||||
| Developmental Stage | Culture Medium Composition | TPC | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg GAE/g | ±DS | mM TEAC | ±DS | mM TEAC | ±DS | mM TEAC | ±DS | ||
| S1 | 0.0 G | 3.27 | ±0.21 | 25.92 | ±2.22 | 25.95 | ±6.58 | 11.61 | ±0.25 |
| 0.5 G | 2.32 | ±0.04 | 4.56 | ±0.00 | 28.02 | ±3.48 | <LOQ | <LOQ | |
| 1.0 G | 3.30 | ±0.40 | 4.95 | ±1.39 | 25.17 | ±5.21 | <LOQ | <LOQ | |
| S2 | 0.0 G | 5.32 | ±0.43 | 23.52 | ±0.82 | 38.06 | ±5.84 | 8.96 | ±0.18 |
| 0.5 G | 8.28 | ±0.44 | 24.19 | ±3.76 | 40.92 | ±3.06 | 9.22 | ±0.18 | |
| 1.0 G | 7.77 | ±0.62 | 27.32 | ±1.10 | 34.36 | ±4.71 | 13.98 | ±0.26 | |
| Statistical analysis | p | p | p | p | |||||
| Developmental Stage (DS) | 0.000 | 0.000 | 0.003 | 0.000 | |||||
| Culture Medium Composition (CMC) | 0.002 | 0.000 | 0.436 | 0.000 | |||||
| DS × CMC | 0.000 | 0.000 | 0.853 | 0.000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirlini, M.; Del Vecchio, L.; Leto, L.; Russo, F.; Dellafiora, L.; Guarrasi, V.; Chiancone, B. Sprouts of Moringa oleifera Lam.: Germination, Polyphenol Content and Antioxidant Activity. Molecules 2022, 27, 8774. https://doi.org/10.3390/molecules27248774
Cirlini M, Del Vecchio L, Leto L, Russo F, Dellafiora L, Guarrasi V, Chiancone B. Sprouts of Moringa oleifera Lam.: Germination, Polyphenol Content and Antioxidant Activity. Molecules. 2022; 27(24):8774. https://doi.org/10.3390/molecules27248774
Chicago/Turabian StyleCirlini, Martina, Lorenzo Del Vecchio, Leandra Leto, Federica Russo, Luca Dellafiora, Valeria Guarrasi, and Benedetta Chiancone. 2022. "Sprouts of Moringa oleifera Lam.: Germination, Polyphenol Content and Antioxidant Activity" Molecules 27, no. 24: 8774. https://doi.org/10.3390/molecules27248774
APA StyleCirlini, M., Del Vecchio, L., Leto, L., Russo, F., Dellafiora, L., Guarrasi, V., & Chiancone, B. (2022). Sprouts of Moringa oleifera Lam.: Germination, Polyphenol Content and Antioxidant Activity. Molecules, 27(24), 8774. https://doi.org/10.3390/molecules27248774