Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions
Abstract
1. Introduction
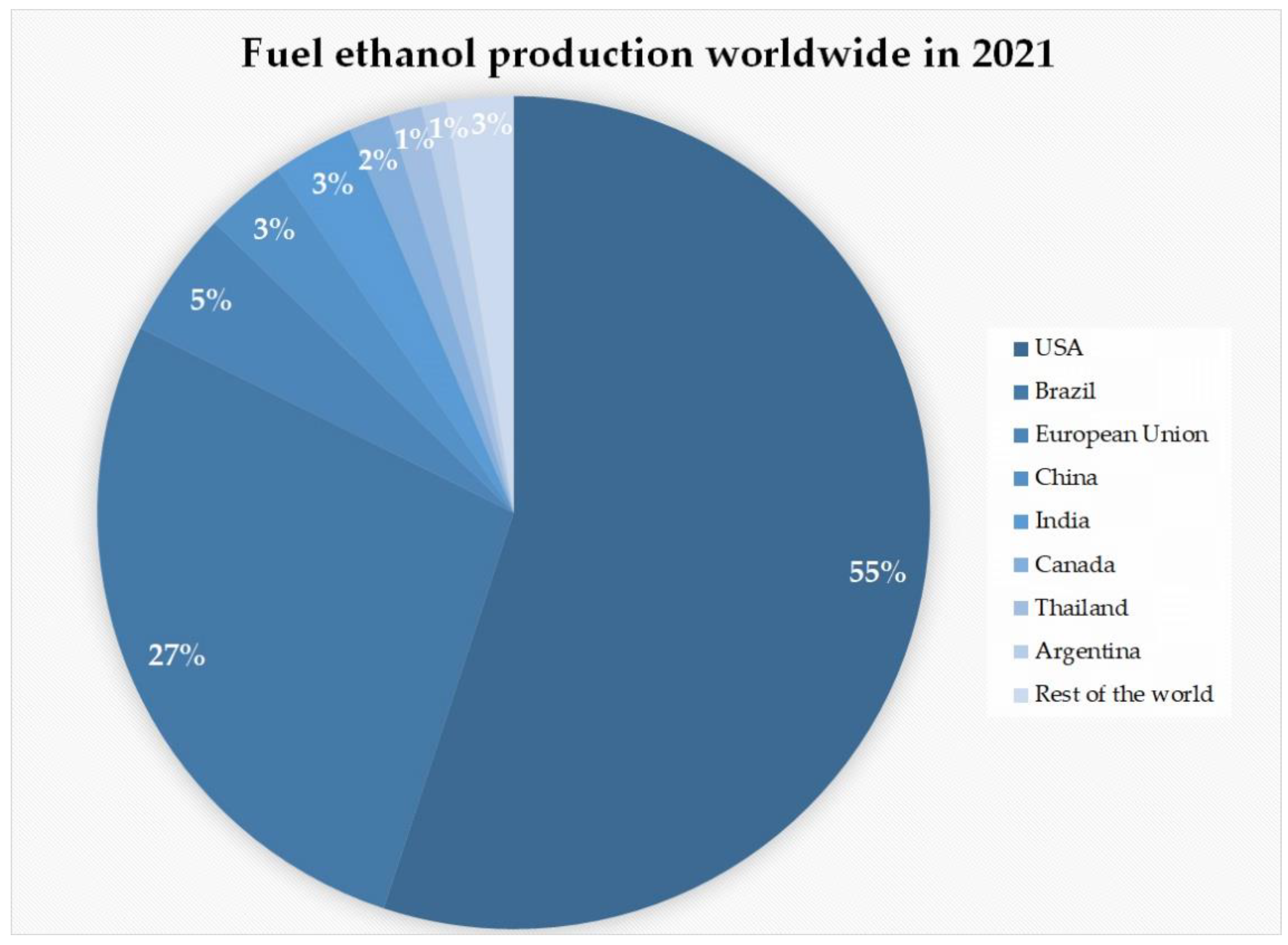
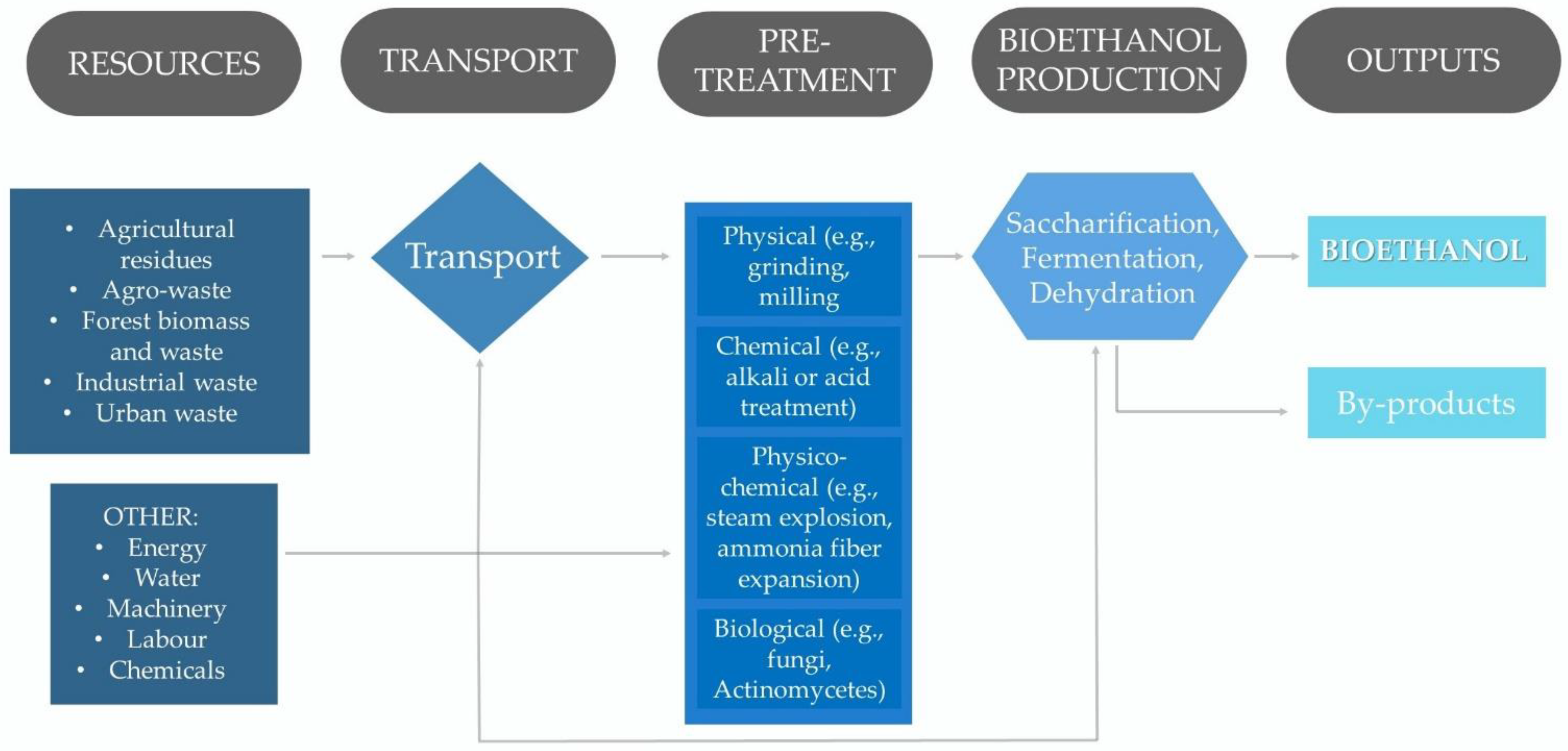
2. Production of Bioethanol from Lignocellulosic Biomass
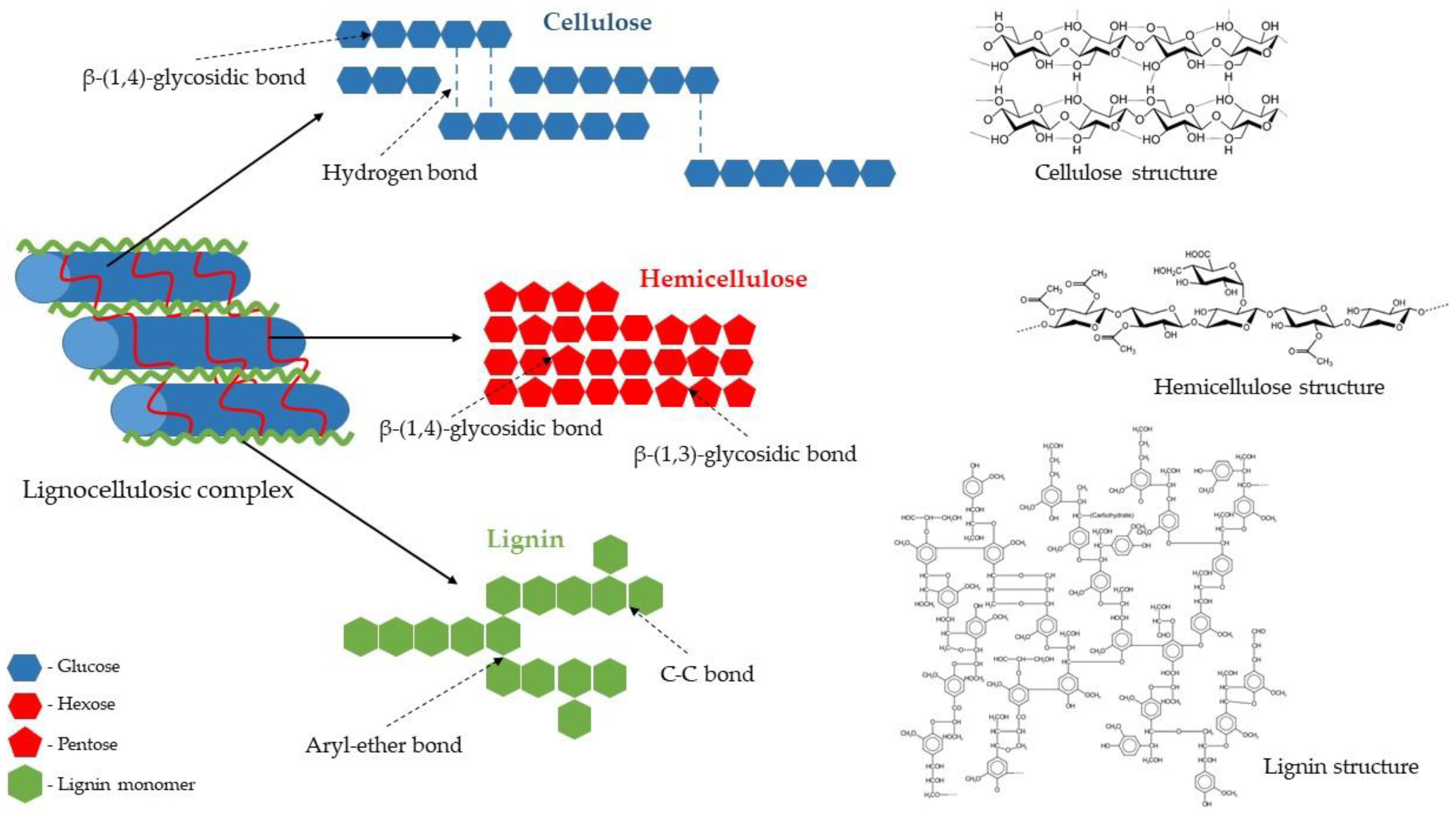
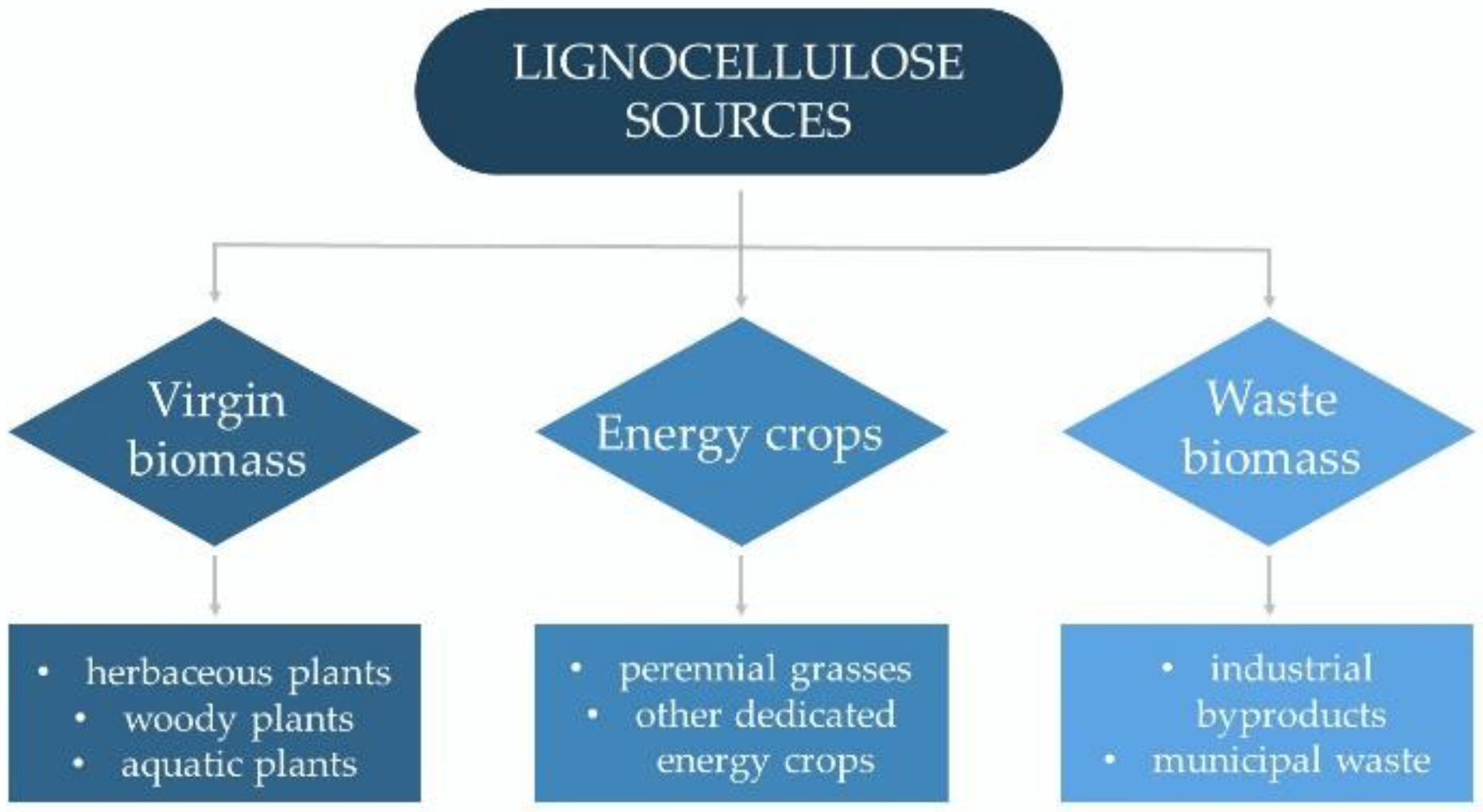
2.1. Lignocellulose Resources
The Structure of the Lignocellulosic Complex
2.2. Pretreatment of a Lignocellulosic Biomass
2.2.1. Physical Pretreatment
2.2.2. Chemical Pretreatment
2.2.3. Physico-Chemical Pretreatment
2.2.4. Biological Pretreatment
2.2.5. Combined Pretreatment Methods
- Biological-alkaline pretreatment combination can enhance the delignification of a lignocellulosic complex and help reduce the chemicals’ concentration, time, and temperature of alkaline treatment, thus lowering operational expenses [83,84,85]. However, the treatment may cause a higher loss of carbohydrates from biomass [85].
- Biological-oxidative pretreatment uses the fact that biomass decay by white-rot fungi involves a Fenton-based oxidation reaction. By mimicking this reaction using other oxidising reagents, e.g., hydrogen peroxide followed a biological pretreatment, it is possible to shorten the residence time and enhance biomass delignification without producing inhibitory by-products, which results in higher sugar yields. This combined pretreatment method seems to be the most effective among biological–chemical treatment combinations [79,83,88,89].
- Biological-steam explosion combinations significantly increase the net sugar yields compared to the processes applied alone. Using lignin-degrading enzymes also reduces energy consumption, the amount of wastewater, the operational costs of steam explosions, and detoxifies the processed biomass [83,92,94].
2.3. Bioethanol Production
2.3.1. Detoxification
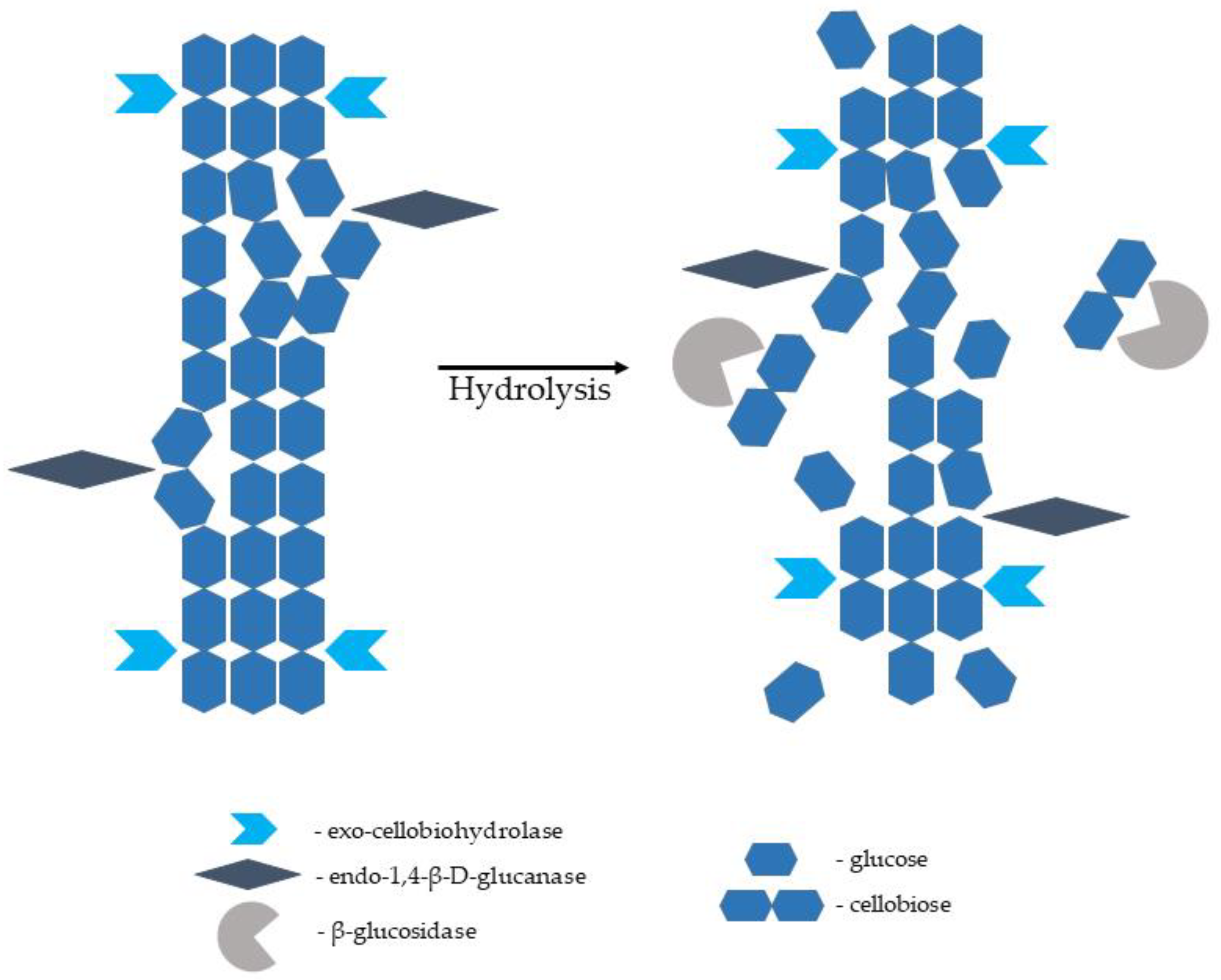
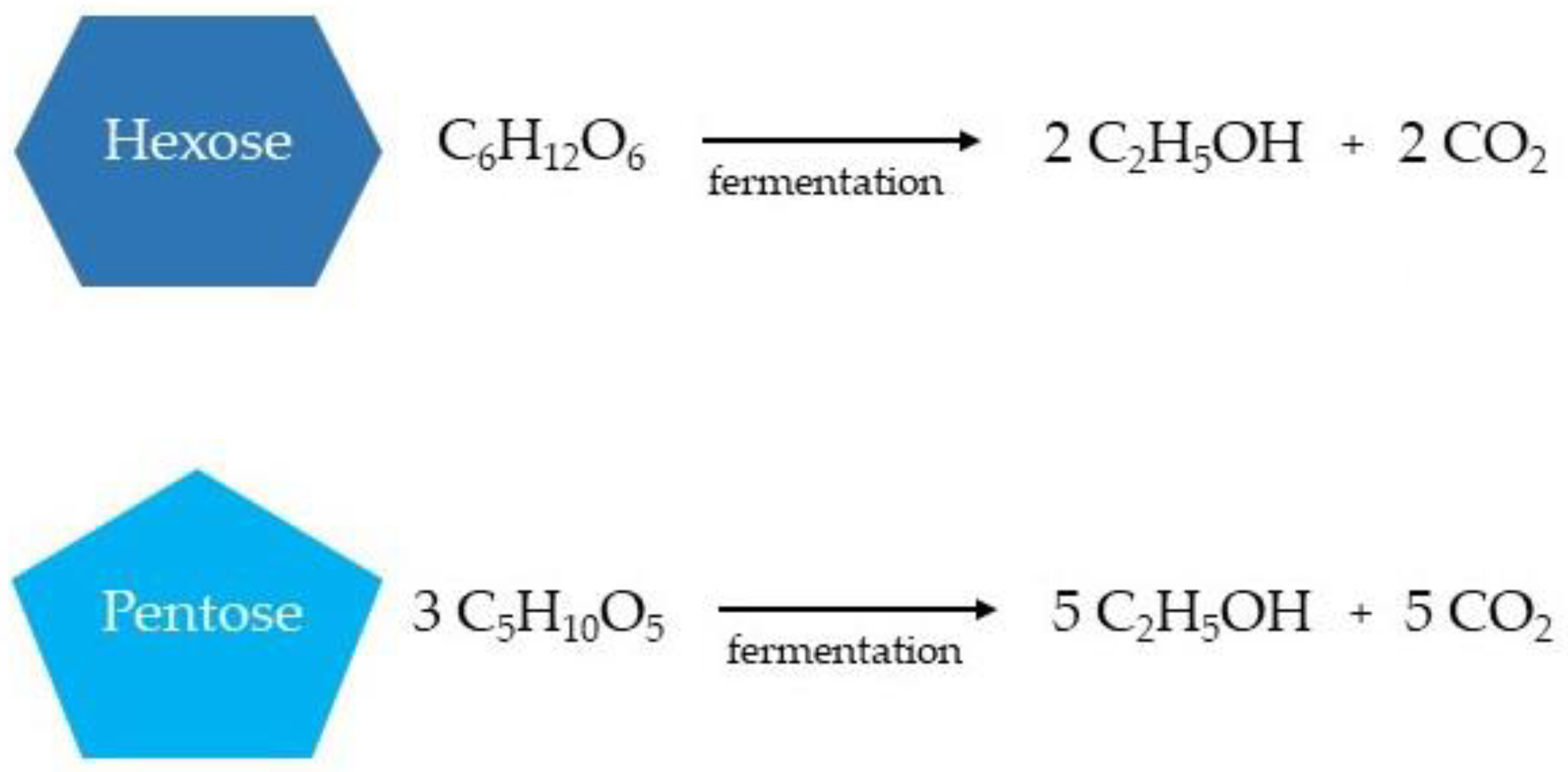
2.3.2. Hydrolysis
- Solid loading—High solid loading reduces hydrolysis installation costs and are necessary to obtain syrups with increased sugar concentrations (80–100 g/L), which determines economically viable distillation (i.e., the ethanol concentration in a fermented broth should be above 4% w/w). It was shown that sugar yield increases with increasing substrate load, but only to some point, after which it decreases. It is mainly because increased cellobiose and glucose concentrations inhibit enzyme activity. Additionally, high solid loading usually translates into a high-viscosity broth, which causes several technical problems due to hampered mixing and impaired mass and heat transfer, affecting the efficiency of enzymes [112,114,115,116,117];
- Enzyme loading—Increased doses of enzymes (or enzyme cocktails) enhance saccharification efficiency providing high glucose yield [12].
- Shaking speed—Optimising shaking/mixing speed is necessary to ensure optimal heat and mass transfer that translates into high glucose yield. Lower speed values result in poor mixing and decreased monosugar yields, while too high of a speed produces shearing forces that may destroy enzymes [117,118,119,120].
- Hydrolysis time—The long time required for complete hydrolysis limits the commercial production of ethanol from lignocellulosic biomass. Therefore, several approaches have attempted to shorten the process by enhancing hydrolysis efficiency, mainly using engineered enzymes/microorganisms or enzyme cocktails and optimising the parameters of the process [121,122].
- Concentration of inhibitors—Inhibitors produced during biomass pretreatment may slow down or even stop enzymatic hydrolysis. Therefore, the detoxification step (see 2.3.1. Detoxification), performed before or during hydrolysis or selecting pretreatment methods producing only a limited amount of inhibitors, is crucial for the process [12,101,102,103,123].
- Effect of various additives—Several different substances were successful as additives in the hydrolysis step to improve glucose yield, including polyethylene glycol (PEG)-based polymers (PEG 600, 4000, 6000), non-ionic surfactants (Tween 80 and Triton X-100), non-catalytic protein (bovine serum albumin (BSA)) or novel chemical surfactants, such as Silwet L-77. Their mode of action is based on blocking the interactions between lignin and enzymes, thus intensifying positive substrate-enzyme interactions and recovering cellulose hydrolysability [124,125,126,127,128,129,130].
2.3.3. Ethanol Fermentation
- Separate hydrolysis and fermentation (SHF)—Hydrolysis and fermentation processes are conducted independently in different units. Carbohydrates from pretreated biomass are degraded to monosugars in a hydrolysis reactor and subsequently converted to ethanol in a fermentation unit. It is a time-consuming and cost-intensive process due to the long residence time needed for complete hydrolysis, high enzyme loading, and material costs required for two separate units, and its main drawback is end-product inhibition (Figure 8A) [157,158,159,160].
- Simultaneous saccharification and fermentation (SSF)—Hydrolysis and fermentation are carried out in the same unit, which improves hydrolysis rates, yields, and product concentrations compared to SHF due to the continuous removal of the sugars by the yeasts, which reduces the end-product inhibition of the enzyme complex. The main drawback is the difference in optimum temperature between saccharification and fermentation and enzyme inhibition by ethanol, microorganisms, and temperature in the reactor (Figure 8B) [160,161,162].
- Simultaneous saccharification and co-fermentation (SSCF)—Hydrolysis and fermentation are carried out in the same unit with concurrent co-fermentation of pentoses using pentose-fermenting strains, which allows converting both hexoses and pentoses from lignocellulosic biomass, thus increasing ethanol yield. This process is suitable for xylose-rich biomass, such as hardwood and agricultural residues; however, the ethanol yield is lower compared to SSF (Figure 8C) [163,164,165,166].
- Consolidated bioprocessing (CBP)—A single-step process where hydrolysis, fermentation, and enzyme production occur in the same unit. The method employs genetically modified microbes or microbial consortia (e.g., some yeast strains and Clostridium thermocellum have already been tested) capable of hydrolysing biomass with enzymes produced on its own and fermenting monosugars to ethanol. The strategy has the potential to revolutionise bioethanol production due to reduced costs for infrastructure and chemicals, making it economically beneficial and environmentally friendly. However, reaching an industrial scale is challenging because of low conversion efficacy, and it still requires further extensive research (Figure 8D) [167,168,169,170].
2.3.4. Distillation and Dehydration
3. Conclusions
- the selection of a suitable pretreatment strategy that is cost-effective and does not impede the overall efficiency of enzymatic saccharification,
- the improvement of the anaerobic digestibility of biomass,
- limiting carbohydrate degradation and the generation of inhibitors during pretreatment to prevent conversion yield loss,
- downsizing the consumption of toxic chemicals, as well as energy and water,
- the improvement and application of novel biocatalysts that can enhance the efficiency of the saccharification process,
- increasing the efficiency of individual enzymes by designing enzymes with enhanced specific activity, thermal stability, and reduced end-product inhibition, and
- reducing the overall footprint of the process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of Fossil Fuels and Future Energy Technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R. An Assessment of the Sustainability of Lignocellulosic Bioethanol Production from Wastes in Iceland. Energies 2018, 11, 1493. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent Developments and Key Barriers to Advanced Biofuels: A Short Review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A Technological Perspective. Energy Environ. Sci. 2008, 1, 542–564. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental Sustainability of Biofuels: A Review. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a Sustainable Future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Efemwenkiekie, U.K.; Oyedepo, S.O.; Idiku, U.D.; Uguru-Okorie, D.C.; Kuhe, A. Comparative Analysis of a Four Stroke Spark Ignition Engine Performance Using Local Ethanol and Gasoline Blends. Procedia Manuf. 2019, 35, 1079–1086. [Google Scholar] [CrossRef]
- Gabisa, E.W.; Gheewala, S.H. Can Substitution of Imported Gasoline by Locally Produced Molasses Ethanol in Ethiopia Be Sustainable? An Eco-Efficiency Assessment. Renew. Sustain. Energy Rev. 2020, 123, 109770. [Google Scholar] [CrossRef]
- Iodice, P.; Senatore, A.; Langella, G.; Amoresano, A. Advantages of Ethanol–Gasoline Blends as Fuel Substitute for Last Generation Si Engines. Environ. Prog. Sustain. Energy 2017, 36, 1173–1179. [Google Scholar] [CrossRef]
- Anderson, S.T. The Demand for Ethanol as a Gasoline Substitute. J. Environ. Econ. Manag. 2012, 63, 151–168. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current State-of-the-Art in Ethanol Production from Lignocellulosic Feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. Bioenerg. Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Vandenberghe, L.P.S.; Soccol, C.R.; Sigoillot, J.-C.; Faulds, C. First Generation Bioethanol. In Green Fuels Technology: Biofuels; Green Energy and Technology; Soccol, C.R., Brar, S.K., Faulds, C., Ramos, L.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 175–212. ISBN 978-3-319-30205-8. [Google Scholar]
- Murawski de Mello, A.F.; Porto de Souza Vandenberghe, L.; Valladares-Diestra, K.K.; Amaro Bittencourt, G.; Martinez Burgos, W.J.; Soccol, C.R. Corn First-Generation Bioethanol Unities with Energy and Dried Grains with Solubles (DDGS) Production. In Liquid Biofuels: Bioethanol; Biofuel and Biorefinery Technologies; Soccol, C.R., Amarante Guimarães Pereira, G., Dussap, C.-G., Porto de Souza Vandenberghe, L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 109–132. ISBN 978-3-031-01241-9. [Google Scholar]
- Mohanty, S.K.; Swain, M.R. Chapter 3—Bioethanol Production From Corn and Wheat: Food, Fuel, and Future. In Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–59. ISBN 978-0-12-813766-6. [Google Scholar]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second Generation Bioethanol Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Baig, K.S.; Wu, J.; Turcotte, G. Future Prospects of Delignification Pretreatments for the Lignocellulosic Materials to Produce Second Generation Bioethanol. Int. J. Energy Res. 2019, 43, 1411–1427. [Google Scholar] [CrossRef]
- Saïdane-Bchir, F.; El Falleh, A.; Ghabbarou, E.; Hamdi, M. 3rd Generation Bioethanol Production from Microalgae Isolated from Slaughterhouse Wastewater. Waste Biomass Valor. 2016, 7, 1041–1046. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A Review on Third Generation Bioethanol Feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Veza, I.; Hoang, A.T.; Abbas, M.M.; Tamaldin, N.; Idris, M.; Djamari, D.W.; Sule, A.; Maulana, E.; Putra, N.R.; Opia, A.C. Microalgae and Macroalgae for Third-Generation Bioethanol Production. In Liquid Biofuels: Bioethanol; Biofuel and Biorefinery Technologies; Soccol, C.R., Amarante Guimarães Pereira, G., Dussap, C.-G., Porto de Souza Vandenberghe, L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 301–331. ISBN 978-3-031-01241-9. [Google Scholar]
- Association, R.F. Annual Ethanol Production. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 28 October 2022).
- European Union: Biofuels Annual. Available online: https://www.fas.usda.gov/data/european-union-biofuels-annual-1 (accessed on 29 October 2022).
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive Assessment of 2G Bioethanol Production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-Generation Biorefineries: A Sustainable Platform for Food, Clean Energy, and Nutraceuticals Production. Biomass Conv. Bioref. 2022, 12, 4215–4230. [Google Scholar] [CrossRef]
- Reports. Available online: https://usdabrazil.org.br/en/reports/ (accessed on 29 October 2022).
- Association, R.F. Ethanol Biorefinery Locations. Available online: https://ethanolrfa.org/resources/ethanol-biorefinery-locations (accessed on 29 October 2022).
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Role of Energy Policy in Renewable Energy Accomplishment: The Case of Second-Generation Bioethanol. Energy Policy 2008, 36, 3360–3365. [Google Scholar] [CrossRef]
- Neto, A.C.; Guimarães, M.J.O.C.; Freire, E. Business Models for Commercial Scale Second-Generation Bioethanol Production. J. Clean. Prod. 2018, 184, 168–178. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic Biomass for Bioethanol: An Overview on Pretreatment, Hydrolysis and Fermentation Processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Arefin, M.A.; Rashid, F.; Islam, A. A Review of Biofuel Production from Floating Aquatic Plants: An Emerging Source of Bio-Renewable Energy. Biofuels Bioprod. Biorefin. 2021, 15, 574–591. [Google Scholar] [CrossRef]
- Ge, X.; Burner, D.M.; Xu, J.; Phillips, G.C.; Sivakumar, G. Bioethanol Production from Dedicated Energy Crops and Residues in Arkansas, USA. Biotechnol. J. 2011, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Wang, X.; Lü, X. A Review on Recycling Techniques for Bioethanol Production from Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Sakakibara, A. A Structural Model of Softwood Lignin. Wood Sci.Technol. 1980, 14, 89–100. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Advances in Polymer Science; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–52. ISBN 978-3-319-26015-0. [Google Scholar]
- Gu, J.; Catchmark, J.M. The Impact of Cellulose Structure on Binding Interactions with Hemicellulose and Pectin. Cellulose 2013, 20, 1613–1627. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Chapter One—Insights into Lignin Degradation and Its Potential Industrial Applications. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 82, pp. 1–28. [Google Scholar]
- Zhang, N.; Li, S.; Xiong, L.; Hong, Y.; Chen, Y. Cellulose-Hemicellulose Interaction in Wood Secondary Cell-Wall. Model. Simul. Mat. Sci. Eng. 2015, 23, 085010. [Google Scholar] [CrossRef]
- Sheng, Y.; Lam, S.S.; Wu, Y.; Ge, S.; Wu, J.; Cai, L.; Huang, Z.; Le, Q.V.; Sonne, C.; Xia, C. Enzymatic Conversion of Pretreated Lignocellulosic Biomass: A Review on Influence of Structural Changes of Lignin. Bioresour. Technol. 2021, 324, 124631. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Yelle, D.J.; Ralph, J.; Frihart, C.R. Characterization of Nonderivatized Plant Cell Walls Using High-Resolution Solution-State NMR Spectroscopy. Magn. Reson. Chem. 2008, 46, 508–517. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic Biomass to Bioethanol, a Comprehensive Review with a Focus on Pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel Ethanol Production from Lignocellulose: A Challenge for Metabolic Engineering and Process Integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef]
- Das, N.; Jena, P.K.; Padhi, D.; Kumar Mohanty, M.; Sahoo, G. A Comprehensive Review of Characterization, Pretreatment and Its Applications on Different Lignocellulosic Biomass for Bioethanol Production. Biomass Conv. Bioref. 2021, 82, 1–25. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V. Pretreatment Techniques for Agricultural Waste. Case Stud. Chem. Environ. Eng. 2022, 6, 100229. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent Advances in Mechanochemical Production of Chemicals and Carbon Materials from Sustainable Biomass Resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a Pretreatment for Lignocellulosic Biomass: Fundamentals and Applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Zheng, J.; Rehmann, L. Extrusion Pretreatment of Lignocellulosic Biomass: A Review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed]
- Rooni, V.; Raud, M.; Kikas, T. The Freezing Pre-Treatment of Lignocellulosic Material: A Cheap Alternative for Nordic Countries. Energy 2017, 139, 1–7. [Google Scholar] [CrossRef]
- Chang, K.-L.; Thitikorn-amorn, J.; Hsieh, J.-F.; Ou, B.-M.; Chen, S.-H.; Ratanakhanokchai, K.; Huang, P.-J.; Chen, S.-T. Enhanced Enzymatic Conversion with Freeze Pretreatment of Rice Straw. Biomass Bioenergy 2011, 35, 90–95. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of Delignification and Subsequent Hydrolysis for the Fermentable Sugar Production from Lignocellulosic Biomass Using Ultrasonic Irradiation. Ultrason. Sonochem. 2018, 40, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different Pretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Pulsed Electric Field Pretreatment of Switchgrass and Wood Chip Species for Biofuel Production. Ind. Eng. Chem. Res. 2011, 50, 10996–11001. [Google Scholar] [CrossRef]
- Broda, M.; Leja, K.; Czaczyk, K.; Grajek, W. The New Methods of Corn Disinfection Used in Bioethanol Production. J. Biobased Mater. Bioenergy 2010, 4, 430–435. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic Solvent Pretreatment of Lignocellulosic Biomass for Biofuels and Biochemicals: A Review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Joy, S.P.; Krishnan, C. Modified Organosolv Pretreatment for Improved Cellulosic Ethanol Production from Sorghum Biomass. Ind. Crops Prod. 2022, 177, 114409. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Naz, S.; Sultan, M.; Griffin, G.; Muhammad, N.; Khan, A.S. Acidic Ionic Liquids: Promising and Cost-Effective Solvents for Processing of Lignocellulosic Biomass. J. Mol. Liq. 2019, 287, 110943. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, K.; Liu, X.; Han, D.; Zhang, Q. Review on Development of Ionic Liquids in Lignocellulosic Biomass Refining. J. Mol. Liq. 2022, 359, 119326. [Google Scholar] [CrossRef]
- Yoon, L.W.; Rafi, I.S.; Ngoh, G.C. Feasibility of Eliminating Washing Step in Bioethanol Production Using Deep Eutectic Solvent Pretreated Lignocellulosic Substrate. Chem. Eng. Res. Des. 2022, 179, 257–264. [Google Scholar] [CrossRef]
- Hassan, E.-S.R.E.; Mutelet, F. Evaluation of Miscanthus Pretreatment Effect by Choline Chloride Based Deep Eutectic Solvents on Bioethanol Production. Bioresour. Technol. 2022, 345, 126460. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent Advances in the Application of Inorganic Salt Pretreatment for Transforming Lignocellulosic Biomass into Reducing Sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef]
- Banerjee, D.; Mukherjee, S.; Pal, S.; Khowala, S. Enhanced Saccharification Efficiency of Lignocellulosic Biomass of Mustard Stalk and Straw by Salt Pretreatment. Ind. Crops Prod. 2016, 80, 42–49. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Zainol, M.M.; Amin, N.A.S. 14—Pretreatment of Agroindustry Waste by Ozonolysis for Synthesis of Biorefinery Products. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 303–336. ISBN 978-0-12-818996-2. [Google Scholar]
- Travaini, R.; Martín-Juárez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An Advantageous Pretreatment for Lignocellulosic Biomass Revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef]
- Dey, P.; Pal, P.; Kevin, J.D.; Das, D.B. Lignocellulosic Bioethanol Production: Prospects of Emerging Membrane Technologies to Improve the Process—A Critical Review. Rev. Chem. Eng. 2020, 36, 333–367. [Google Scholar] [CrossRef]
- Balan, V.; Bals, B.; Chundawat, S.P.S.; Marshall, D.; Dale, B.E. Lignocellulosic Biomass Pretreatment Using AFEX. In Biofuels: Methods and Protocols; Methods in Molecular Biology; Mielenz, J.R., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 61–77. ISBN 978-1-60761-214-8. [Google Scholar]
- Zhao, C.; Shao, Q.; Chundawat, S.P.S. Recent Advances on Ammonia-Based Pretreatments of Lignocellulosic Biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Pal, R.K.; Zhao, C.; Campbell, T.; Teymouri, F.; Videto, J.; Nielson, C.; Wieferich, B.; Sousa, L.; Dale, B.E. Ammonia Fiber Expansion (AFEX) Pretreatment of Lignocellulosic Biomass. J. Vis. Exp. 2020, 158, e57488. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, N.; Faik, A.; Goetz, D.J.; Gu, T. Supercritical Carbon Dioxide Pretreatment of Corn Stover and Switchgrass for Lignocellulosic Ethanol Production. Bioresour. Technol. 2011, 102, 6995–7000. [Google Scholar] [CrossRef] [PubMed]
- Gu, T. Pretreatment of Lignocellulosic Biomass Using Supercritical Carbon Dioxide as a Green Solvent. In Green Biomass Pretreatment for Biofuels Production; SpringerBriefs in Molecular Science; Gu, T., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 107–125. ISBN 978-94-007-6052-3. [Google Scholar]
- Badgujar, K.C.; Dange, R.; Bhanage, B.M. Recent Advances of Use of the Supercritical Carbon Dioxide for the Biomass Pre-Treatment and Extraction: A Mini-Review. J. Indian Chem. Soc. 2021, 98, 100018. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, W.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X.; Zhou, G.; Yuan, Z. Liquid Hot Water Pretreatment of Lignocellulosic Biomass for Bioethanol Production Accompanying with High Valuable Products. Biores. Technol. 2016, 199, 68–75. [Google Scholar] [CrossRef]
- Arvaniti, E.; Bjerre, A.B.; Schmidt, J.E. Wet Oxidation Pretreatment of Rape Straw for Ethanol Production. Biomass Bioenergy 2012, 39, 94–105. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Chapter 7—Biological Pretreatment of Lignocellulosic Biomass—Current Trends and Future Perspectives. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. ISBN 978-0-12-815162-4. [Google Scholar]
- López-Abelairas, M.; Álvarez Pallín, M.; Salvachúa, D.; Lú-Chau, T.; Martínez, M.J.; Lema, J.M. Optimisation of the Biological Pretreatment of Wheat Straw with White-Rot Fungi for Ethanol Production. Bioprocess Biosyst. Eng. 2013, 36, 1251–1260. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Chapter 24—Biological Pretreatment of Lignocellulosic Biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 561–585. ISBN 978-0-12-802323-5. [Google Scholar]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de Fatima Rodrigues de Souza, R.; da Silva Aquino, K.A.; Morais, M.A.M., Jr.; Menezes, R.S.C. Alkaline Hydrogen Peroxide Pretreatment of Lignocellulosic Biomass: Status and Perspectives. Biomass Conv. Bioref. 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber Degradation and Carbohydrate Production by Combined Biological and Chemical/Physicochemical Pretreatment Methods of Lignocellulosic Biomass—A Review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef]
- Si, M.; Liu, D.; Liu, M.; Yan, X.; Gao, C.; Chai, L.; Shi, Y. Complementary Effect of Combined Bacterial-Chemical Pretreatment to Promote Enzymatic Digestibility of Lignocellulose Biomass. Bioresour. Technol. 2019, 272, 275–280. [Google Scholar] [CrossRef]
- Yu, H.; Du, W.; Zhang, J.; Ma, F.; Zhang, X.; Zhong, W. Fungal Treatment of Cornstalks Enhances the Delignification and Xylan Loss during Mild Alkaline Pretreatment and Enzymatic Digestibility of Glucan. Bioresour. Technol. 2010, 101, 6728–6734. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Lu-Chau, T.A.; Gullón, B.; Ruiz, E.; Romero, I.; Castro, E.; Lema, J.M. Application of a Combined Fungal and Diluted Acid Pretreatment on Olive Tree Biomass. Ind. Crops Prod. 2018, 121, 10–17. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L.; Liu, X.; Shi, Y. Bacteria-Enhanced Dilute Acid Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-Rot Fungi Pretreatment Combined with Alkaline/Oxidative Pretreatment to Improve Enzymatic Saccharification of Industrial Hemp. Bioresour. Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kandhola, G.; Rajan, K.; Labbé, N.; Chmely, S.; Heringer, N.; Kim, J.-W.; Hood, E.E.; Carrier, D.J. Beneficial Effects of Trametes Versicolor Pretreatment on Saccharification and Lignin Enrichment of Organosolv-Pretreated Pinewood. RSC Adv. 2017, 7, 45652–45661. [Google Scholar] [CrossRef]
- Zhuo, S.; Yan, X.; Liu, D.; Si, M.; Zhang, K.; Liu, M.; Peng, B.; Shi, Y. Use of Bacteria for Improving the Lignocellulose Biorefinery Process: Importance of Pre-Erosion. Biotechnol. Biofuels 2018, 11, 146. [Google Scholar] [CrossRef]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving Enzymatic Hydrolysis of Lignocellulosic Biomass by Bio-Coordinated Physicochemical Pretreatment—A Review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.-H.; Yu, Y. Recent Advances and Challenges of Inter-Disciplinary Biomass Valorization by Integrating Hydrothermal and Biological Techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. Exploring Laccase and Mediators Behavior during Saccharification and Fermentation of Steam-Exploded Wheat Straw for Bioethanol Production. J. Chem. Technol. Biotechnol. 2016, 91, 1816–1825. [Google Scholar] [CrossRef]
- Ramadoss, G.; Muthukumar, K. Mechanistic Study on Ultrasound Assisted Pretreatment of Sugarcane Bagasse Using Metal Salt with Hydrogen Peroxide for Bioethanol Production. Ultrason. Sonochem. 2016, 28, 207–217. [Google Scholar] [CrossRef]
- Moodley, P.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Progress in the Development of Alkali and Metal Salt Catalysed Lignocellulosic Pretreatment Regimes: Potential for Bioethanol Production. Bioresour. Technol. 2020, 310, 123372. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Yang, G.H.; Ang, L.Y.; New, E.K.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2018, 249, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.C.; Ong, V.Z.; Wu, T.Y. Potential Use of Alkaline Hydrogen Peroxide in Lignocellulosic Biomass Pretreatment and Valorization—A Review. Renew. Sustain. Energy Rev. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball Milling as an Important Pretreatment Technique in Lignocellulose Biorefineries: A Review. Biomass Conv. Bioref. 2021, 1–24. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Zhang, C.-C.; Nan, J.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Advances in Pretreatment of Lignocellulosic Biomass for Bioenergy Production: Challenges and Perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A Review of Biological Delignification and Detoxification Methods for Lignocellulosic Bioethanol Production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- da Nogueira, C.; de Araújo Padilha, C.E.; de Medeiros Dantas, J.M.; de Medeiros, F.G.M.; de Araújo Guilherme, A.; de Santana Souza, D.F.; dos Santos, E.S. In-Situ Detoxification Strategies to Boost Bioalcohol Production from Lignocellulosic Biomass. Renew. Energy 2021, 180, 914–936. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Meyer, A.S. Enzymatic Cellulose Hydrolysis: Enzyme Reusability and Visualization of β-Glucosidase Immobilized in Calcium Alginate. Molecules 2014, 19, 19390–19406. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P.W. Applications of Fungal Cellulases in Biofuel Production: Advances and Limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhang, Y.-H.P. Cellulases: Characteristics, Sources, Production, and Applications. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA; pp. 131–146. ISBN 978-1-118-64204-7.
- Singhania, R.R.; Adsul, M.; Pandey, A.; Patel, A.K. 4—Cellulases. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–101. ISBN 978-0-444-63662-1. [Google Scholar]
- Mohanram, S.; Amat, D.; Choudhary, J.; Arora, A.; Nain, L. Novel Perspectives for Evolving Enzyme Cocktails for Lignocellulose Hydrolysis in Biorefineries. Sustain. Chem. Processes 2013, 1, 15. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Börjesson, J.; Bruun, M.H.; Tjerneld, F.; Jørgensen, H. Use of Surface Active Additives in Enzymatic Hydrolysis of Wheat Straw Lignocellulose. Enzym. Microb. Technol. 2007, 40, 888–895. [Google Scholar] [CrossRef]
- Malherbe, S.; Cloete, T.E. Lignocellulose Biodegradation: Fundamentals and Applications. Rev. Environ. Sci. Biotechnol. 2002, 1, 105–114. [Google Scholar] [CrossRef]
- Bhattacharya, A.S.; Bhattacharya, A.; Pletschke, B.I. Synergism of Fungal and Bacterial Cellulases and Hemicellulases: A Novel Perspective for Enhanced Bio-Ethanol Production. Biotechnol. Lett. 2015, 37, 1117–1129. [Google Scholar] [CrossRef]
- Abdou Alio, M.; Tugui, O.-C.; Rusu, L.; Pons, A.; Vial, C. Hydrolysis and Fermentation Steps of a Pretreated Sawmill Mixed Feedstock for Bioethanol Production in a Wood Biorefinery. Bioresour. Technol. 2020, 310, 123412. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-Catalyzed Ethanol Pretreatment of Sugarcane Bagasse Boosts Sugar Yields with Low Enzyme Loadings and Short Hydrolysis Time. Bioresour. Technol. 2018, 249, 395–401. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Zhang, H.; Zheng, H.; Huang, H. Factors to Decrease the Cellulose Conversion of Enzymatic Hydrolysis of Lignocellulose at High Solid Concentrations. Cellulose 2014, 21, 2409–2417. [Google Scholar] [CrossRef]
- da Silva, A.S.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and Advances in High-Solids Enzymatic Hydrolysis of Lignocellulosic Biomass: A Critical Review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, L.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Pretreating Wheat Straw by Phosphoric Acid plus Hydrogen Peroxide for Enzymatic Saccharification and Ethanol Production at High Solid Loading. Bioresour. Technol. 2017, 238, 174–181. [Google Scholar] [CrossRef]
- Santos, C.A.; Morais, M.A.B.; Terrett, O.M.; Lyczakowski, J.J.; Zanphorlin, L.M.; Ferreira-Filho, J.A.; Tonoli, C.C.C.; Murakami, M.T.; Dupree, P.; Souza, A.P. An Engineered GH1 β-Glucosidase Displays Enhanced Glucose Tolerance and Increased Sugar Release from Lignocellulosic Materials. Sci. Rep. 2019, 9, 4903. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, R.; Shi, K.; Yuan, Y.; Dale, B.E.; Gao, Z.; Jin, M. Mixing Alkali Pretreated and Acid Pretreated Biomass for Cellulosic Ethanol Production Featuring Reduced Chemical Use and Decreased Inhibitory Effect. Ind. Crops Prod. 2018, 124, 719–725. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Ingesson, H.; Zacchi, G.; Yang, B.; Esteghlalian, A.R.; Saddler, J.N. The Effect of Shaking Regime on the Rate and Extent of Enzymatic Hydrolysis of Cellulose. J. Biotechnol. 2001, 88, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Fenila, F.; Shastri, Y. Control of Enzymatic Hydrolysis of Lignocellulosic Biomass. Resour.-Effic. Technol. 2016, 2, S96–S104. [Google Scholar] [CrossRef]
- Viikari, L.; Vehmaanperä, J.; Koivula, A. Lignocellulosic Ethanol: From Science to Industry. Biomass Bioenergy 2012, 46, 13–24. [Google Scholar] [CrossRef]
- Devi, A.; Singh, A.; Bajar, S.; Pant, D.; Din, Z.U. Ethanol from Lignocellulosic Biomass: An in-Depth Analysis of Pre-Treatment Methods, Fermentation Approaches and Detoxification Processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Y.; Wu, S.; Yuan, Z. Improving Corn Stover Enzymatic Saccharification via Ferric Chloride Catalyzed Dimethyl Sulfoxide Pretreatment and Various Additives. Ind. Crops Prod. 2019, 140, 111663. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Q.; Fang, Z.; Cong, W.; Miao, Z. High-Concentrated Substrate Enzymatic Hydrolysis of Pretreated Rice Straw with Glycerol and Aluminum Chloride at Low Cellulase Loadings. Bioresour. Technol. 2019, 294, 122164. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the Action of Tween 20 Non-Ionic Surfactant during Enzymatic Hydrolysis of Lignocellulose: Pretreatment, Hydrolysis Conditions and Lignin Structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef]
- Sun, D.; Yang, Q.; Wang, Y.; Gao, H.; He, M.; Lin, X.; Lu, J.; Wang, Y.; Kang, H.; Alam, A.; et al. Distinct Mechanisms of Enzymatic Saccharification and Bioethanol Conversion Enhancement by Three Surfactants under Steam Explosion and Mild Chemical Pretreatments in Bioenergy Miscanthus. Ind. Crops Prod. 2020, 153, 112559. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kaushal, J.; Singh, G.; Kaur, A.; Arya, S.K. Melioration of Enzymatic Ethanol Production from Alkali Pre-Treated Paddy Straw Promoted by Addition of Surfactant. Biocatal. Biotransform. 2022, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Han, X.; Wang, Y.; Rao, S.; Zhang, Q.; Zhou, J.; Li, J.; Liu, S.; Du, G. Recent Progress in Key Lignocellulosic Enzymes: Enzyme Discovery, Molecular Modifications, Production, and Enzymatic Biomass Saccharification. Bioresour. Technol. 2022, 363, 127986. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhao, C.; Huang, X.; Zhang, H.; Xie, J. Enhanced Digestibility and Fermentability of Sugarcane Bagasse in Biofuel Production by Surfactant-Assisted Dilute Acid Pretreatment. Ind. Crops Prod. 2021, 172, 114006. [Google Scholar] [CrossRef]
- Dien, B.S.; Hespell, R.B.; Wyckoff, H.A.; Bothast, R.J. Fermentation of Hexose and Pentose Sugars Using a Novel Ethanologenic Escherichia Coli Strain. Enzym. Microb. Technol. 1998, 23, 366–371. [Google Scholar] [CrossRef]
- du Preez, J.C.; Bosch, M.; Prior, B.A. The Fermentation of Hexose and Pentose Sugars by Candida Shehatae and Pichia Stipitis. Appl. Microbiol. Biotechnol. 1986, 23, 228–233. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; dos Santos, E.S.; Teixeira, J.A.; de Macedo, G.R. Bioethanol Production by Saccharomyces Cerevisiae, Pichia Stipitis and Zymomonas Mobilis from Delignified Coconut Fibre Mature and Lignin Extraction According to Biorefinery Concept. Renew. Energy 2016, 94, 353–365. [Google Scholar] [CrossRef]
- Ma’As, M.F.; Ghazali, H.M.; Chieng, S. Bioethanol Production from Brewer’s Rice by Saccharomyces Cerevisiae and Zymomonas Mobilis: Evaluation of Process Kinetics and Performance. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–14. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Wirawan, F.; Cheng, C.-L.; Lo, Y.-C.; Chen, C.-Y.; Chang, J.-S.; Leu, S.-Y.; Lee, D.-J. Continuous Cellulosic Bioethanol Co-Fermentation by Immobilized Zymomonas Mobilis and Suspended Pichia Stipitis in a Two-Stage Process. Appl. Energy 2020, 266, 114871. [Google Scholar] [CrossRef]
- De Bari, I.; De Canio, P.; Cuna, D.; Liuzzi, F.; Capece, A.; Romano, P. Bioethanol Production from Mixed Sugars by Scheffersomyces Stipitis Free and Immobilized Cells, and Co-Cultures with Saccharomyces Cerevisiae. New Biotechnol. 2013, 30, 591–597. [Google Scholar] [CrossRef]
- Bailey, J.E. Toward a Science of Metabolic Engineering. Science 1991, 252, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenerg. Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of Microbial Strains for Biofuel Production: Metabolic Engineering, Applications, and Challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Pereira, F.; Johansson, B.; Domingues, L. Metabolic Engineering of Saccharomyces Cerevisiae Ethanol Strains PE-2 and CAT-1 for Efficient Lignocellulosic Fermentation. Bioresour. Technol. 2015, 179, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-S.; Cate, J.H. Metabolic Engineering of Yeast for Lignocellulosic Biofuel Production. Curr. Opin. Chem. Biol. 2017, 41, 99–106. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, S.; Lee, S.-M. Metabolic Engineering for the Utilization of Carbohydrate Portions of Lignocellulosic Biomass. Metab. Eng. 2022, 71, 2–12. [Google Scholar] [CrossRef]
- Jeong, D.; Oh, E.J.; Ko, J.K.; Nam, J.-O.; Park, H.-S.; Jin, Y.-S.; Lee, E.J.; Kim, S.R. Metabolic Engineering Considerations for the Heterologous Expression of Xylose-Catabolic Pathways in Saccharomyces Cerevisiae. PLoS ONE 2020, 15, e0236294. [Google Scholar] [CrossRef]
- Anasontzis, G.E.; Kourtoglou, E.; Villas-Boâs, S.G.; Hatzinikolaou, D.G.; Christakopoulos, P. Metabolic Engineering of Fusarium Oxysporum to Improve Its Ethanol-Producing Capability. Front. Microbiol. 2016, 7, 632. [Google Scholar] [CrossRef]
- Yao, S.; Mikkelsen, M.J. Metabolic Engineering to Improve Ethanol Production in Thermoanaerobacter Mathranii. Appl. Microbiol. Biotechnol. 2010, 88, 199–208. [Google Scholar] [CrossRef]
- Jojima, T.; Noburyu, R.; Sasaki, M.; Tajima, T.; Suda, M.; Yukawa, H.; Inui, M. Metabolic Engineering for Improved Production of Ethanol by Corynebacterium Glutamicum. Appl. Microbiol. Biotechnol. 2015, 99, 1165–1172. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization Technologies and Support Materials Suitable in Alcohol Beverages Production: A Review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Recent Advances in Nano-Carrier Immobilized Enzymes and Their Applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Kyriakou, M.; Patsalou, M.; Xiaris, N.; Tsevis, A.; Koutsokeras, L.; Constantinides, G.; Koutinas, M. Enhancing Bioproduction and Thermotolerance in Saccharomyces Cerevisiae via Cell Immobilization on Biochar: Application in a Citrus Peel Waste Biorefinery. Renew. Energy 2020, 155, 53–64. [Google Scholar] [CrossRef]
- Karagoz, P.; Bill, R.M.; Ozkan, M. Lignocellulosic Ethanol Production: Evaluation of New Approaches, Cell Immobilization and Reactor Configurations. Renew. Energy 2019, 143, 741–752. [Google Scholar] [CrossRef]
- Soares, L.B.; da Silveira, J.M.; Biazi, L.E.; Longo, L.; de Oliveira, D.; Furigo Júnior, A.; Ienczak, J.L. An Overview on Fermentation Strategies to Overcome Lignocellulosic Inhibitors in Second-Generation Ethanol Production Using Cell Immobilization. Crit. Rev. Biotechnol. 2022, 1–22. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. I: Inhibition and Detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Flevaris, K.; Chatzidoukas, C. Optimal Fed-Batch Bioreactor Operating Strategies for the Microbial Production of Lignocellulosic Bioethanol and Exploration of Their Economic Implications: A Step Forward towards Sustainability and Commercialization. J. Clean. Prod. 2021, 295, 126384. [Google Scholar] [CrossRef]
- Ghorbanpour Khamseh, A.A.; Miccio, M. Comparison of Batch, Fed-Batch and Continuous Well-Mixed Reactors for Enzymatic Hydrolysis of Orange Peel Wastes. Process Biochem. 2012, 47, 1588–1594. [Google Scholar] [CrossRef]
- Jahnavi, G.; Prashanthi, G.S.; Sravanthi, K.; Rao, L.V. Status of Availability of Lignocellulosic Feed Stocks in India: Biotechnological Strategies Involved in the Production of Bioethanol. Renew. Sustain. Energy Rev. 2017, 73, 798–820. [Google Scholar] [CrossRef]
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic Biomass for Bioethanol: Recent Advances, Technology Trends, and Barriers to Industrial Development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzymatic-Based Hydrolysis Processes for Ethanol from Lignocellulosic Materials: A Review. BioResources 2007, 2, 707–738. [Google Scholar]
- Srivastava, N.; Rawat, R.; Singh Oberoi, H.; Ramteke, P.W. A Review on Fuel Ethanol Production from Lignocellulosic Biomass. Int. J. Green Energy 2015, 12, 949–960. [Google Scholar] [CrossRef]
- Wright, J.D.; Wyman, C.E.; Grohmann, K. Simultaneous Saccharification and Fermentation of Lignocellulose. Appl. Biochem. Biotechnol. 1988, 18, 75–90. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Chowdary, G.V. Optimization of Simultaneous Saccharification and Fermentation for the Production of Ethanol from Lignocellulosic Biomass. J. Agric. Food Chem. 2000, 48, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Roberto, I.C.; Castro, R.C.A.; Silva, J.P.A.; Mussatto, S.I. Ethanol Production from High Solid Loading of Rice Straw by Simultaneous Saccharification and Fermentation in a Non-Conventional Reactor. Energies 2020, 13, 2090. [Google Scholar] [CrossRef]
- Bondesson, P.-M.; Galbe, M. Process Design of SSCF for Ethanol Production from Steam-Pretreated, Acetic-Acid-Impregnated Wheat Straw. Biotechnol. Biofuels 2016, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Nielsen, F.; Albers, E.; Lambert, A.; Wännström, S.; Welin, L.; Zacchi, G.; Olsson, L. Simultaneous Saccharification and Co-Fermentation for Bioethanol Production Using Corncobs at Lab, PDU and Demo Scales. Biotechnol. Biofuels 2013, 6, 2. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Chen, H.-Z. Simultaneous Saccharification and Co-Fermentation for Improving the Xylose Utilization of Steam Exploded Corn Stover at High Solid Loading. Bioresour. Technol. 2016, 201, 15–26. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Wang, J.; Fan, Y.; Shi, W.; Liu, X.; Shun, Q. Simultaneous Saccharification and Co-Fermentation of Corn Stover Pretreated by H2O2 Oxidative Degradation for Ethanol Production. Energy 2019, 168, 946–952. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kondo, A. Consolidated Bioprocessing and Simultaneous Saccharification and Fermentation of Lignocellulose to Ethanol with Thermotolerant Yeast Strains. Process Biochem. 2012, 47, 1287–1294. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Singh, A.; Haldar, D.; Soam, S.; Chen, C.-W.; Tsai, M.-L.; Dong, C.-D. Consolidated Bioprocessing of Lignocellulosic Biomass: Technological Advances and Challenges. Bioresour. Technol. 2022, 354, 127153. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Beula Isabel, J.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Sivashanmugam, P.; Aminabhavi, T.M. Recent Advances in Consolidated Bioprocessing for Conversion of Lignocellulosic Biomass into Bioethanol—A Review. Chem. Eng. J. 2022, 453, 139783. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Liu, W.; Xu, H.; Cao, Y. Consolidated Bioprocess for Bioethanol Production from Lignocellulosic Biomass Using Clostridium Thermocellum DSM 1237. BioResources 2020, 15, 8355–8368. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Li, W.-C.; Zhu, J.-Q.; Liu, L.; Li, B.-Z.; Yuan, Y.-J. Process Analysis and Optimization of Simultaneous Saccharification and Co-Fermentation of Ethylenediamine-Pretreated Corn Stover for Ethanol Production. Biotechnol. Biofuels 2018, 11, 118. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, F.; Zhang, S.; Gong, Q.; Chen, G. Ethanol Production by Simultaneous Saccharification and Cofermentation of Pretreated Corn Stalk. J. Basic Microbiol. 2019, 59, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Madson, P.W.; Lococo, D.B. Recovery of Volatile Products from Dilute High-Fouling Process Streams. Appl. Biochem. Biotechnol. 2000, 84, 1049–1061. [Google Scholar] [CrossRef]
- Yang, R.-J.; Liu, C.-C.; Wang, Y.-N.; Hou, H.-H.; Fu, L.-M. A Comprehensive Review of Micro-Distillation Methods. Chem. Eng. J. 2017, 313, 1509–1520. [Google Scholar] [CrossRef]
- Kang, K.E.; Jeong, J.-S.; Kim, Y.; Min, J.; Moon, S.-K. Development and Economic Analysis of Bioethanol Production Facilities Using Lignocellulosic Biomass. J. Biosci. Bioeng. 2019, 128, 475–479. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Van Hooijdonk, G.; Faaij, A.P. Ethanol from Lignocellulosic Biomass: Techno-Economic Performance in Short-, Middle-and Long-Term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Fan, S.; Xiao, Z.; Liu, Y.; Liu, J.; Qiu, B.; Wang, Y. Bioethanol Production in Vacuum Membrane Distillation Bioreactor by Permeate Fractional Condensation and Mechanical Vapor Compression with Polytetrafluoroethylene (PTFE) Membrane. Bioresour. Technol. 2018, 268, 708–714. [Google Scholar] [CrossRef]
- Shirazi, M.A.; Kargari, A. Concentrating of Sugar Syrup in Bioethanol Production Using Sweeping Gas Membrane Distillation. Membranes 2019, 9, 59. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and Theoretical Modeling of Membrane Distillation: A Review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Loulergue, P.; Balannec, B.; Fouchard-Le Graët, L.; Cabrol, A.; Sayed, W.; Djelal, H.; Amrane, A.; Szymczyk, A. Air-Gap Membrane Distillation for the Separation of Bioethanol from Algal-Based Fermentation Broth. Sep. Purif. Technol. 2019, 213, 255–263. [Google Scholar] [CrossRef]
- Gaykawad, S.S.; Zha, Y.; Punt, P.J.; van Groenestijn, J.W.; van der Wielen, L.A.M.; Straathof, A.J.J. Pervaporation of Ethanol from Lignocellulosic Fermentation Broth. Bioresour. Technol. 2013, 129, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for Bioethanol Production by Pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- Trinh, L.T.P.; Lee, Y.-J.; Park, C.S.; Bae, H.-J. Aqueous Acidified Ionic Liquid Pretreatment for Bioethanol Production and Concentration of Produced Ethanol by Pervaporation. J. Ind. Eng. Chem. 2019, 69, 57–65. [Google Scholar] [CrossRef]
- Jain, A.; Dhabhai, R.; Dalai, A.K.; Chaurasia, S.P. Bioethanol Production in a Pervaporation Membrane Bioreactor. In Membrane Technology; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-10566-6. [Google Scholar]

| Country/Calendar Year | 2014 r | 2015 r | 2016 r | 2017 r | 2018 r | 2019 e | 2020 e | 2021 f |
|---|---|---|---|---|---|---|---|---|
| France | 1018 | 1039 | 987 | 1000 | 1138 | 1299 | 1049 | 1095 |
| Germany | 920 | 870 | 882 | 810 | 799 | 676 | 875 | 950 |
| Hungary | 456 | 591 | 633 | 633 | 645 | 689 | 639 | 640 |
| Netherlands | 519 | 563 | 443 | 532 | 563 | 570 | 538 | 570 |
| Spain | 454 | 494 | 328 | 377 | 522 | 547 | 487 | 480 |
| Belgium | 557 | 557 | 570 | 620 | 646 | 620 | 380 | 380 |
| Poland | 181 | 214 | 241 | 258 | 259 | 286 | 277 | 285 |
| Austria | 230 | 223 | 224 | 235 | 251 | 254 | 241 | 255 |
| United Kingdom | 329 | 538 | 658 | 684 | 443 | 190 | 127 | 190 |
| Total | 5190 | 5165 | 5159 | 5373 | 5497 | 5281 | 4747 | 5000 |
| Company | City | State | Feedstock | Production Capacity (MGY) | Under Construction (MGY) |
|---|---|---|---|---|---|
| NewEnergyBlue LLC | Mason City | IA | Cellulosic Biomass | - | 20 |
| Project LIBERTY | Emmetsburg | IA | Cellulosic Biomass | 25 | - |
| VERBIO North America Corp. | Nevada | IA | Corn/Cellulosic Biomass | - | 60 |
| Quad County Corn Processors | Galva | IA | Corn/Cellulosic Biomass | 38 | - |
| Ace Ethanol LLC | Stanley | WI | Corn/Cellulosic Biomass | 54 | - |
| POET Biorefining-Iowa Falls LLC | Iowa Falls | IA | Corn/Cellulosic Biomass | 115 | - |
| Louis Dreyfus Grand Junction LLC | Grand Junction | IA | Corn/Cellulosic Biomass | 125 | - |
| POET Biorefining-Shell Rock LLC | Shell Rock | IA | Corn/Cellulosic Biomass | 140 | - |
| PureField Ingredients LLC | Russell | KS | Corn/Sorghum/Cellul. Biomass | 55 | - |
| Pelican Acquisition LLC | Stockton | CA | Corn/Sorghum/Cellul. Biomass | 60 | - |
| ELEMENT LLC | Colwich | KS | Corn/Sorghum/Cellul. Biomass | 70 | - |
| LanzaTech Freedom Pines Fuels LLC | Soperton | GA | Industrial Off-Gases/Biomass/Biogas | - | 10 |
| Total | - | - | - | 682 | 90 |
| Country | Feedstock | Capacity (Million Litres Per Year) | Year of Opening |
|---|---|---|---|
| Finland | Sawdust | 10 | 2018 |
| Italy | Biomass | 28 | 2020 |
| Austria | Wood sugar | 30 | 2020 |
| Romania | Wheat straw | 65 | 2021 |
| Bulgaria | Corn stover | 50 | 2021 |
| Total | - | 183 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. https://doi.org/10.3390/molecules27248717
Broda M, Yelle DJ, Serwańska K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules. 2022; 27(24):8717. https://doi.org/10.3390/molecules27248717
Chicago/Turabian StyleBroda, Magdalena, Daniel J. Yelle, and Katarzyna Serwańska. 2022. "Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions" Molecules 27, no. 24: 8717. https://doi.org/10.3390/molecules27248717
APA StyleBroda, M., Yelle, D. J., & Serwańska, K. (2022). Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules, 27(24), 8717. https://doi.org/10.3390/molecules27248717