Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Growth Medium from Grass Clippings
2.2. Cultivation of Cupriavidus Necator pKR-hum
2.3. Carbon and Nitrogen Analysis
2.4. Quantification of α-Humulene
3. Results and Discussion
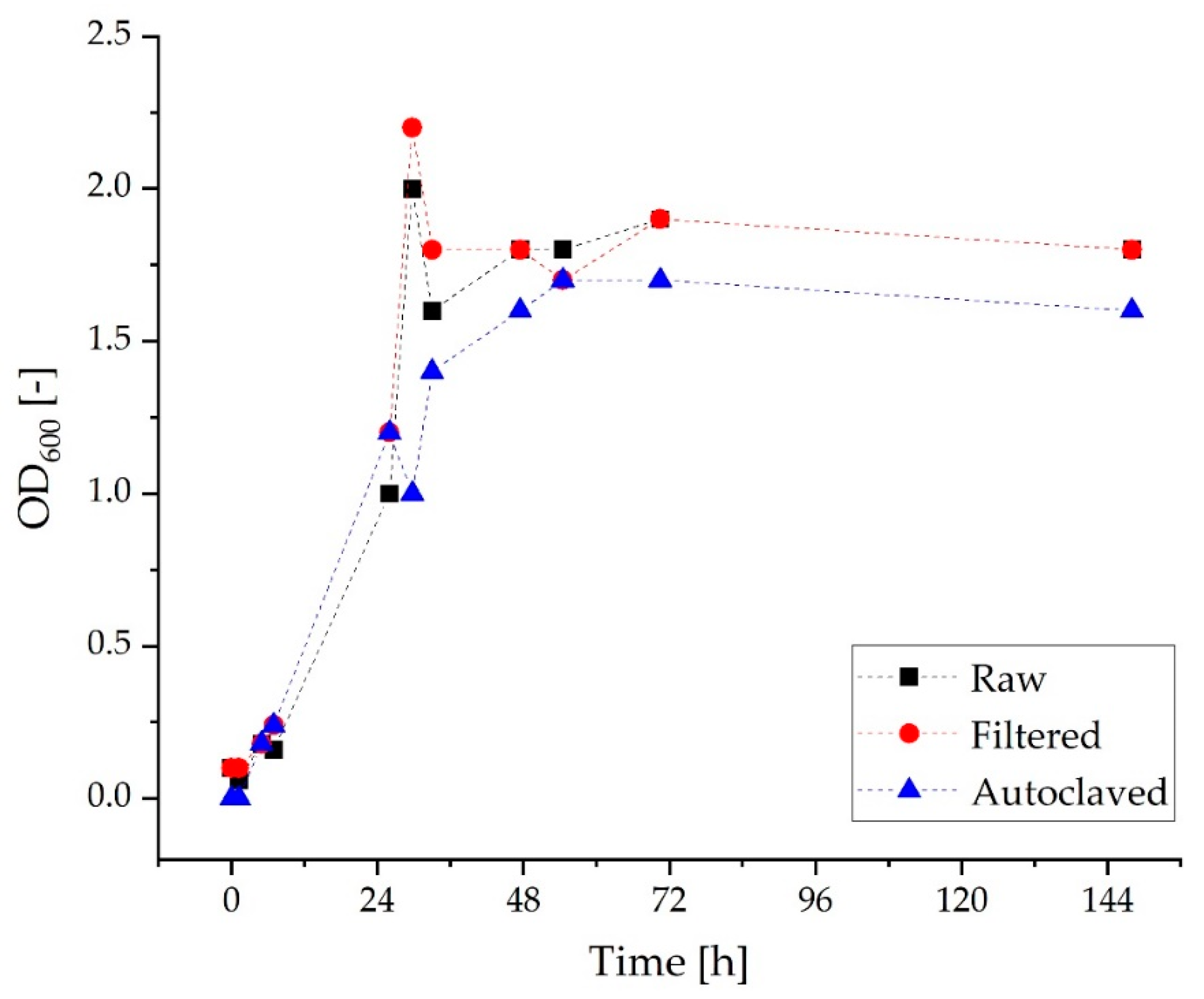
3.1. Screening Studies
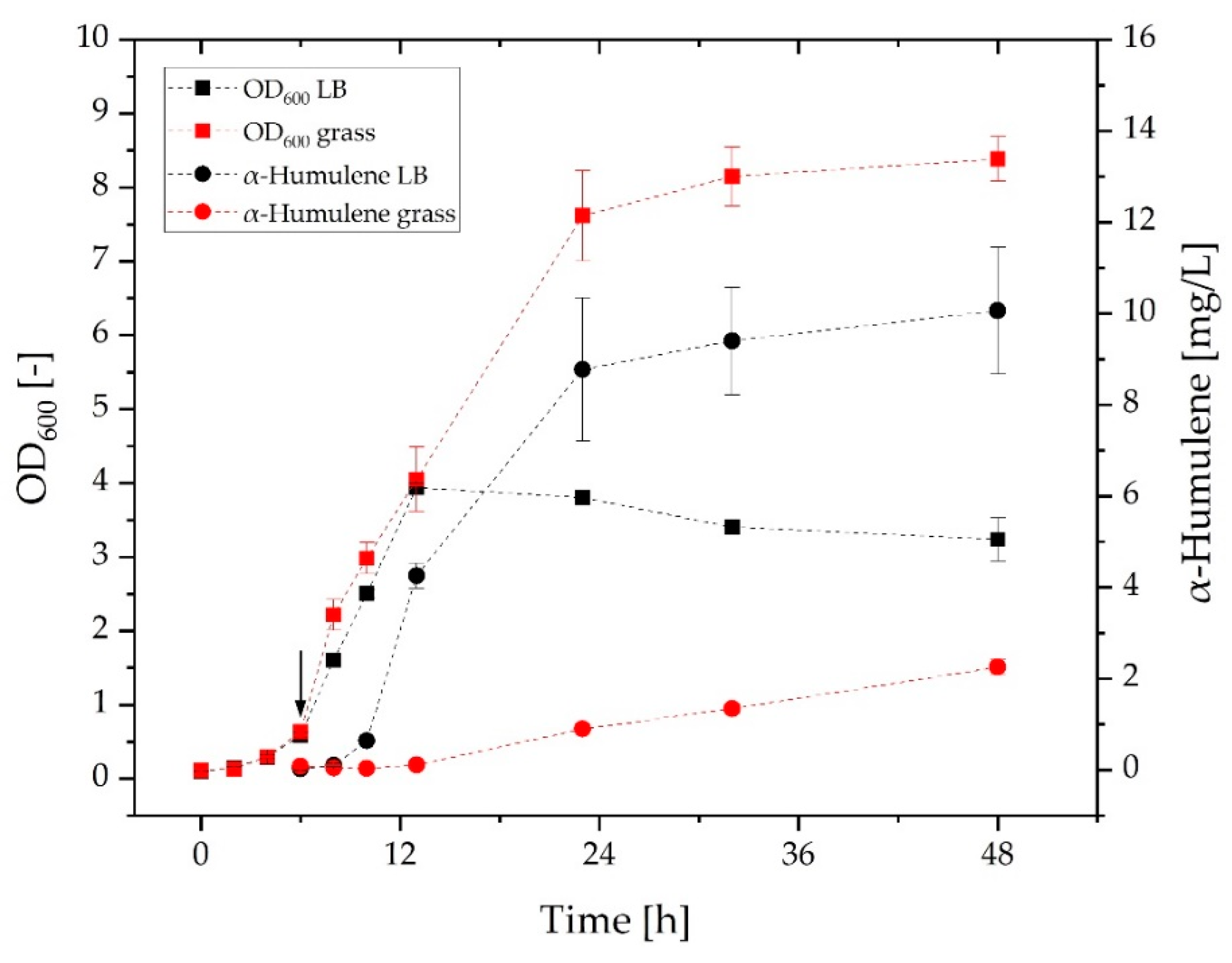
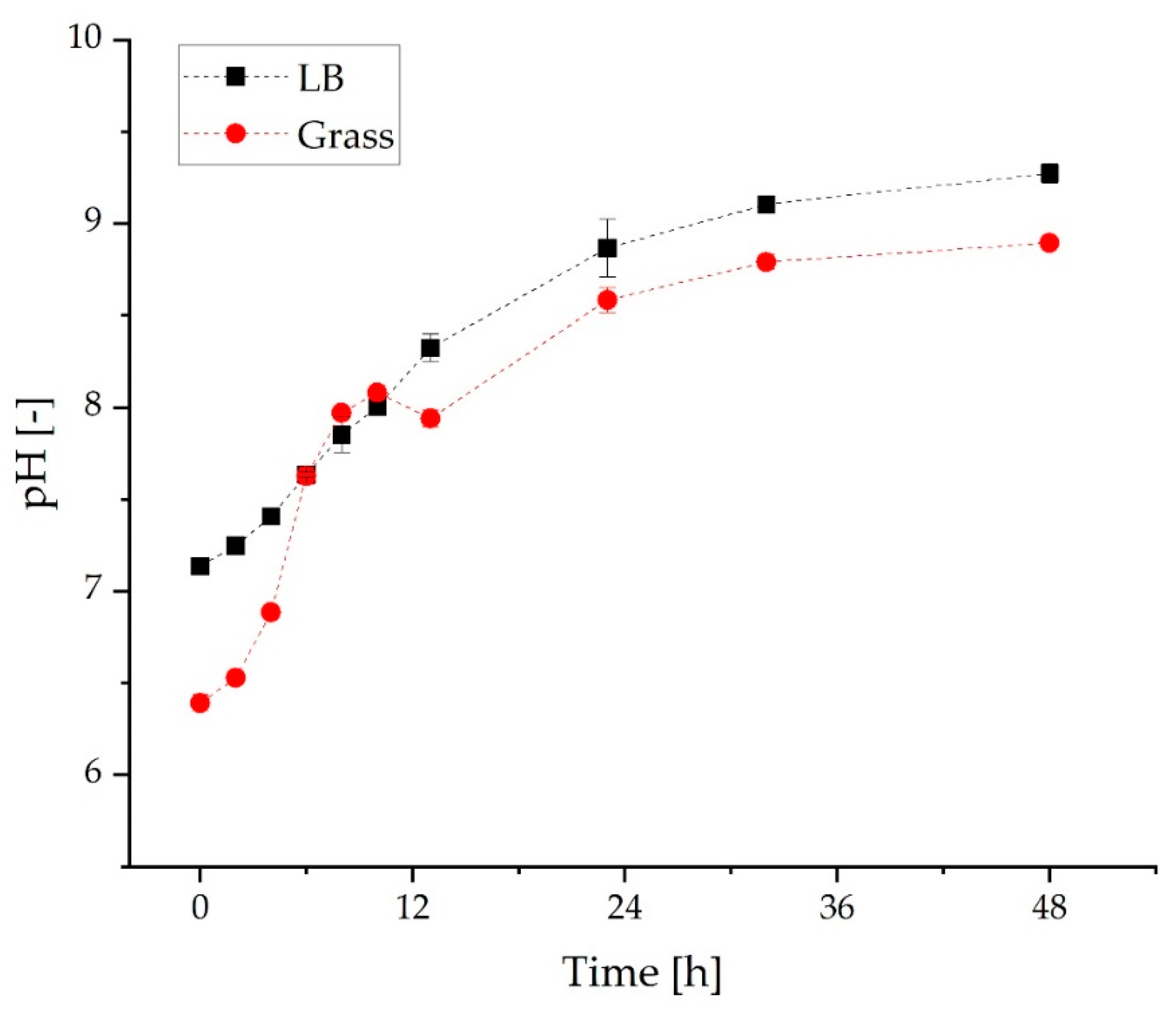
3.2. Comparison of Growth and α-Humulene Production in Grass Medium and LB Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Medick, J.; Teichmann, I.; Kemfert, C. Hydrothermal Carbonization (HTC) of Green Waste: An Environmental and Economic Assessment of HTC Coal in the Metropolitan Region of Berlin, Germany. SSRN J. 2017. DIW Berlin Discussion Paper No. 1690. [Google Scholar] [CrossRef]
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material utilization of green waste: A review on potential valorization methods. Bioresour. Bioprocess. 2021, 8, 1–26. [Google Scholar] [CrossRef]
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotechnol. 2016, 9, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Muñoz-Dorado, J.; de La Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, K.; Alvarado-Morales, M.; Kuglarz, M.; Angelidaki, I. Miscanthus straw as substrate for biosuccinic acid production: Focusing on pretreatment and downstream processing. Bioresour. Technol. 2019, 278, 82–91. [Google Scholar] [CrossRef]
- Kuglarz, M.; Rom, M. Influence of Carbon Dioxide and Nitrogen Source on Sustainable Production of Succinic Acid from Miscanthus Hydrolysates. IJESD 2019, 10, 362–367. [Google Scholar] [CrossRef]
- West, T.P. Xylitol production by Candida species grown on a grass hydrolysate. World J. Microbiol. Biotechnol. 2009, 25, 913–916. [Google Scholar] [CrossRef]
- Neeru, C.; Chandrajit, B.; Vidyasagar, J. Biological Production of Xylitol from Corn Husk and Switchgrass by Pichia stiptis. Res. J. Chem. Sci. 2013, 3, 58–64. [Google Scholar]
- Friedrich, C.G.; Friedrich, B.; Bowien, B. Formation of Enzymes of Autotrophic Metabolism During Heterotrophic Growth of Alcaligenes eutrophus. Microbiology 1981, 122, 69–78. [Google Scholar] [CrossRef]
- Makkar, N.S.; Casida, L.E. Cupriavidus necator gen. nov., sp. nov.; a Nonobligate Bacterial Predator of Bacteria in Soil. Int. J. Syst. Bacteriol. 1987, 37, 323–326. [Google Scholar] [CrossRef]
- Ng, K.-S.; Ooi, W.-Y.; Goh, L.-K.; Shenbagarathai, R.; Sudesh, K. Evaluation of jatropha oil to produce poly(3-hydroxybutyrate) by Cupriavidus necator H16. Polym. Degrad. Stab. 2010, 95, 1365–1369. [Google Scholar] [CrossRef]
- Batcha, A.F.M.; Prasad, D.M.R.; Khan, M.R.; Abdullah, H. Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator H16 from jatropha oil as carbon source. Bioprocess Biosyst. Eng. 2014, 37, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Mifune, J.; Nakamura, S.; Fukui, T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from vegetable oil. Polym. Degrad. Stab. 2010, 95, 1305–1312. [Google Scholar] [CrossRef]
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86. [Google Scholar] [CrossRef]
- Verlinden, R.A.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef]
- Martino, L.; Cruz, M.V.; Scoma, A.; Freitas, F.; Bertin, L.; Scandola, M.; Reis, M.A.M. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int. J. Biol. Macromol. 2014, 71, 117–123. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Alexandri, M.; Strati, A.; Gardeli, C.; Papanikolaou, S.; Komaitis, M.; Kookos, I.K.; Koutinas, A.A. Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly(3-hydroxybutyrate). Ind. Crops Prod. 2015, 71, 106–113. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Hafuka, A.; Sakaida, K.; Satoh, H.; Takahashi, M.; Watanabe, Y.; Okabe, S. Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresour. Technol. 2011, 102, 3551–3553. [Google Scholar] [CrossRef]
- Passanha, P.; Esteves, S.R.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J. Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour. Technol. 2013, 147, 345–352. [Google Scholar] [CrossRef]
- García, I.L.; López, J.A.; Dorado, M.P.; Kopsahelis, N.; Alexandri, M.; Papanikolaou, S.; Villar, M.A.; Koutinas, A.A. Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresour. Technol. 2013, 130, 16–22. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.-K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]
- Haas, C. Production of PHB from Chicory Roots—Comparison of Three Cupriavidus necator Strains. Chem. Biochem. Eng. Q. 2015, 29, 99–112. [Google Scholar] [CrossRef]
- Agustín Martinez, G.; Bertin, L.; Scoma, A.; Rebecchi, S.; Braunegg, G.; Fava, F. Production of polyhydroxyalkanoates from dephenolised and fermented olive mill wastewaters by employing a pure culture of Cupriavidus necator. Biochem. Eng. J. 2015, 97, 92–100. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N. Production of polyhydroxybutyrate from wheat bran hydrolysate using Ralstonia eutropha through microbial fermentation. J. Biotechnol. 2016, 237, 13–17. [Google Scholar] [CrossRef]
- Crépin, L.; Lombard, E.; Guillouet, S.E. Metabolic engineering of Cupriavidus necator for heterotrophic and autotrophic alka(e)ne production. Metab. Eng. 2016, 37, 92–101. [Google Scholar] [CrossRef]
- Marc, J.; Grousseau, E.; Lombard, E.; Sinskey, A.J.; Gorret, N.; Guillouet, S.E. Over expression of GroESL in Cupriavidus necator for heterotrophic and autotrophic isopropanol production. Metab. Eng. 2017, 42, 74–84. [Google Scholar] [CrossRef]
- Lu, J.; Brigham, C.J.; Gai, C.S.; Sinskey, A.J. Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2012, 96, 283–297. [Google Scholar] [CrossRef]
- Müller, J.; MacEachran, D.; Burd, H.; Sathitsuksanoh, N.; Bi, C.; Yeh, Y.-C.; Lee, T.S.; Hillson, N.J.; Chhabra, S.R.; Singer, S.W.; et al. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl. Environ. Microbiol. 2013, 79, 4433–4439. [Google Scholar] [CrossRef]
- Przybylski, D.; Rohwerder, T.; Dilßner, C.; Maskow, T.; Harms, H.; Müller, R.H. Exploiting mixtures of H2, CO2, and O2 for improved production of methacrylate precursor 2-hydroxyisobutyric acid by engineered Cupriavidus necator strains. Appl. Microbiol. Biotechnol. 2015, 99, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Lütte, S.; Pohlmann, A.; Zaychikov, E.; Schwartz, E.; Becher, J.R.; Heumann, H.; Friedrich, B. Autotrophic production of stable-isotope-labeled arginine in Ralstonia eutropha strain H16. Appl. Environ. Microbiol. 2012, 78, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Sydow, A.; Faust, S.; Huth, I.; Holtmann, D. CO2 to Terpenes: Autotrophic and Electroautotrophic α-Humulene Production with Cupriavidus necator. Angew. Chem. Int. Ed. Engl. 2018, 57, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Lin, F.-Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. Engl. 2012, 51, 1124–1137. [Google Scholar] [CrossRef]
- Milker, S.; Holtmann, D. First time β-farnesene production by the versatile bacterium Cupriavidus necator. Microb. Cell Fact. 2021, 20, 89. [Google Scholar] [CrossRef]
- Schwab, W.; Fuchs, C.; Huang, F.-C. Transformation of terpenes into fine chemicals. Eur. J. Lipid Sci. Technol. 2013, 115, 3–8. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Sonntag, F.; Kroner, C.; Lubuta, P.; Peyraud, R.; Horst, A.; Buchhaupt, M.; Schrader, J. Engineering Methylobacterium extorquens for de novo synthesis of the sesquiterpenoid α-humulene from methanol. Metab. Eng. 2015, 32, 82–94. [Google Scholar] [CrossRef]
- Alemdar, S.; König, J.C.; Hartwig, S.; Frister, T.; Scheper, T.; Beutel, S. Bioproduction of α-humulene in metabolically engineered Escherichia coli and application in zerumbone synthesis. Eng. Life Sci. 2017, 17, 900–907. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Kim, D.; Lee, E.Y. Unlocking the biosynthesis of sesquiterpenoids from methane via the methylerythritol phosphate pathway in methanotrophic bacteria, using α-humulene as a model compound. Metab. Eng. 2020, 61, 69–78. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by alpha-humulene as possible mechanism of action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Mendes de Lacerda Leite, G.; de Oliveira Barbosa, M.; Pereira Lopes, M.J.; de Araújo Delmondes, G.; Bezerra, D.S.; Araújo, I.M.; Carvalho de Alencar, C.D.; Melo Coutinho, H.D.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and toxicological activities of α-humulene and its isomers: A systematic review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- Hadri, A.; Rio, M.; Sanz, J.; Coloma, A.; Idaomar, M.; Ozonas, B.; Benedi, J.; Reus, M. Cytotoxic activity of alpha-humulene and trans-caryophyllene from Salvia officinalis in animal and human tumor cells. An. Real Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Jang, H.-I.; Rhee, K.-J.; Eom, Y.-B. Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can. J. Microbiol. 2020, 66, 389–399. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Hermann, C.; Horvat, P.; Martinz, J.; Neto, J.; Pereira, L.; Varila, P.; Braunegg, G. Biotechnological production of poly(3-hydroxybutyrate) with Wautersia eutropha by application of green grass juice and silage juice as additional complex substrates. Biocatal. Biotransform. 2005, 23, 329–337. [Google Scholar] [CrossRef]
- Boakye-Boaten, N.A.; Xiu, S.; Shahbazi, A.; Wang, L.; Li, R.; Schimmel, K. Uses of miscanthus press juice within a green biorefinery platform. Bioresour. Technol. 2016, 207, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.; Kiel, P. Integrated utilisation of green biomass in the green biorefinery. Ind. Crops Prod. 2000, 11, 129–137. [Google Scholar] [CrossRef]
- Leiß, S.; Venus, J.; Kamm, B. Fermentative Production of L-Lysine-L-lactate with Fractionated Press Juices from the Green Biorefinery. Chem. Eng. Technol. 2010, 33, 2102–2105. [Google Scholar] [CrossRef]
- Cerrone, F.; Davis, R.; Kenny, S.T.; Woods, T.; O’Donovan, A.; Gupta, V.K.; Tuohy, M.; Babu, R.P.; O’Kiely, P.; O’Connor, K. Use of a mannitol rich ensiled grass press juice (EGPJ) as a sole carbon source for polyhydroxyalkanoates (PHAs) production through high cell density cultivation. Bioresour. Technol. 2015, 191, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Schoenenwald, A.K.J.; Dörrstein, J.; Sterba, J.; Kahoun, D.; Fojtíková, P.; Vilímek, J.; Schieder, D.; Zollfrank, C.; Sieber, V. Biosynthesis of poly-3-hydroxybutyrate from grass silage by a two-stage fermentation process based on an integrated biorefinery concept. Bioresour. Technol. 2018, 269, 237–245. [Google Scholar] [CrossRef]
- Sydow, A.; Krieg, T.; Ulber, R.; Holtmann, D. Growth medium and electrolyte-How to combine the different requirements on the reaction solution in bioelectrochemical systems using Cupriavidus necator. Eng. Life Sci. 2017, 17, 781–791. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Sangkharak, K.; Paichid, N.; Yunu, T.; Klomklao, S.; Prasertsan, P. Utilisation of tuna condensate waste from the canning industry as a novel substrate for polyhydroxyalkanoate production. Biomass Conv. Bioref. 2021, 11, 2053–2064. [Google Scholar] [CrossRef]
- Vu, D.H.; Mahboubi, A.; Root, A.; Heinmaa, I.; Taherzadeh, M.J.; Åkesson, D. Thorough Investigation of the Effects of Cultivation Factors on Polyhydroalkanoates (PHAs) Production by Cupriavidus necator from Food Waste-Derived Volatile Fatty Acids. Fermentation 2022, 8, 605. [Google Scholar] [CrossRef]
- Zainab-L, I.; Uyama, H.; Li, C.; Shen, Y.; Sudesh, K. Production of Polyhydroxyalkanoates from Underutilized Plant Oils by Cupriavidus necator. Clean-Soil Air Water 2018, 46, 1700542. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from landscape management of grassland used for biogas production: Effects of harvest date and silage additives on feedstock quality and methane yield. Grass Forage Sci. 2014, 69, 549–566. [Google Scholar] [CrossRef]
- Milker, S.; Sydow, A.; Torres-Monroy, I.; Jach, G.; Faust, F.; Kranz, L.; Tkatschuk, L.; Holtmann, D. Gram-scale production of the sesquiterpene α-humulene with Cupriavidus necator. Biotechnol. Bioeng. 2021, 118, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Bokinsky, G.; Peralta-Yahya, P.P.; George, A.; Holmes, B.M.; Steen, E.J.; Dietrich, J.; Lee, T.S.; Tullman-Ercek, D.; Voigt, C.A.; Simmons, B.A.; et al. Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 19949–19954. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langsdorf, A.; Drommershausen, A.-L.; Volkmar, M.; Ulber, R.; Holtmann, D. Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium. Molecules 2022, 27, 8684. https://doi.org/10.3390/molecules27248684
Langsdorf A, Drommershausen A-L, Volkmar M, Ulber R, Holtmann D. Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium. Molecules. 2022; 27(24):8684. https://doi.org/10.3390/molecules27248684
Chicago/Turabian StyleLangsdorf, Alexander, Anna-Lena Drommershausen, Marianne Volkmar, Roland Ulber, and Dirk Holtmann. 2022. "Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium" Molecules 27, no. 24: 8684. https://doi.org/10.3390/molecules27248684
APA StyleLangsdorf, A., Drommershausen, A.-L., Volkmar, M., Ulber, R., & Holtmann, D. (2022). Fermentative α-Humulene Production from Homogenized Grass Clippings as a Growth Medium. Molecules, 27(24), 8684. https://doi.org/10.3390/molecules27248684







