Can the Fluxionality in Borospherene Influence the Confinement-Induced Bonding between Two Noble Gas Atoms?
Abstract
1. Introduction
2. Methods and Computational Details
3. Results and Discussion
3.1. Structure and Stability
3.2. Bonding
3.3. ADMP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Weiske, T.; Böhme, D.K.; Hrušák, J.; Krätschmer, W.; Schwarz, H. Endohedral cluster compounds: Inclusion of helium within C and C through collision experiments. Angew. Chem. Int. Ed. 1991, 30, 884–886. [Google Scholar] [CrossRef]
- Weiske, T.; Hrušák, J.; Böhme, D.K.; Schwarz, H. Formation of endohedral carbon-cluster noble-gas compounds with high-energy bimolecular reactions: C60Hen+ (n = 1, 2). Chem. Phys. Lett. 1991, 186, 459–462. [Google Scholar] [CrossRef]
- Weiske, T.; Wong, T.; Krätschmer, W.; Terlouw, J.K.; Schwarz, H. The neutralization of HeC in the gas phase: Compelling evidence for the existence of an endohedral structure for He@C60. Angew. Chem. Int. Ed. 1992, 31, 183–185. [Google Scholar] [CrossRef]
- Weiske, T.; Böhme, D.K.; Schwarz, H. Injection of helium atoms into doubly and triply charged C60 cations. J. Phys. Chem. 1991, 95, 8451–8452. [Google Scholar] [CrossRef]
- Ross, M.M.; Callahan, J.H. Formation and characterization of C60He+. J. Phys. Chem. 1991, 95, 5720–5723. [Google Scholar] [CrossRef]
- Weiske, T.; Hrušák, J.; Bohme, D.K.; Schwarz, H. Endohedral fullerene-noble gas clusters formed with high-energy bimolecular reactions of Cxn+ (x = 60, 70; n = 1, 2, 3). Helv. Chim. Acta 1992, 75, 79–89. [Google Scholar] [CrossRef]
- Wong, T.; Terlouw, J.K.; Weiske, T.; Schwarz, H. The neutralization—Reionization mass spectrum of C60+. Int. J. Mass. Spectrom. Ion Process. 1992, 113, R23–R29. [Google Scholar] [CrossRef]
- Mowrey, R.C.; Ross, M.M.; Callahan, J.H. Molecular dynamics simulations and experimental studies of the formation of endohedral complexes of buckminsterfullerene. J. Phys. Chem. 1992, 96, 4755–4761. [Google Scholar] [CrossRef]
- Wan, Z.; Christian, J.F.; Anderson, S.L. Ne++C60: Collision energy and impact parameter dependence for endohedral complex formation, fragmentation, and charge transfer. J. Chem. Phys. 1992, 96, 3344–3347. [Google Scholar] [CrossRef]
- Hrušák, J.; Böhme, D.K.; Weiske, T.; Schwarz, H. Ab initio MO calculation on the energy barrier for the penetration of a benzene ring by a helium atom. Model studies for the formation of endohedral He@C60+ complexes by high-energy bimolecular reactions. Chem. Phys. Lett. 1992, 193, 97–100. [Google Scholar] [CrossRef]
- Christian, J.F.; Wan, Z.; Anderson, S.L. Ne++C60 collisions: The dynamics of charge and energy transfer, fragmentation, and endohedral complex formation. J. Chem. Phys. 1993, 99, 3468–3479. [Google Scholar] [CrossRef]
- Kleiser, R.; Sprang, H.; Furrer, S.; Campbell, E.E.B. He capture by negatively charged Buckminsterfullerene. Zeitschrift für Physik D. At. Mol. Clust. 1993, 28, 89–90. [Google Scholar] [CrossRef]
- Saunders, M.; Jimenez-Vazquez, H.A.; Cross, R.J.; Poreda, R.J. Stable compounds of helium and neon: He@C60 and Ne@C60. Science 1993, 259, 1428–1430. [Google Scholar] [CrossRef]
- Saunders, M.; Jiménez-Vázquez, H.A.; James Cross, R.; Mroczkowski, S.; Gross, M.; Giblin, D.E.; Poreda, R.J. Incorporation of Helium, Neon, Argon, Krypton, and Xenon into Fullerenes Using High Pressure. J. Am. Chem. Soc. 1994, 116, 2193–2194. [Google Scholar] [CrossRef]
- Shimshi, R.; Cross, R.J.; Saunders, M. Beam implantation: A new method for preparing cage molecules containing atoms at high incorporation levels. J. Am. Chem. Soc. 1997, 119, 1163–1164. [Google Scholar] [CrossRef]
- Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 46, 6083–6094. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, Y.; Tanabe, F.; Murata, M.; Murata, Y.; Komatsu, K. Rational synthesis, enrichment, and 13C NMR spectra of endohedral C60 and C70 encapsulating a helium atom. Chem. Commun. 2010, 46, 4532–4534. [Google Scholar] [CrossRef]
- Liu, S.; Sun, S. Recent Progress in the Studies of Endohedral Metallofullerenes. J. Organomet. Chem. 2000, 599, 74–86. [Google Scholar] [CrossRef]
- Sternfeld, T.; Hoffman, R.E.; Saunders, M.; Cross, R.J.; Syamala, M.S.; Rabinovitz, M. Two Helium Atoms inside Fullerenes: Probing the Internal Magnetic Field in C606− and C706−. J. Am. Chem. Soc. 2002, 124, 8786–8787. [Google Scholar] [CrossRef]
- Krapp, A.; Frenking, G. Is This a Chemical Bond? A Theoretical Study of Ng2@C60 (Ng = He, Ne, Ar, Kr, Xe). Chem. Eur. J. 2007, 13, 8256–8270. [Google Scholar] [CrossRef]
- Straka, M.; Vaara, J. Density Functional Calculations Of 3 He Chemical Shift in Endohedral Helium Fullerenes: Neutral, Anionic, and Di-Helium Species. J. Phys. Chem. A 2006, 110, 12338–12341. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, R.B.; Scuseria, G.E. Noble Gas Endohedral Complexes of C60 Buckminsterfullerene. J. Phys. Chem. A 1998, 102, 3458. [Google Scholar] [CrossRef][Green Version]
- Yu Nikolaienko, T.; Kryachko, E.S.; Dolgonos, G.A. On the Existence of He-He Bond in the Endohedral Fullerene He2@C60. J. Comput. Chem. 2018, 39, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yu, S.; Wang, X.; He, Y.; Huang, W.; Yang, M. Dipole Polarizabilities of Noble Gas Endohedral Fullerenes. Chem. Phys. Lett. 2008, 456, 223–226. [Google Scholar] [CrossRef]
- Giblin, D.E.; Gross, M.L.; Saunders, M.; Jiménez-Vázquez, H.; Cross, R.J. Incorporation of helium into endohedral complexes of C60 and C70 containing noble-gas atoms: A tandem mass spectrometry study. J. Am. Chem. Soc. 1997, 119, 9883–9890. [Google Scholar] [CrossRef]
- Khong, A.; Jiménez-Vázquez, H.A.; Saunders, M.; Cross, R.J.; Laskin, J.; Peres, T.; Chava Lifshitz, R.S.; Amos, B.S. An NMR study of He2 inside C70. J. Am. Chem. Soc. 1998, 120, 6380–6383. [Google Scholar] [CrossRef]
- Laskin, J.; Peres, T.; Lifshitz, C.; Saunders, M.; Cross, R.J.; Khong, A. An artificial molecule of Ne2 inside C70. Chem. Phys. Lett. 1998, 285, 7–9. [Google Scholar] [CrossRef]
- Strenalyuk, T.; Haaland, A. Chemical Bonding in the Inclusion Complex of He in Adamantane (He@adam): The Origin of the Barrier to Dissociation. Chem. Eur. J. 2008, 14, 10223–10226. [Google Scholar] [CrossRef]
- Cross, R.J.; Saunders, M.; Prinzbach, H. Putting Helium inside Dodecahedrane. Org. Lett. 1999, 1, 1479–1481. [Google Scholar] [CrossRef]
- Jiménez-Vázquez, H.A.; Tamariz, J.; James Cross, R. Binding Energy in and Equilibrium Constant of Formation for the Dodecahedrane Compounds He@C20H20 and Ne@C20H20. J. Phys. Chem. A 2001, 105, 1315–1319. [Google Scholar] [CrossRef]
- Zou, W.; Liu, Y.; Liu, W.; Wang, T.; Boggs, J.E. He@Mo6Cl8F6: A Stable Complex of Helium. J. Phys. Chem. A 2010, 114, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Khatua, M.; Pan, S.; Chattaraj, P.K. Confinement Induced Binding of Noble Gas Atoms. J. Chem. Phys. 2014, 140, 164306. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guajardo, G.; Cabellos, J.L.; Díaz-Celaya, A.; Pan, S.; Islas, R.; Chattaraj, P.K.; Heine, T.; Merino, G. Dynamical Behavior of Borospherene: A Nanobubble. Sci. Rep. 2015, 5, 11287. [Google Scholar] [CrossRef]
- Pan, S.; Ghara, M.; Kar, S.; Zarate, X.; Merino, G.; Chattaraj, P.K. Noble Gas Encapsulated B40 Cage. Phys. Chem. Chem. Phys. 2018, 20, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Stankevich, L.V.; Chistyakov, A.L.; Gal’pern, E.G. Polyhedral Boron Nitride Molecules. Russ. Chem. Bull. 1993, 42, 1634–1636. [Google Scholar] [CrossRef]
- Xia, X.; Jelski, D.A.; Bowser, J.R.; George, T.F. MNDO Study of Boron-Nitrogen Analogues of Buckminsterfullerene. J. Am. Chem. Soc. 1992, 114, 6493–6496. [Google Scholar] [CrossRef]
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural Bond Orbital Analysis of Near-Hartree–Fock Water Dimer. J. Chem. Phys. 1998, 78, 4066. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural Bond Orbital Analysis Program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules a Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Morokuma, K. Why Do Molecules Interact? The Origin of Electron Donor-Acceptor Complexes, Hydrogen Bonding and Proton Affinity. Acc. Chem. Res. 1977, 10, 294–300. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the Calculation of Bonding Energies by the Hartree Fock Slater Method. Theor. Chim. Acta 1977, 46, 1–10. [Google Scholar] [CrossRef]
- von Hopffgarten, M.; Frenking, G. Energy Decomposition Analysis. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 43–62. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Millam, J.M.; Iyengar, S.S.; Voth, G.A.; Daniels, A.D.; Scuseria, G.E.; Frisch, M.J. Ab Initio Molecular Dynamics: Propagating the Density Matrix with Gaussian Orbitals. J. Chem. Phys. 2001, 114, 9758–9763. [Google Scholar] [CrossRef]
- Iyengar, S.S.; Schlegel, H.B.; Millam, J.M.; Voth, G.A.; Scuseria, G.E.; Frisch, M.J. Ab Initio Molecular Dynamics: Propagating the Density Matrix with Gaussian Orbitals. II. Generalizations Based on Mass-Weighting, Idempotency, Energy Conservation and Choice of Initial Conditions. J. Chem. Phys. 2001, 115, 10291–10302. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Iyengar, S.S.; Li, X.; Millam, J.M.; Voth, G.A.; Scuseria, G.E.; Frisch, M.J. Ab Initio Molecular Dynamics: Propagating the Density Matrix with Gaussian Orbitals. III. Comparison with Born–Oppenheimer Dynamics. J. Chem. Phys. 2002, 117, 8694–8704. [Google Scholar] [CrossRef]
- da Chai, J.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, K.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F.J.M.P. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF 2014.01; SCM: Amsterdam, The Netherlands, 2014. [Google Scholar]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. B.01; Gaussian Inc.: Wallingford CT, USA, 2016. [Google Scholar]
- Yin, D.; Yang, Y.; Yang, Y.; Fang, H. A Novel Fullerene-like B30N30 Structure: Stability and Electronic Property. Carbon 2016, 102, 273–278. [Google Scholar] [CrossRef]
- Khatua, M.; Pan, S.; Chattaraj, P.K. Movement of Ng2 Molecules Confined in a C60 Cage: An Ab Initio Molecular Dynamics Study. Chem. Phys. Lett. 2014, 610–611, 351–356. [Google Scholar] [CrossRef]
- Ogilvie, J.F.; Wang, F.Y.H. Potential-Energy Functions of Diatomic Molecules of the Noble Gases I. Like Nuclear Species. J. Mol. Struct. 1992, 273, 277–290. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Matta, C.F.; Hernández-Trujillo, J.; Tang, T.H.; Bader, R.F.W. Hydrogen–Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Haaland, A.; Shorokhov, D.J.; Tverdova, N.V. Topological Analysis of Electron Densities: Is the Presence of an Atomic Interaction Line in an Equilibrium Geometry a Sufficient Condition for the Existence of a Chemical Bond? Chem. Eur. J. 2004, 10, 4416–4421. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Fang, D.C. Properties of Atoms in Molecules: Caged Atoms and the Ehrenfest Force. J. Chem. Theory. Comput. 2005, 1, 403–414. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. Hydrogen–Hydrogen Bonding in Planar Biphenyl, Predicted by Atoms-In-Molecules Theory, Does Not Exist. Chem. Eur. J. 2006, 12, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Pauli Repulsions Exist Only in the Eye of the Beholder. Chem. Eur. J. 2006, 12, 2896–2901. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Solà, M.; Bickelhaupt, F.M. A Model of the Chemical Bond Must Be Rooted in Quantum Mechanics, Provide Insight, and Possess Predictive Power. Chem. Eur. J. 2006, 12, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density—Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Pauling, L. The Formulas of Antimonic Acid and the Antimonates. J. Am. Chem. Soc. 1933, 55, 1895–1900. [Google Scholar] [CrossRef]
- Ziółkowski, M.; Grabowski, S.J.; Leszczynski, J. Cooperativity in Hydrogen-Bonded Interactions: Ab Initio and “Atoms in Molecules” Analyses. J. Phys. Chem. A 2006, 110, 6514–6521. [Google Scholar] [CrossRef]
- Pal, R.; Chattaraj, P.K. Possible Effects of Fluxionality of a Cavitand on Its Catalytic Activity through Confinement. Phys. Chem. Chem. Phys. 2021, 23, 15817–15834. [Google Scholar] [CrossRef]
- Stone, A.J.; Wales, D.J. Theoretical Studies of Icosahedral C60 and Some Related Species. Chem. Phys. Lett. 1986, 128, 501–503. [Google Scholar] [CrossRef]

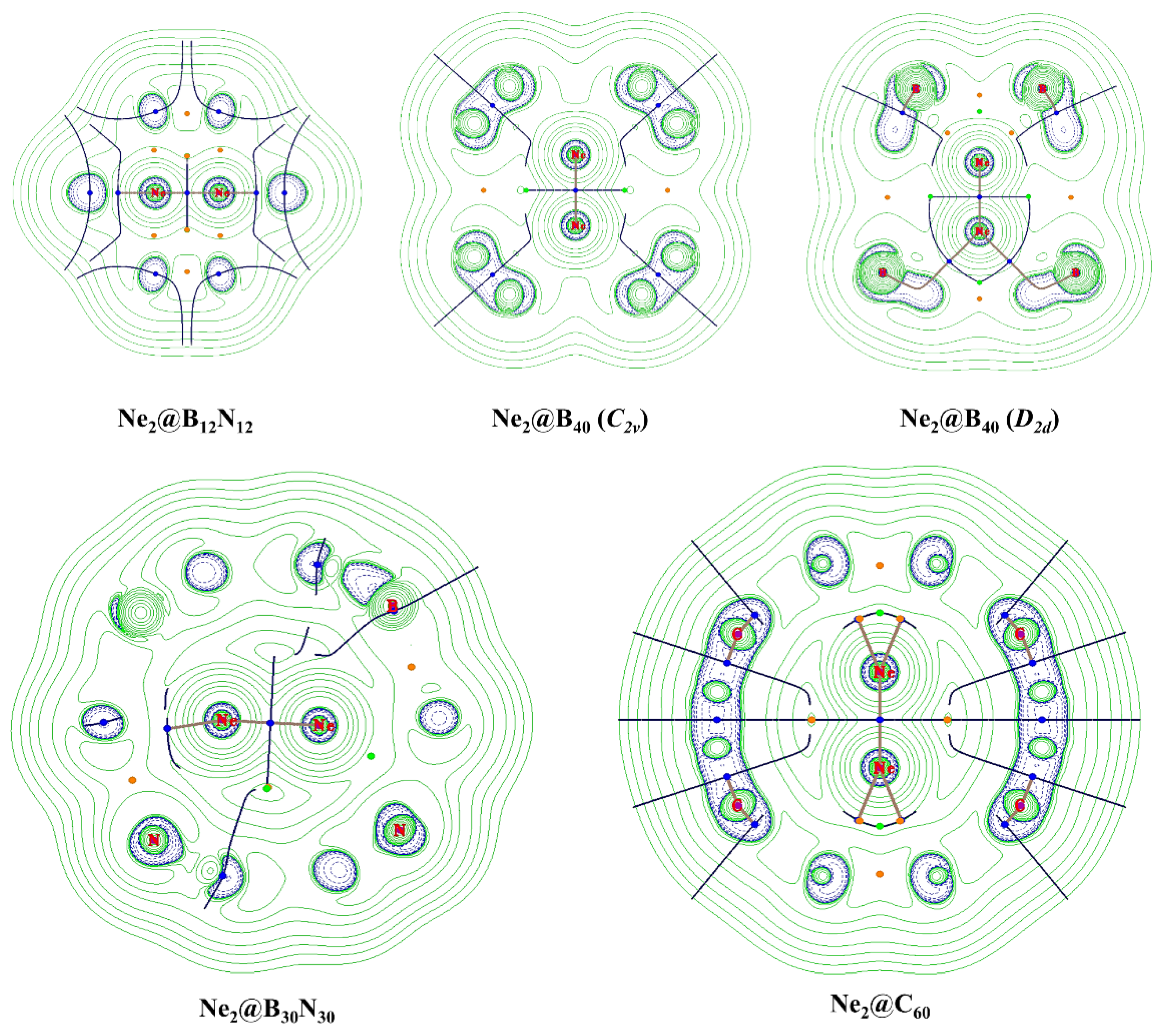
| Systems | Edist | Processes | D0 | D0BSSE | ΔS | ΔG |
|---|---|---|---|---|---|---|
| Ne2@B40 (C2v) | 6.395 | Ne2@B40 → Ne2 + B40 | −70.5 | −81.5 | 0.048 | −83.5 |
| Ne2@B40 → Ne + Ne@B40 | −69.3 | −80.3 | 0.030 | −77.4 | ||
| Ne2@B40 (D2d) | 8.106 | Ne2@B40 → Ne2 + B40 | −79.4 | −90.6 | 0.049 | −92.7 |
| Ne2@B40 → Ne + Ne@B40 | −78.1 | −89.4 | 0.031 | −86.5 | ||
| Ne2@B12N12 | 84.687 | Ne2@B12N12 → Ne2 + B12N12 | −449.8 | −462.2 | 0.047 | −462.7 |
| Ne2@B12N12 → Ne + Ne@B12N12 | −352.1 | −364.5 | 0.026 | −359.7 | ||
| Ne2@B30N30 | 0.604 | Ne2@B30N30 → Ne2 + B30N30 | 5.3 | −11.4 | 0.035 | −5.1 |
| Ne2@B30N30 → Ne + Ne@B30N30 | −6.7 | −23.5 | 0.030 | −15.1 | ||
| Ne2@C60 | 0.983 | Ne2@C60 → Ne2 + C60 | −6.9 | −19.4 | 0.040 | −31.0 |
| Ne2@C60 → Ne + Ne@C60 | −13.5 | −26.0 | 0.033 | −35.1 |
| Processes | Reactions | ER (kcal mol−1) |
|---|---|---|
| 2Ne → [Ne2] | 12.3 | |
| 14.7 | ||
| 54.8 | ||
| 3.7 | ||
| 5.8 | ||
| cage → [cage] | 6.4 | |
| 8.1 | ||
| 84.7 | ||
| 0.6 | ||
| 1.0 | ||
| [Ne2] + [cage] → [Ne2@cage] | [Ne2] + [B40] → [Ne2@B40] (C2v) | 50.3 |
| [Ne2] + [B40] → [Ne2@B40] (D2d) | 55.1 | |
| [Ne2] + [B12N12] → [Ne2@B12N12] | 312.8 | |
| [Ne2] + [B30N30] → [Ne2@B30N30] | −10.9 | |
| [Ne2] + [C60] → [Ne2@C60] | 11.5 | |
| 2Ne + cage → [Ne2@cage] | 2Ne + B40 → [Ne2@B40] (C2v) | 69.0 |
| 2Ne + B40 → [Ne2@B40] (D2d) | 77.8 | |
| 2Ne + B12N12 → [Ne2@B12N12] | 452.2 | |
| 2Ne + B30N30 → [Ne2@B30N30] | −6.6 | |
| 2Ne + C60 → [Ne2@C60] | 18.3 |
| Systems | qNe(1) | qNe(2) | WBINe-Ne | Total WBINe |
|---|---|---|---|---|
| Ne2@B12N12 | 0.137 | 0.137 | 0.0027 | 0.2736 |
| Ne2@B40 (C2v) | 0.047 | 0.047 | 0.0003 | 0.1015 |
| Ne2@B40 (D2d) | 0.046 | 0.046 | 0.0004 | 0.1001 |
| Ne2@C60 | 0.010 | 0.010 | 0.0251 | 0.0231 |
| Ne2@B30N30 | 0.041 | 0.042 | 0.0003 | 0.0837 |
| Systems | ρ | ∇2ρ(rc) | G(rc) | V(rc) | −G(rc)/V(rc) | H(rc) | G(rc)/ρ(rc) | ELF |
|---|---|---|---|---|---|---|---|---|
| Ne2@B12N12 | 0.130 | 1.255 | 0.313 | −0.312 | 1.002 | 0.001 | 2.412 | 0.085 |
| Ne2@B40 (C2v) | 0.053 | 0.446 | 0.103 | −0.095 | 1.089 | 0.008 | 1.933 | 0.042 |
| Ne2@B40 (D2d) | 0.059 | 0.497 | 0.116 | −0.108 | 1.074 | 0.008 | 1.974 | 0.046 |
| Ne2@C60 | 0.036 | 0.298 | 0.064 | −0.054 | 1.184 | 0.010 | 1.810 | 0.029 |
| Ne2@B30N30 | 0.029 | 0.234 | 0.049 | −0.040 | 1.237 | 0.009 | 1.716 | 0.024 |
| Systems | ΔEint | ΔEPauli | ΔEelstat | ΔEorb | ΔEdisp |
|---|---|---|---|---|---|
| Ne2@B12N12 | 326.0 | 666.0 | −249.4 (73.4) | −82.9 (24.4) | −7.7 (2.3) |
| Ne2@B40 (C2v) | 59.0 | 132.9 | −51.0 (69.0) | −11.7 (15.8) | −11.2 (15.2) |
| Ne2@B40 (D2d) | 64.0 | 142.4 | −54.5 (69.4) | −12.7 (16.2) | −11.3 (14.3) |
| Ne2@C60 | 7.6 | 35.0 | −14.2 (51.6) | −2.2 (8.0) | −11.1 (40.4) |
| Ne2@B30N30 | 4.2 | 28.1 | −11.6 (48.4) | −2.0 (8.2) | −10.4 (43.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, R.; Chattaraj, P.K. Can the Fluxionality in Borospherene Influence the Confinement-Induced Bonding between Two Noble Gas Atoms? Molecules 2022, 27, 8683. https://doi.org/10.3390/molecules27248683
Pal R, Chattaraj PK. Can the Fluxionality in Borospherene Influence the Confinement-Induced Bonding between Two Noble Gas Atoms? Molecules. 2022; 27(24):8683. https://doi.org/10.3390/molecules27248683
Chicago/Turabian StylePal, Ranita, and Pratim Kumar Chattaraj. 2022. "Can the Fluxionality in Borospherene Influence the Confinement-Induced Bonding between Two Noble Gas Atoms?" Molecules 27, no. 24: 8683. https://doi.org/10.3390/molecules27248683
APA StylePal, R., & Chattaraj, P. K. (2022). Can the Fluxionality in Borospherene Influence the Confinement-Induced Bonding between Two Noble Gas Atoms? Molecules, 27(24), 8683. https://doi.org/10.3390/molecules27248683






