Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease
Abstract
1. Introduction
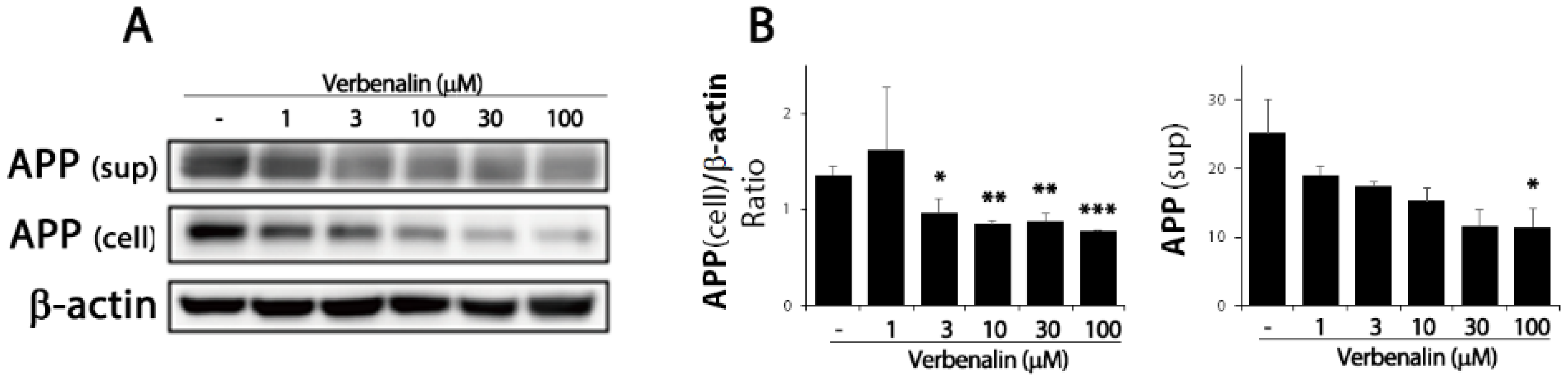
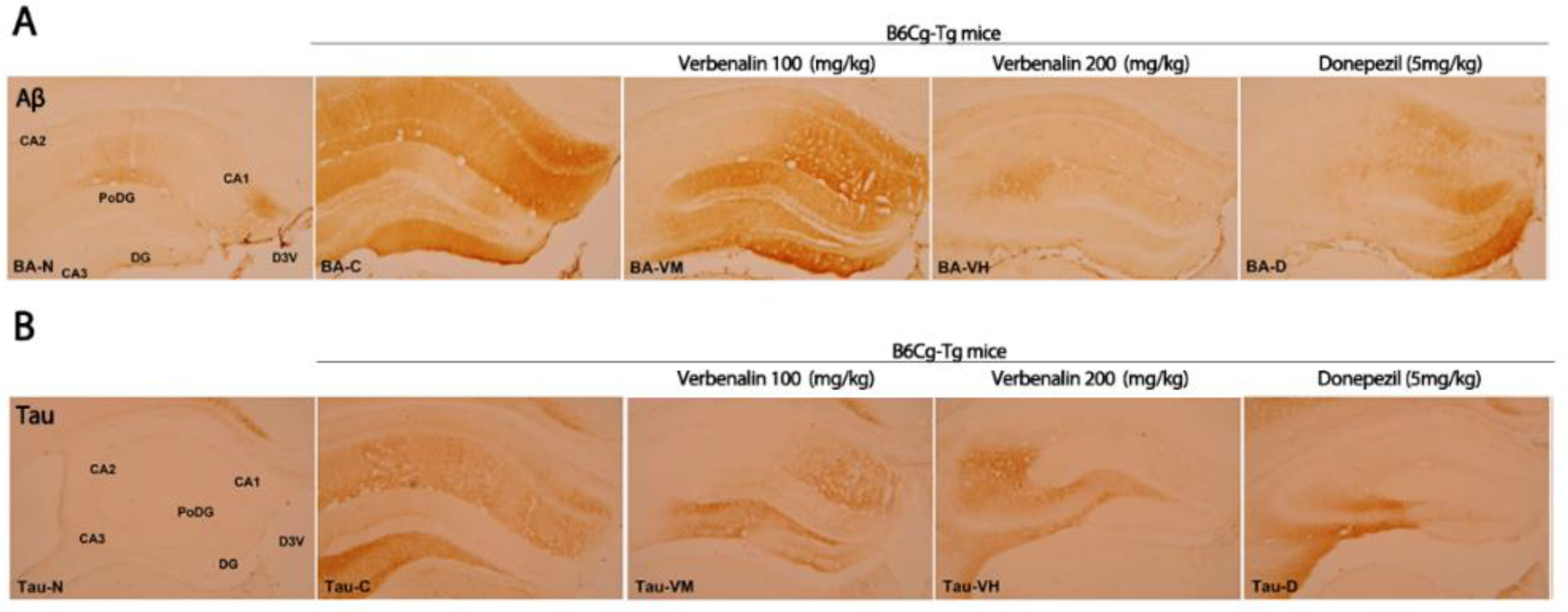
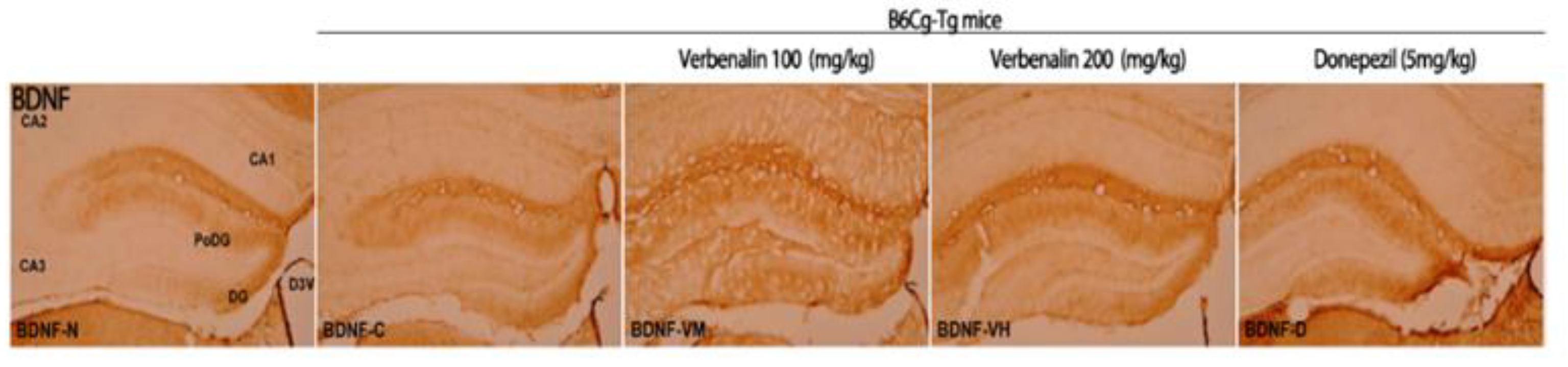
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Western Blotting
4.3. Supernatant Immunoblot Analysis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Animal Care
4.6. Immunohistochemistry (IHC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, X.; Wang, R. Experimental models of Alzheimer’s disease for deciphering the pathogenesis and therapeutic screening (Review). Int. J. Mol. Med. 2016, 37, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Wildburger, N.C.; Esparza, T.J.; LeDuc, R.D.; Fellers, R.T.; Thomas, P.M.; Cairns, N.J.; Kelleher, N.L.; Bateman, R.J.; Brody, D.L. Diversity of Amyloid-beta Proteoforms in the Alzheimer’s Disease Brain. Sci. Rep. 2017, 7, 9520. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 2022, 20, e3001694. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? Geroscience 2022, 44, 1879–1883. [Google Scholar] [CrossRef]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.; McDade, E.M.; Klunk, W.E.; Cohen, A.D.; Mathis, C.A. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014, 71, 562–568. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Miao, M.; Qiao, J.; Bai, M.; Li, R. The protective role of verbenalin in rat model of focal cerebral ischemia reperfusion. Saudi J. Biol. Sci. 2018, 25, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Kondo, S.; Nishimura, Y.; Tsukamoto, Y.; Huang, Z.-L.; Urade, Y. Hastatoside and verbenalin are sleep-promoting components in Verbena officinalis. Sleep Biol. Rhythm. 2009, 7, 211–217. [Google Scholar] [CrossRef]
- Bilia, A.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F. HPLC–DAD–ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Yu, M.S.; Yuen, W.H.; Chang, R.C. Novel neuroprotective effects of the aqueous extracts from Verbena officinalis Linn. Neuropharmacology 2006, 50, 641–650. [Google Scholar] [CrossRef]
- Ferdousi, F.; Kondo, S.; Sasaki, K.; Uchida, Y.; Ohkohchi, N.; Zheng, Y.W.; Isoda, H. Microarray analysis of verbenalin-treated human amniotic epithelial cells reveals therapeutic potential for Alzheimer’s Disease. Aging 2020, 12, 5516. [Google Scholar] [CrossRef]
- Mori, Y.; Tsuji, M.; Oguchi, T.; Kasuga, K.; Kimura, A.; Futamura, A.; Sugimoto, A.; Kasai, H.; Kuroda, T.; Yano, S.; et al. Serum BDNF as a Potential Biomarker of Alzheimer’s Disease: Verification Through Assessment of Serum, Cerebrospinal Fluid, and Medial Temporal Lobe Atrophy. Front. Neurol. 2021, 12, 653267. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Maruszak, A.; Thuret, S. Why looking at the whole hippocampus is not enough—A critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front. Cell. Neurosci. 2014, 8, 95. [Google Scholar] [CrossRef]
- Dalton, M.A.; Zeidman, P.; Barry, D.N.; Williams, E.; Maguire, E.A. Segmenting subregions of the human hippocampus on structural magnetic resonance image scans: An illustrated tutorial. Brain Neurosci. Adv. 2017, 1, 2398212817701448. [Google Scholar] [CrossRef]
- d‘Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.L.; Copani, A.; Rizzarelli, E. A promising connection between BDNF and Alzheimer’s disease. Aging 2018, 10, 1791–1792. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Luna, R.; Colin-Barenque, L. Amyloid beta: Multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell. Longev. 2014, 2014, 795375. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Pezzotti, M.; Orsini, F.; Sampaolese, B.; Mezzogori, D.; Grassi, C.; Giardina, B.; Misiti, F. Alzheimer’s amyloid beta-peptide (1-42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: An intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 2006, 342, 206–213. [Google Scholar] [CrossRef]
- Klöppel, S.; Abdulkadir, A.; Jack, C.R., Jr.; Koutsouleris, N.; Mourão-Miranda, J.; Vemuri, P. Diagnostic neuroimaging across diseases. Neuroimage 2012, 61, 457–463. [Google Scholar] [CrossRef]
- Klein-Koerkamp, Y.; Heckemann, R.; Ramdeen, K.; Moreaud, O.; Keignart, S.; Krainik, A.; Hammers, A.; Baciu, M.; Hot, P.; Alzheimer’s disease Neuroimaging Initiative. Amygdalar atrophy in early Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 239–252. [Google Scholar] [CrossRef]
- Ledig, C.; Schuh, A.; Guerrero, R.; Heckemann, R.A.; Rueckert, D. Structural brain imaging in Alzheimer’s disease and mild cognitive impairment: Biomarker analysis and shared morphometry database. Sci. Rep. 2018, 8, 11258. [Google Scholar] [CrossRef]
- La Joie, R.; Perrotin, A.; de La Sayette, V.; Egret, S.; Doeuvre, L.; Belliard, S.; Eustache, F.; Desgranges, B.; Chételat, G. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin. 2013, 3, 155–162. [Google Scholar] [CrossRef]

| C57BL/6 | B6Cg-Tg | ||||
|---|---|---|---|---|---|
| - | - | Verbenalin 100 mg/kg | Verbenalin 200 mg/kg | Donepezil 5 mg/kg | |
| CA 1 | + | +++ | +++ | - | + |
| CA 2 | + | ++ | - | - | - |
| CA 3 | - | + | + | + | - |
| PoDG | - | +++ | +++ | - | + |
| DG | - | +++ | +++ | - | +++ |
| Total | 2 | 12 | 4 | 1 | 5 |
| C57BL/6 | B6Cg-Tg | ||||
|---|---|---|---|---|---|
| - | - | Verbenalin 100 mg/kg | Verbenalin 200 mg/kg | Donepezil 5 mg/kg | |
| CA 1 | - | ++ | + | - | - |
| CA 2 | - | ++ | - | - | - |
| CA 3 | - | +++ | ++ | ++ | +++ |
| PoDG | - | ++ | - | - | + |
| DG | - | + | + | - | - |
| Total | 0 | 10 | 4 | 2 | 4 |
| C57BL/6 | B6Cg-Tg | ||||
|---|---|---|---|---|---|
| - | - | Verbenalin 100 mg/kg | Verbenalin 200 mg/kg | Donepezil 5 mg/kg | |
| CA 1 | - | - | + | + | - |
| CA 2 | - | - | - | - | - |
| CA 3 | - | - | + | + | - |
| PoDG | ++ | + | ++ | ++ | ++ |
| DG | ++ | + | ++ | ++ | ++ |
| Total | 4 | 2 | 6 | 6 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Kim, S.; Lee, C.; Park, J.; Yang, G.; Yook, T. Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease. Molecules 2022, 27, 8678. https://doi.org/10.3390/molecules27248678
Lim J, Kim S, Lee C, Park J, Yang G, Yook T. Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease. Molecules. 2022; 27(24):8678. https://doi.org/10.3390/molecules27248678
Chicago/Turabian StyleLim, Juhee, Seokhee Kim, Changhyun Lee, Jeongwoo Park, Gabsik Yang, and Taehan Yook. 2022. "Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease" Molecules 27, no. 24: 8678. https://doi.org/10.3390/molecules27248678
APA StyleLim, J., Kim, S., Lee, C., Park, J., Yang, G., & Yook, T. (2022). Verbenalin Reduces Amyloid-Beta Peptide Generation in Cellular and Animal Models of Alzheimer’s Disease. Molecules, 27(24), 8678. https://doi.org/10.3390/molecules27248678





