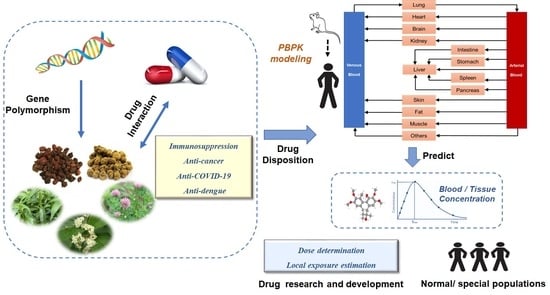
Utilization of Physiologically Based Pharmacokinetic Modeling in Pharmacokinetic Study of Natural Medicine: An Overview
Abstract
1. Introduction
2. Overview of Pharmacokinetic Study of Natural Medicine Components
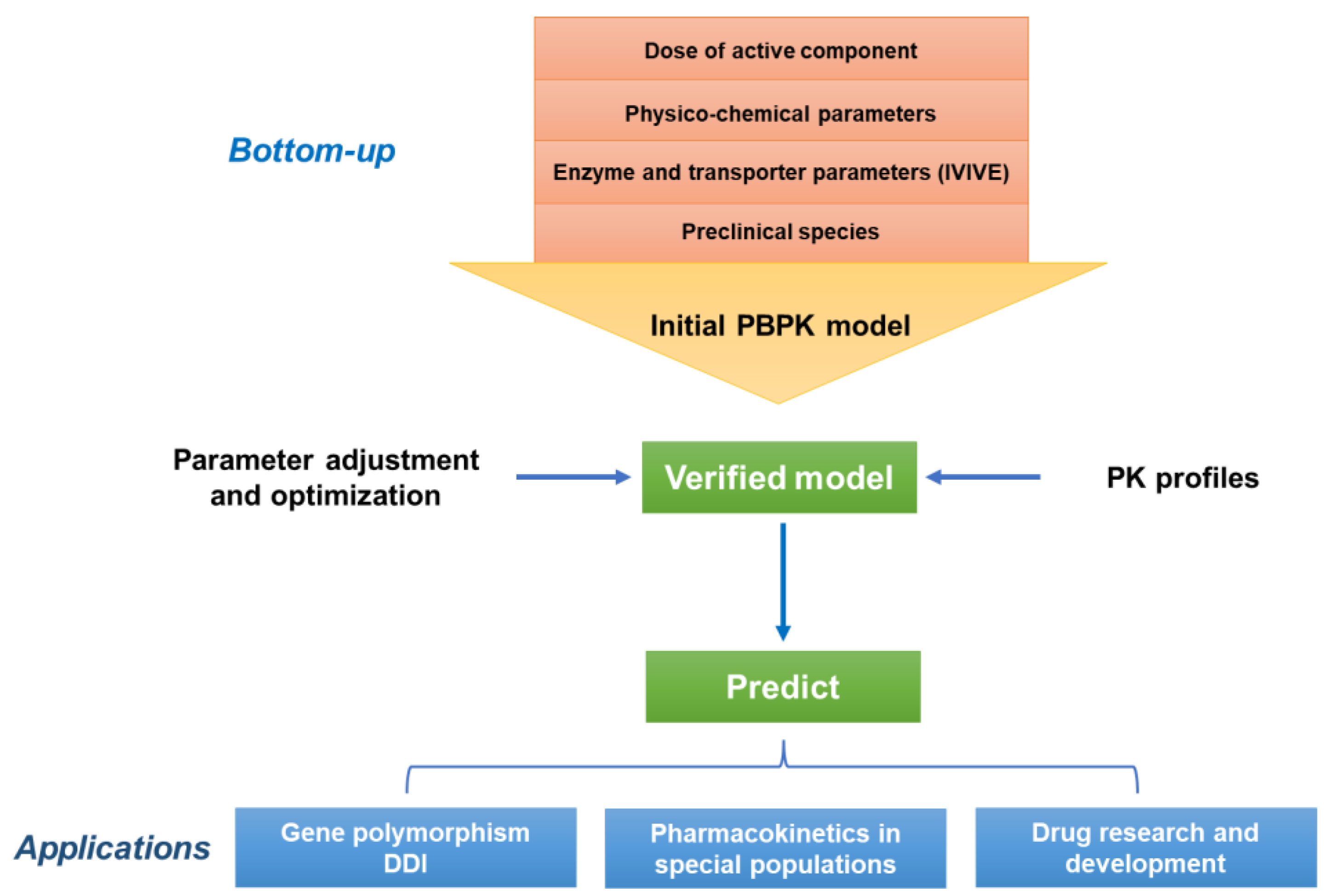
3. PBPK Modeling and Its Advantages in Pharmacokinetic Studies of Natural Medicine
3.1. Overview of the PBPK Modeling
3.2. Advantages of PBPK Modeling in Pharmacokinetic Studies of Natural Medicine
3.2.1. The Drug Exposure of Natural Medicine Components in Target Organs and Tissues Can Be Studied by PBPK Modeling
3.2.2. The PBPK Model Is an Effective Tool for Studying DDIs
3.2.3. The PBPK Model Has Unique Advantages for Solving the Problem of Safe Use of Natural Medicine in Special Populations
3.2.4. The PBPK Model Has More Potential in the Research and Development of New Drugs Based on Natural Medicine
4. Applications of the PBPK Model in the Study of Pharmacokinetics of Natural Medicine
4.1. The Enzyme-Mediated Pharmacokinetic Changes in Natural Drugs
4.1.1. The effects of Gene Polymorphism on the Pharmacokinetics of Natural Drugs
4.1.2. Study the Drug Interaction with Natural Medicine in Oncology Using PBPK Modeling
4.1.3. Study of the Drug Interactions with Natural Medicine in Immunosuppression by PBPK Modeling
4.2. Pharmacokinetics of Natural Medicine Active Components in Special Populations
4.2.1. THC/11-OH-THC PBPK Modeling in Pregnant Women
4.2.2. Evaluating the Safety of Tripterygium Wilfordii in Patients with Liver Injury by PBPK Modeling
4.3. PBPK Modeling Can Help Speed Up the Research and Development of New Natural Medicines and Add New Indications
4.3.1. Dosing Determination for Natural Medicine Using PBPK Modeling
4.3.2. Local Exposure Estimation of Natural Medicine Using PBPK Modeling
4.3.3. Extrapolation to Humans Pharmacokinetically and Pharmacodynamically Using PBPK Modeling
4.4. Other Applications of PBPK Modeling in the Pharmacokinetic Study of Natural Medicine
5. Discussion
6. Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, T.; Tan, Z.; Peng, J.; Guo, Y.; Chen, Y.; Zhou, H.; Ouyang, D. The pharmacogenetics of natural products: A pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 2019, 146, 104283. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Grimstein, M.; Yang, Y.; Zhang, X.; Grillo, J.; Huang, S.M.; Zineh, I.; Wang, Y. Physiologically Based Pharmacokinetic Modeling in Regulatory Science: An Update From the U.S. Food and Drug Administration’s Office of Clinical Pharmacology. J. Pharm. Sci. 2019, 108, 21–25. [Google Scholar] [CrossRef]
- Lesko, L.J. Perspective on model-informed drug development. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1127–1129. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Z.; Yan, X.; Zhu, M.; Fang, C.; Feng, M.; Huang, B.; Liu, J.; Li, Q.; Xiao, H. Effect of Chuanxiong Rhizoma on the absorption and clearance of four coumarins in Angelicae Dahuricae Radix. Acta Pharm. Sin. 2021, 56, 1804–1810. (In Chinese) [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Zou, Y.; Liu, S.; Wang, W.; Chen, Q.; Liu, G.; Liu, Y.; Lu, C.; Jia, J. Pharmacokinetics and Safety of Huperzine A in Chinese Elderly Subjects. Her. Med. 2021, 40, 764–767. (In Chinese) [Google Scholar]
- Gong, Z.; Lin, Z.; Han, L. Analysis of research status based on projects in pharmacokinetics of traditional Chinese medicine funded by National Natural Science Foundation of China. China J. Chin. Mater. Med. 2021, 46, 1010–1016. (In Chinese) [Google Scholar] [CrossRef]
- Ling, X.; Chen, Y.; Wang, P.; Li, C.; Li, X. Effects of flavonoids from Chinese traditional Medicine on other drugs mediated by metabolic enzymes and transporters. China Pharm. 2021, 32, 2287–2293. (In Chinese) [Google Scholar]
- Zhang, W.; Liu, X.; Wen, C.; Jiang, X.; Wang, L. Review of the in vitro inhibitory effects of traditional Chinese medicine and its components on CYP450 enzymes in liver microsomes. Acta Pharm. Sin. 2022, 57, 303–312. (In Chinese) [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Ma, W.; Zhen, X.; Lv, S.; Guo, Y. Advances in pharmacokinetics of Traditional Chinese medicine. J. Hebei Tradit. Chin. Med. Pharmacol. 2018, 33, 52–55. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C. Difficulty and hot-points on pharmacokinetics studies of traditional Chinese medicine. Acta Pharm. Sin. 2005, 40, 395–401. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Y.; Feng, J.; Sun, H.; Ning, L.; He, F. Survey on pharmacokinetics of Gastrodia elata Blume. Chin. J. Ethnomedicine Ethnopharmacy 2021, 30, 71–77. (In Chinese) [Google Scholar]
- Guo, S.; Wang, Q.; Lin, S.; Que, H.; Yang, J. Pharmacokinetics of triptolide as active metabolite of iso-tripthiocyanatolide in SD rats. Chin. J. Clin. Pharmacol. 2012, 28, 355–357. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Luo, H.; Huang, C.; Zhu, J. Determination of Serum Concentration of Triptolide in Patients with Rheumatoid Arthritis and lts Pharmacokinetics Study. Chin. J. Inf. Tradit. Chin. Med. 2014, 21, 85–87. (In Chinese) [Google Scholar]
- Jones, H.M.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.A.; Snoeys, J.; Upreti, V.V.; Zheng, M.; Hall, S.D. Physiologically based pharmacokinetic modeling in drug discovery and development: A pharmaceutical industry perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Xiang, X.; Quan, Y.; Jin, S. Application of Physiologically Based Pharmacokinetic Modeling in Pharmaceutics. Chin. J. Pharm. 2019, 50, 383–391. (In Chinese) [Google Scholar] [CrossRef]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef]
- Jamei, M. Recent Advances in Development and Application of Physiologically-Based Pharmacokinetic (PBPK) Models: A Transition from Academic Curiosity to Regulatory Acceptance. Curr. Pharmacol. Rep. 2016, 2, 161–169. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Wang, X. Study on physiologically based pharmacokinetic modelling and its application. Master’s Thesis, Zhejiang University, Hangzhou, China, 2006. (In Chinese). [Google Scholar]
- Jiang, X.L.; Samant, S.; Lewis, J.P.; Horenstein, R.B.; Shuldiner, A.R.; Yerges-Armstrong, L.M.; Peletier, L.A.; Lesko, L.J.; Schmidt, S. Development of a physiology-directed population pharmacokinetic and pharmacodynamic model for characterizing the impact of genetic and demographic factors on clopidogrel response in healthy adults. Eur. J. Pharm. Sci. 2016, 82, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; He, Q.; Zhu, X.; Zhu, M.; Wang, Y.; Wu, X.A.; Lv, Q.; Xiang, X. Application of physiologically based pharmacokinetic modelling for the prediction of drug-drug interactions involving anlotinib as a perpetrator of cytochrome P450 enzymes. Basic Clin. Pharmacol. Toxicol. 2022, 130, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Li, S.; Jin, S.; Lin, X.; Zhang, M.; Cai, W.; Jiao, Z.; Xiang, X. Investigating the interaction between nifedipine- and ritonavir-containing antiviral regimens: A physiologically based pharmacokinetic/pharmacodynamic analysis. Br. J. Clin. Pharmacol. 2021, 87, 2790–2806. [Google Scholar] [CrossRef]
- George, B.; Lumen, A.; Nguyen, C.; Wesley, B.; Wang, J.; Beitz, J.; Crentsil, V. Application of physiologically based pharmacokinetic modeling for sertraline dosing recommendations in pregnancy. NPJ Syst. Biol. Appl. 2020, 6, 36. [Google Scholar] [CrossRef]
- Wagner, C.; Zhao, P.; Pan, Y.; Hsu, V.; Grillo, J.; Huang, S.M.; Sinha, V. Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to Support Dose Selection: Report of an FDA Public Workshop on PBPK. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Offman, E.; Edginton, A.N. A PBPK workflow for first-in-human dose selection of a subcutaneously administered pegylated peptide. J. Pharmacokinet. Pharmacodyn. 2015, 42, 135–150. [Google Scholar] [CrossRef]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer Zu Schwabedissen, H.E. Clinical relevance of St. John’s wort drug interactions revisited. Br. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef]
- Brantley, S.J.; Argikar, A.A.; Lin, Y.S.; Nagar, S.; Paine, M.F. Herb-drug interactions: Challenges and opportunities for improved predictions. Drug Metab. Dispos. 2014, 42, 301–317. [Google Scholar] [CrossRef]
- Gufford, B.T.; Barr, J.T.; González-Pérez, V.; Layton, M.E.; White, J.R., Jr.; Oberlies, N.H.; Paine, M.F. Quantitative prediction and clinical evaluation of an unexplored herb-drug interaction mechanism in healthy volunteers. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 701–710. [Google Scholar] [CrossRef]
- Dong, J.; Olaleye, O.E.; Jiang, R.; Li, J.; Lu, C.; Du, F.; Xu, F.; Yang, J.; Wang, F.; Jia, W.; et al. Glycyrrhizin has a high likelihood to be a victim of drug-drug interactions mediated by hepatic organic anion-transporting polypeptide 1B1/1B3. Br. J. Pharmacol. 2018, 175, 3486–3503. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; Boddy, A.V.; McLachlan, A.J. Physiologically-Based Pharmacokinetic Predictions of the Effect of Curcumin on Metabolism of Imatinib and Bosutinib: In Vitro and In Vivo Disconnect. Pharm. Res. 2020, 37, 128. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; Boddy, A.V.; McLachlan, A.J. Potential for pharmacokinetic interactions between Schisandra sphenanthera and bosutinib, but not imatinib: In vitro metabolism study combined with a physiologically-based pharmacokinetic modelling approach. Br. J. Clin. Pharmacol. 2020, 86, 2080–2094. [Google Scholar] [CrossRef]
- Mazzari, A.L.; Prieto, J.M. Herbal medicines in Brazil: Pharmacokinetic profile and potential herb-drug interactions. Front. Pharmacol. 2014, 5, 162. [Google Scholar] [CrossRef][Green Version]
- Le Foll, B.; Tyndale, R.F. Cannabinoids: Friend or foe? Clin. Pharmacol. Ther. 2015, 97, 528–531. [Google Scholar] [CrossRef]
- Wolowich, W.R.; Greif, R.; Kleine-Brueggeney, M.; Bernhard, W.; Theiler, L. Minimal Physiologically Based Pharmacokinetic Model of Intravenously and Orally Administered Delta-9-Tetrahydrocannabinol in Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 691–711. [Google Scholar] [CrossRef]
- Wilsey, B.L.; Deutsch, R.; Samara, E.; Marcotte, T.D.; Barnes, A.J.; Huestis, M.A.; Le, D. A preliminary evaluation of the relationship of cannabinoid blood concentrations with the analgesic response to vaporized cannabis. J. Pain. Res. 2016, 9, 587–598. [Google Scholar] [CrossRef]
- Zakaria, Z.; Badhan, R.K.S. The impact of CYP2B6 polymorphisms on the interactions of efavirenz with lumefantrine: Implications for paediatric antimalarial therapy. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 119, 90–101. [Google Scholar] [CrossRef]
- Tanveer, A.; Hussain, K.; Tasneem, H.; Arif, I.; Rashid, M.; Abbas, N.; Shamim, R.; Shah, P.A.; Bukhari, N.I. Prediction of CYP-mediated silybin A-losartan pharmacokinetic interactions using physiological based pharmacokinetic modeling. J. Pharmacokinet. Pharmacodyn. 2022, 49, 311–323. [Google Scholar] [CrossRef]
- Pilla Reddy, V.; Jo, H.; Neuhoff, S. Food constituent- and herb-drug interactions in oncology: Influence of quantitative modelling on Drug labelling. Br. J. Clin. Pharmacol. 2021, 87, 3988–4000. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, T.; Chang, T.T.; Chiang, J.H.; Chang, C.M.; Hsieh, C.Y.; Yen, H.R. Adjunctive Chinese Herbal Medicine therapy improves survival of patients with chronic myeloid leukemia: A nationwide population-based cohort study. Cancer Med. 2016, 5, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Adiwidjaja, J.; Boddy, A.V.; McLachlan, A.J. Physiologically based pharmacokinetic model predictions of natural product-drug interactions between goldenseal, berberine, imatinib and bosutinib. Eur. J. Clin. Pharmacol. 2022, 78, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, F.; Gao, S.; Chen, L.; Feng, G.; Yin, J.; Chen, W. Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain. J. Ethnopharmacol. 2018, 210, 223–231. [Google Scholar] [CrossRef]
- Chen, L.; Ji, N.; Zhang, M.; Chen, W. The Influence of Wuzhi Capsule on the Pharmacokinetics of Cyclophosphamide. Recent Pat. Anticancer Drug Discov. 2021, 17, 195–203. [Google Scholar] [CrossRef]
- Zhang, H.; Bu, F.; Li, L.; Jiao, Z.; Ma, G.; Cai, W.; Zhuang, X.; Lin, H.S.; Shin, J.G.; Xiang, X. Prediction of Drug-Drug Interaction between Tacrolimus and Principal Ingredients of Wuzhi Capsule in Chinese Healthy Volunteers Using Physiologically-Based Pharmacokinetic Modelling. Basic Clin. Pharmacol. Toxicol. 2018, 122, 331–340. [Google Scholar] [CrossRef]
- Tang, J.T.; Andrews, L.M.; van Gelder, T.; Shi, Y.Y.; van Schaik, R.H.; Wang, L.L.; Hesselink, D.A. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: Recent developments and ethnic considerations. Expert Opin. Drug Metab. Toxicol. 2016, 12, 555–565. [Google Scholar] [CrossRef]
- He, Q.; Bu, F.; Zhang, H.; Wang, Q.; Tang, Z.; Yuan, J.; Lin, H.S.; Xiang, X. Investigation of the Impact of CYP3A5 Polymorphism on Drug-Drug Interaction between Tacrolimus and Schisantherin A/Schisandrin A Based on Physiologically-Based Pharmacokinetic Modeling. Pharmaceuticals 2021, 14, 198. [Google Scholar] [CrossRef]
- He, Q.; Bu, F.; Wang, Q.; Li, M.; Lin, J.; Tang, Z.; Mak, W.Y.; Zhuang, X.; Zhu, X.; Lin, H.S.; et al. Examination of the Impact of CYP3A4/5 on Drug-Drug Interaction between Schizandrol A/Schizandrol B and Tacrolimus (FK-506): A Physiologically Based Pharmacokinetic Modeling Approach. Int. J. Mol. Sci. 2022, 23, 4485. [Google Scholar] [CrossRef]
- Fan, J.; Chen, L.; Lu, X.; Li, M.; Zhu, L. The Pharmacokinetic Prediction of Cyclosporin A after Coadministration with Wuzhi Capsule. AAPS Pharm. Sci. Tech. 2019, 20, 247. [Google Scholar] [CrossRef]
- Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.; Knight, E.J.; Leemaqz, S.; Poston, L.; McCowan, L.; Kenny, L.; Myers, J.; Walker, J.J.; et al. The deleterious effects of cannabis during pregnancy on neonatal outcomes. Med. J. Aust. 2020, 212, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Stickrath, E. Marijuana Use in Pregnancy: An Updated Look at Marijuana Use and Its Impact on Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Patilea-Vrana, G.I.; Unadkat, J.D. Development and Verification of a Linked Δ (9)-THC/11-OH-THC Physiologically Based Pharmacokinetic Model in Healthy, Nonpregnant Population and Extrapolation to Pregnant Women. Drug Metab. Dispos. 2021, 49, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wu, T. Study on Metabolism and Disposition of TP in vivo and in vitro and its interaction with CYP enzyme. Master’s Thesis, Academy of Military Sciences, Beijing, China, 2018. (In Chinese). [Google Scholar]
- Tam, Y.K.; Lin, Y.-C.J.; Sloley, B.D.; Tseng, C.-Y. Development of a phytoestrogen product for the prevention or treatment of osteoporosis using red clover. US patent 11123389, 21 September 2021. [Google Scholar]
- Talapphetsakun, T.; Viyoch, J.; Waranuch, N.; Sermsappasuk, P. The Development of a Physiologically Based Pharmacokinetic (PBPK) Model of Andrographolide in Mice and Scaling it up to Rats, Dogs, and Humans. Curr. Drug Metab. 2022, 23, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Segura, N.A.; Gomez-Verjan, J.C. In Silico Screening of Natural Products Isolated from Mexican Herbal Medicines against COVID-19. Biomolecules 2021, 11, 216. [Google Scholar] [CrossRef]
- Ya, K.; Methaneethorn, J.; Tran, Q.B.; Trakulsrichai, S.; Wananukul, W.; Lohitnavy, M. Development of a Physiologically Based Pharmacokinetic Model of Mitragynine, Psychoactive Alkaloid in Kratom (Mitragyna Speciosa Korth.), In Rats and Humans. J. Psychoact. Drugs 2021, 53, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, K.; Liu, F.; Li, Y.; Zhong, Z.; Hong, S.; Liu, X.; Liu, L. Predicting Antitumor Effect of Deoxypodophyllotoxin in NCI-H460 Tumor-Bearing Mice on the Basis of In Vitro Pharmacodynamics and a Physiologically Based Pharmacokinetic-Pharmacodynamic Model. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 897–907. [Google Scholar] [CrossRef]
- Rox, K.; Heyner, M.; Krull, J.; Harmrolfs, K.; Rinne, V.; Hokkanen, J.; Perez Vilaro, G.; Díez, J.; Müller, R.; Kröger, A.; et al. Physiologically Based Pharmacokinetic/Pharmacodynamic Model for the Treatment of Dengue Infections Applied to the Broad Spectrum Antiviral Soraphen A. ACS Pharmacol. Transl. Sci. 2021, 4, 1499–1513. [Google Scholar] [CrossRef]
- Martins, F.S.; Sy, S.K.B.; Fonseca, M.J.V.; de Freitas, O. Pharmacokinetics, Pharmacodynamics and Dermal Distribution of 5-Methoxypsoralen Based on a Physiologically Based Pharmacokinetic Model to Support Phytotherapy Using Brosimum gaudichaudii. Planta Med. 2020, 86, 276–283. [Google Scholar] [CrossRef]
- Han, D.G.; Seo, S.W.; Choi, E.; Kim, M.S.; Yoo, J.W.; Jung, Y.; Yoon, I.S. Impact of route-dependent phase-II gut metabolism and enterohepatic circulation on the bioavailability and systemic disposition of resveratrol in rats and humans: A comprehensive whole body physiologically-based pharmacokinetic modeling. Biomed. Pharmacother. 2022, 151, 113141. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Bian, X.; Yao, L.; Li, M.; Lou, Y.R.; Yuan, J.; Lin, H.S.; Liu, L.; Han, B.; et al. Prediction of oral hepatotoxic dose of natural products derived from traditional Chinese medicines based on SVM classifier and PBPK modeling. Arch. Toxicol. 2021, 95, 1683–1701. [Google Scholar] [CrossRef]
- Yanagi, M.; Kamiya, Y.; Murayama, N.; Banju, K.; Shimizu, M.; Yamazaki, H. Metabolic profiles for the pyrrolizidine alkaloid neopetasitenine and its metabolite petasitenine in humans extrapolated from rat in vivo and in vitro data sets using a simplified physiologically based pharmacokinetic model. J. Toxicol. Sci. 2021, 46, 391–399. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Shi, X.; Wen, Y.; Yang, L.; Dong, L. Applicability analysis and evaluation of aglycones in single-pass intestinal perfusion technique based on PBPK model. China J. Chin. Mater. Med. 2019, 44, 3645–3652. (In Chinese) [Google Scholar] [CrossRef]
- Piersma, A.H.; Ezendam, J.; Luijten, M.; Muller, J.J.; Rorije, E.; van der Ven, L.T.; van Benthem, J. A critical appraisal of the process of regulatory implementation of novel in vivo and in vitro methods for chemical hazard and risk assessment. Crit. Rev. Toxicol. 2014, 44, 876–894. [Google Scholar] [CrossRef]
- Yuan, Y.; He, Q.; Zhang, S.; Li, M.; Tang, Z.; Zhu, X.; Jiao, Z.; Cai, W.; Xiang, X. Application of Physiologically Based Pharmacokinetic Modeling in Preclinical Studies: A Feasible Strategy to Practice the Principles of 3Rs. Front. Pharmacol. 2022, 13, 895556. [Google Scholar] [CrossRef]
- Hess, H.M.; Miller, R.K. 2.19—Herbs during pregnancy. In Drugs During Pregnancy and Lactation, 3rd ed.; Schaefer, C., Peters, P., Miller, R.K., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 511–525. [Google Scholar]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six—Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: San Diego, CA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
| Applications | Examples | Regimen Adjustment | References | ||
|---|---|---|---|---|---|
| The enzyme-mediated pharmacokinetic changes in natural drugs | The effect of gene polymorphism on the pharmacokinetics of natural drugs | The special pharmacokinetics of THC in individuals homozygous for CYP2C9*3 | NA | [36] | |
| The impact of CYP2B6 polymorphisms on the interactions between efavirenz and lu-mefantrine | Artemether and lumefantrine regi-men should be ex-tended from 3 days to 7 days in *6/*6 genotype groups | [38] | |||
| The drug interaction with natural medicine in oncology | Perpetrator drugs: natural medicine (active components) | Victim drug: chemical drugs | Regimen adjustment | References | |
| Grapefruit juice (bergamottin); Turmeric (curcumin); St. John’s grass (hyperforin) | Acalabrutinib; Osimertinib; Olaparib | NA | [40] | ||
| Turmeric (curcumin) | Imatinib; Bosutinib | NA | [32] | ||
| Schisandra sphenanthera (SIA, STA and schisandrol B) | Imatinib; Bosutinib | Bosutinib should be reduced from 400 to 150–200 mg s after coadministration with Schisandra lignans | [33] | ||
| Goldenseal; Berberine | Imatinib; Bosutinib | NA | [44] | ||
| Wuzhi Capsule (SIA and STA) | Cyclophosphamide | NA | [46] | ||
| The drug interaction with natural medicine in transplant | Wuzhi Capsule (SIA and STA) | Tacrolimus | NA | [49] | |
| Wuzhi Capsule (SZA and SZB) | Tacrolimus | NA | [50] | ||
| Wuzhi Capsule (SIA and STA) | Cyclosporine A | NA | [51] | ||
| Pharmacokinetics of natural medicine active components in special populations | Natural medicine (active components/metabolites) | Special populations | Regimen recommendation | References | |
| Cannabis (THC and 11-OH-THC) | Pregnant women | NA | [54] | ||
| Tripterygium wilfordii (triptolide) | Patients with liver injury | NA | [55] | ||
| Speed up the research and development of new drugs | Dosing determination for natural medicine using PBPK modeling | Research and development of a phytoestrogen product for the prevention or treatment of osteoporosis using red clover | 5–200 mg daily dosage of total isoflavone | [56] | |
| Predict the concentration of andrographolide in the lungs to initially evaluate the potential efficacy of the proposed COVID-19 regimen | 200 mg of andrographolide orally q8h | [57] | |||
| Local exposure estimation of natural medicine | In-silico screening of natural medicine isolated from Mexican herbal medicines against COVID-19 | Cichoriin IV at 100 mg/kg | [58] | ||
| Predict the blood and brain concentration–time curves of mitragynine in rats and humans | NA | [59] | |||
| Predict the PK of deoxypodophyllotoxin in mice to accelerate the screening of anticancer drug | NA | [60] | |||
| Extrapolation to humans pharmacokinetically | The treatment of dengue infections applied to Soraphen A | NA | [61] | ||
| PK and PD assessment of 5-methoxypsoralen in humans to support psoralen and ultraviolet type A therapy | Supported a once-every-two-day regimen for optimal melanin production | [62] | |||
| Other applications of PBPK modeling in the pharmacokinetic study of natural medicine | The effects of intestinal metabolism and enterohepatic circulation on bioavailability and systemic disposal of resveratrol in rats and humans | NA | [63] | ||
| Prediction of oral hepatotoxic dose of natural products derived from TCM based on support vector machine classifier and PBPK modeling | regimen of oxymatrine estimated by this method (367 mg thrice a day) was close to the clinically recommended regimen (200–300 mg thrice a day) | [64] | |||
| Exposure, toxicity and risk assessment of Pyrrolizidine alkaloids in food and phyto-medicine | NA | [65] | |||
| Study the permeability of aglycones in TCM and predict human absorption | NA | [66] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; He, Q.; Yao, L.; Li, M.; Lin, J.; Tang, Z.; Zhu, X.; Xiang, X. Utilization of Physiologically Based Pharmacokinetic Modeling in Pharmacokinetic Study of Natural Medicine: An Overview. Molecules 2022, 27, 8670. https://doi.org/10.3390/molecules27248670
Jia Q, He Q, Yao L, Li M, Lin J, Tang Z, Zhu X, Xiang X. Utilization of Physiologically Based Pharmacokinetic Modeling in Pharmacokinetic Study of Natural Medicine: An Overview. Molecules. 2022; 27(24):8670. https://doi.org/10.3390/molecules27248670
Chicago/Turabian StyleJia, Qiuyu, Qingfeng He, Li Yao, Min Li, Jiaying Lin, Zhijia Tang, Xiao Zhu, and Xiaoqiang Xiang. 2022. "Utilization of Physiologically Based Pharmacokinetic Modeling in Pharmacokinetic Study of Natural Medicine: An Overview" Molecules 27, no. 24: 8670. https://doi.org/10.3390/molecules27248670
APA StyleJia, Q., He, Q., Yao, L., Li, M., Lin, J., Tang, Z., Zhu, X., & Xiang, X. (2022). Utilization of Physiologically Based Pharmacokinetic Modeling in Pharmacokinetic Study of Natural Medicine: An Overview. Molecules, 27(24), 8670. https://doi.org/10.3390/molecules27248670