Reinvestigating the Preferential Enrichment of DL-Arginine Fumarate: New Thoughts on the Mechanism of This Far from Equilibrium Crystallization Phenomenon
Abstract
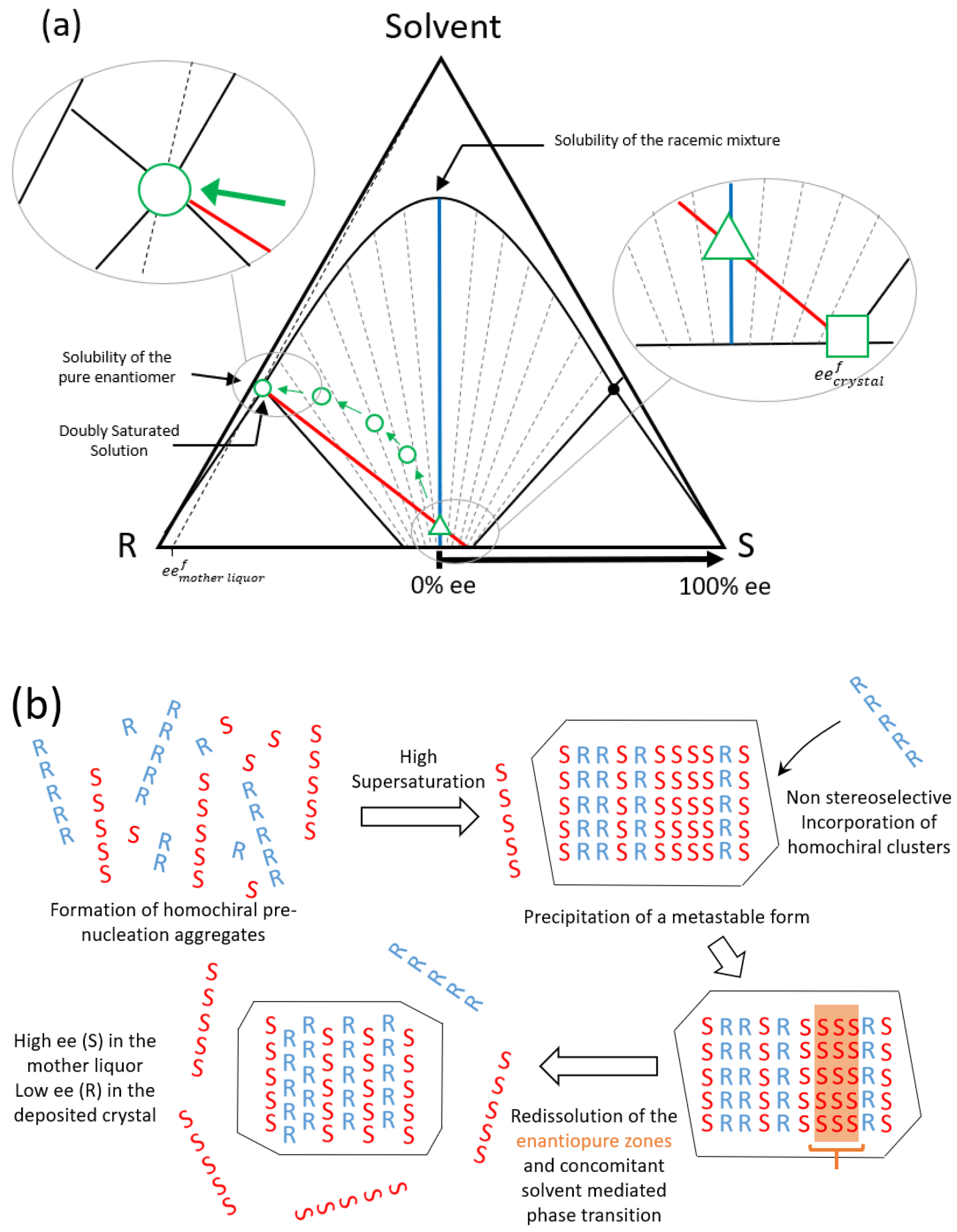
1. Introduction
2. Results
2.1. Performance of the Standard Protocol for the PE of Arginine Fumarate
- Concerning stirring, if the use of magnetic stirring systematically drove the system to equilibrium (i.e., crystal in equilibrium with the mother liquor at ca. 70–80%ee—red equilibrium line in Figure 2) it was observed that the use of a rocking plate can improve slightly the speed of the process (see Table S1 in Supplementary Materials) but may also stochastically induce failure. This illustrates the out-of-equilibrium nature of PE and that the process should be performed under stagnant conditions.
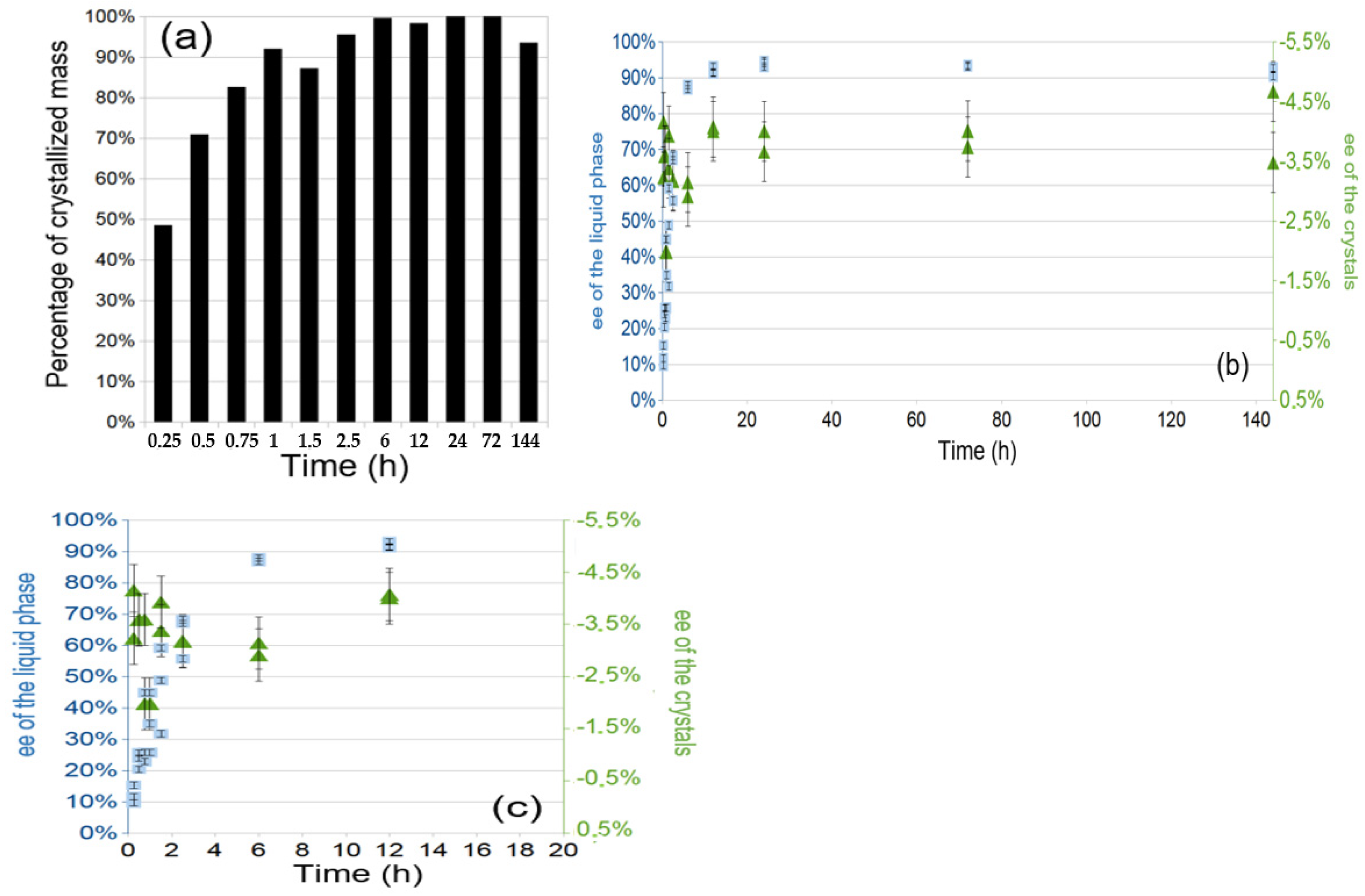
- To assess the kinetic stability of the final state of a typical PE experiment, the process duration was extended up to 1 year. The enantiomeric composition of the mother liquor and deposited crystal remained constant after several months and the onset of the return to equilibrium was only observed after 1 year (see Table S2).
- The process was performed with initial supersaturation of β = 2, 4 and 6. Successful PE was observed at β = 4 and 6, but the experiments at β = 2 failed (see Table S3), suggesting the existence of a supersaturation threshold below which PE cannot occur. Our experience suggests that the higher the supersaturation, the faster the process, although experiments with β > 8 were difficult to handle due to spontaneous crystallization before reaching T = 5 °C or directly in the mixing set-up. We did not investigate further such situations.
- The starting ee was varied from 0 to ca. (+) 20%ee. When the initial ee is exactly 0%, no one can predict the final sign of the mother liquor and the opposite sign of the solid. In the 0–1% range, the PE effect is weak. Above 1% there is a take-off in the final ee of the mother liquor which approaches 90% and then exceeds 95%. There is a threshold of the initial ee at ca. 11% above which the ee of the deposited crystals was of the same sign as that of the mother liquor.
2.2. Monitoring of the PE Phenomenon of Arginine Fumarate Using the Standard Protocol
2.3. Doping the Process with 13C6-L-Arginine.HCl
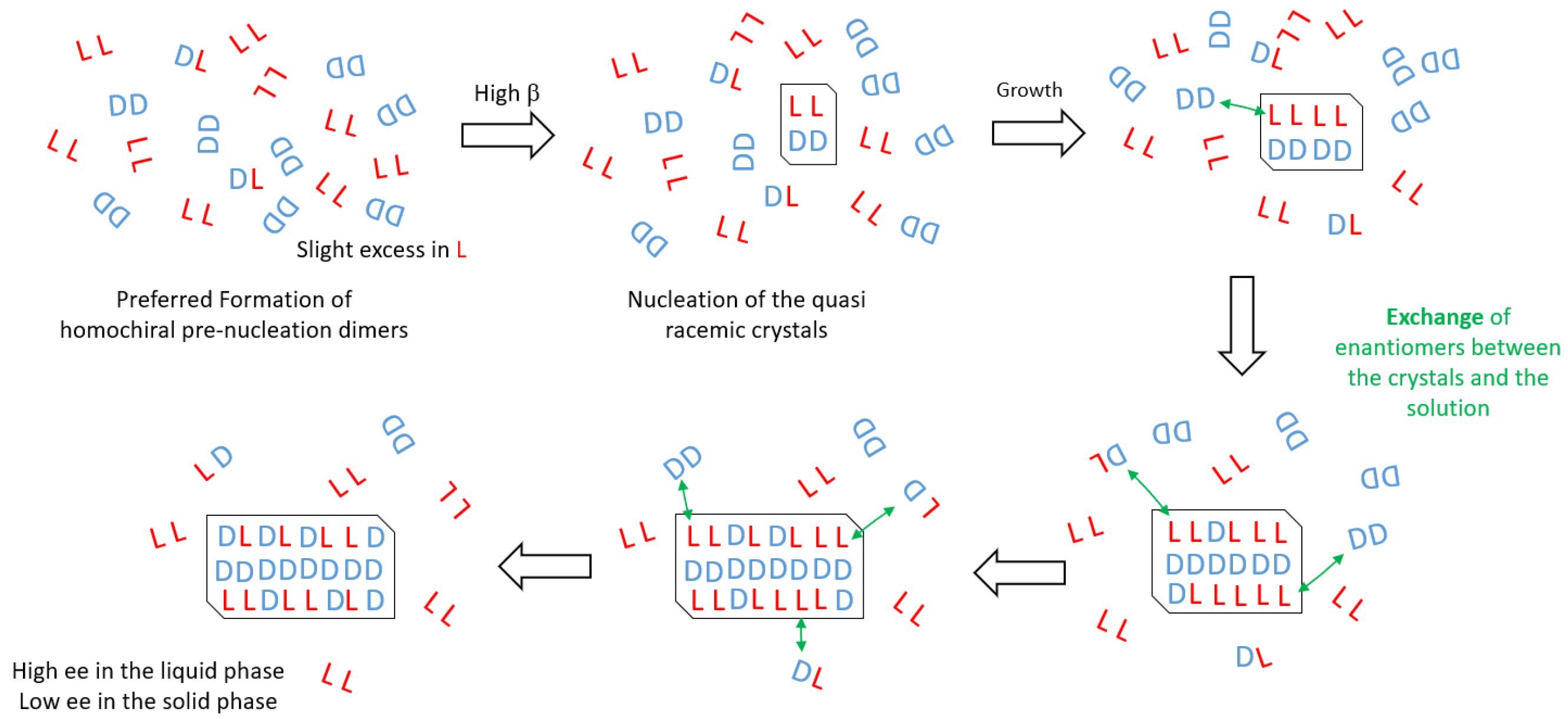
3. Discussion
4. Materials and Methods
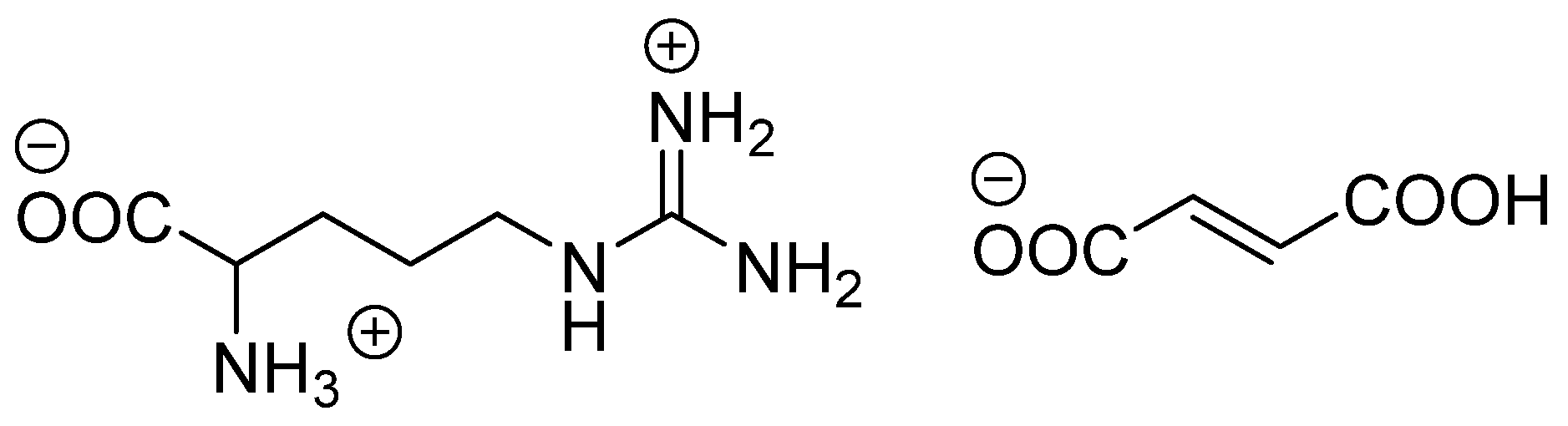
4.1. Materials
4.2. Recrystallization of DL-Arginine Fumarate
4.3. Procedure for PE Experiments
4.4. Procedure for Monitoring
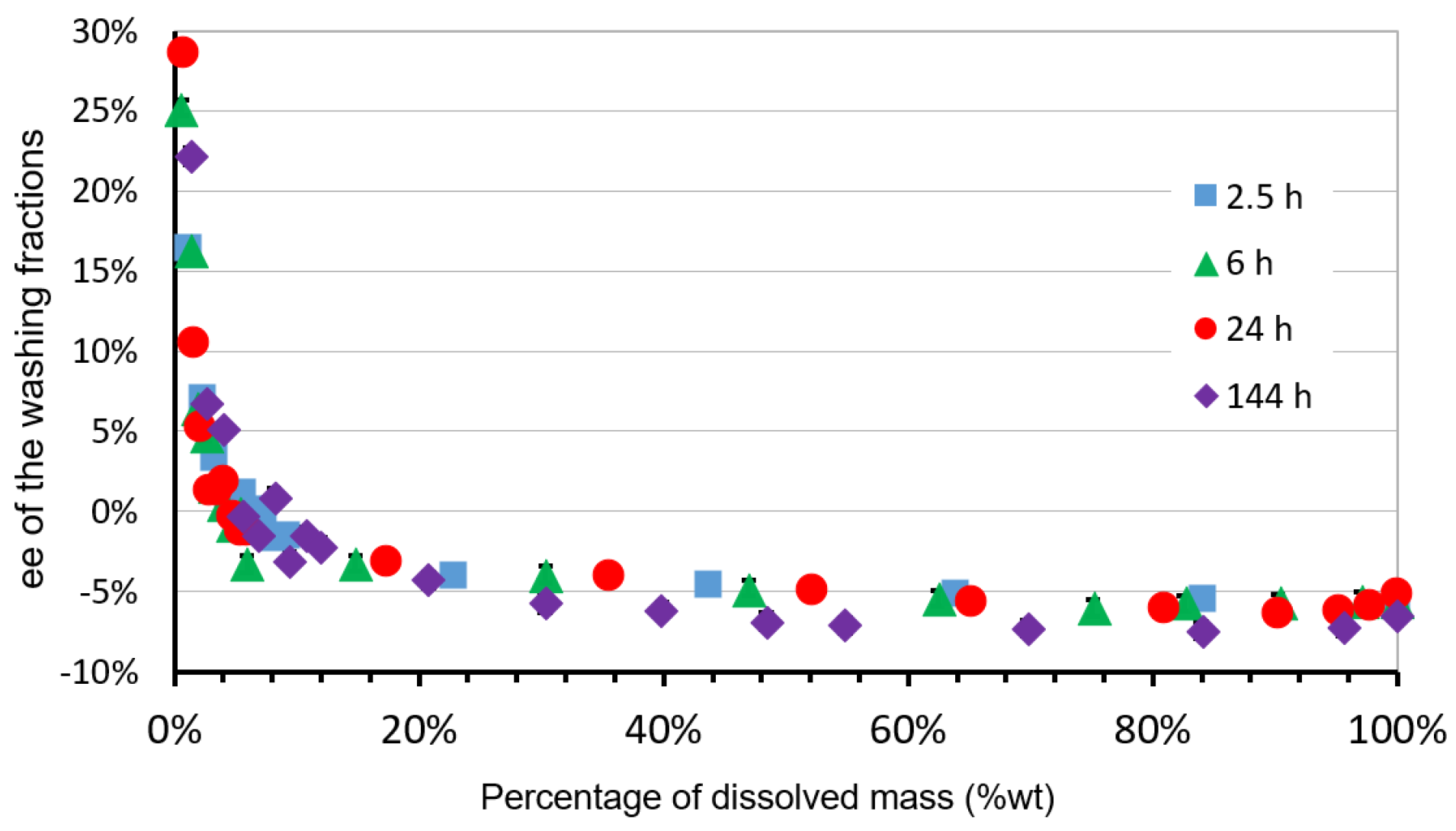
4.5. Procedure for Successive Dissolution
4.6. Procedure for the Addition of Labeled L-Arginine
4.7. HPLC-UV Method
4.8. HPLC-MS/MS Method
4.9. Experimental Set-Up for In Situ XRPD Measurements
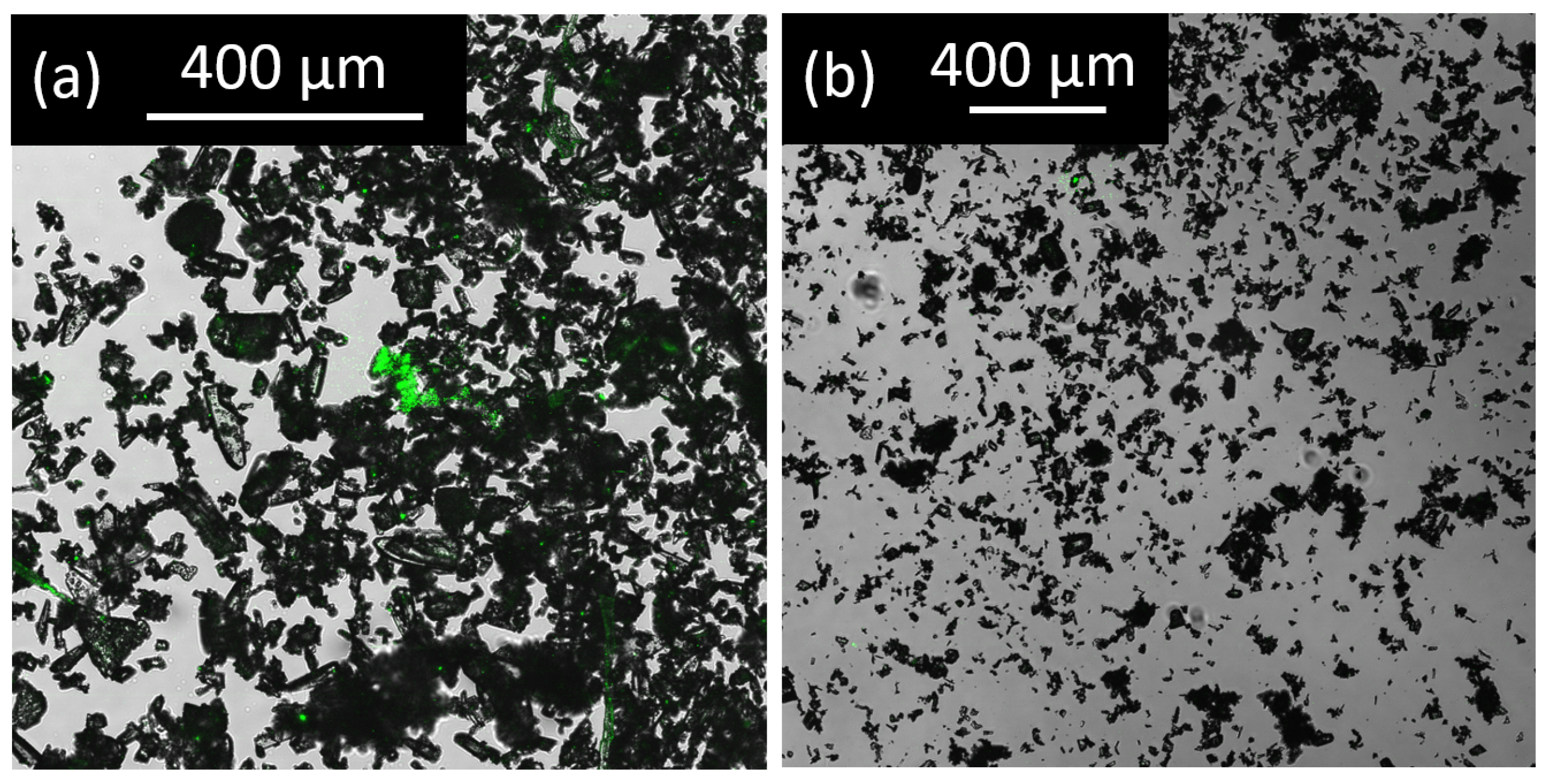
4.10. SHG Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of Chirality in Drug Design and Development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Haupert, L.M.; Simpson, G.J. Chirality in Nonlinear Optics. Annu. Rev. Phys. Chem. 2009, 60, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.J.W.; Gu, C.G. Effect of Crystal Structure and Physical Properties on the Release of Chiral Drugs. In Chirality in Drug Design and Development; Reddy, I.K., Mehvar, R., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 1–33. [Google Scholar] [CrossRef]
- Saigo, K.; Sakai, K. Resolution of Chiral Drugs and Drug Intermediates by Crystallisation. In Chirality in Drug Research; Francotte, E., Lindner, W., Eds.; VCH Verlag GmbH & Co.: Weinheim, Germany, 2006; Volume 33, pp. 127–154. [Google Scholar] [CrossRef]
- Andersson, S.; Allenamrk, S.G. Preparative Chiral Chromatographic Resolution of Enantiomers in Drug Discovery. J. Biochem. Biophys. Methods 2002, 54, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fogassy, E.; Nógrádi, M.; Kozma, D.; Egri, G.; Pálovicsc, E.; Kissa, V. Optical Resolution Methods. Org. Biomol. Chem. 2006, 16, 3011–3030. [Google Scholar] [CrossRef] [PubMed]
- Coquerel, G. Preferential Crystallization. In Novel Optical Resolution Technologies; Sakai, K., Hirayama, N., Tamura, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 269, pp. 1–51. [Google Scholar]
- Intaraboonrod, K.; Lerdwiriyanupap, T.; Hoquante, M.; Coquerel, G.; Flood, A.E. Temperature Cycled Induced Deracemization. Mendeleev. Commun. 2020, 30, 395–405. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S. Enantiomers, Racemates and Resolutions; Krieger Publishing Company: Malabar, FL, USA, 1994. [Google Scholar] [CrossRef]
- Harfouche, L.C.; Brandel, C.; Cartigny, Y.; Petit, S.; Coquerel, G. Resolution by Preferential Crystallization of Proxyphylline by Using Its Salicylic Acid Monohydrate Co-Crystal. Chem. Eng. Technol. 2020, 43, 1093–1098. [Google Scholar] [CrossRef]
- Mbodji, A.; Gbabode, G.; Sanselme, S.; Couvrat, N.; Leeman, M.; Dupray, V.; Kellogg, R.M.; Coquerel, G. Family of Conglomerate-Forming Systems Composed of Chlocyphos and Alkyl-amine. Assessment of Their Resolution Performances by Using Various Modes of Preferential Crystallization. Cryst. Growth Des. 2019, 19, 5173–5183. [Google Scholar] [CrossRef]
- Coquerel, G. Solubility of chiral species as function of the enantiomeric excess. J. Pharm. Pharmacol. 2015, 67, 869–878. [Google Scholar] [CrossRef]
- Tamura, R.; Fujimoto, D.; Lepp, Z.; Misaki, K.; Miura, H.; Takahashi, H.; Ushio, T.; Nakai, T.; Hirotsu, K. Mechanism of Preferential Enrichment, an Unusual Enantiomeric Resolution Phenomenon Caused by Polymorphic Transition during Crystallization of Mixed Crystals Composed of Two Enantiomers. J. Am. Chem. Soc. 2002, 124, 13130–13153. [Google Scholar] [CrossRef]
- Iwama, S.; Horiguchi, M.; Sato, H.; Uchida, Y.; Takahashi, H.; Tsue, H.; Tamura, R. Observation of the Preferential Enrichment Phenomenon for Essential r-Amino Acids with a Racemic Crystal Structure. Cryst. Growth Des. 2010, 10, 2668–2675. [Google Scholar] [CrossRef]
- Gonnade, R.G.; Iwama, S.; Sugiwake, R.; Manoj, K.; Takahashi, H.; Tsue, H.; Tamura, R. Occurrence of spontaneous resolution of ketoprofen with a racemic crystal structure by simple crystallization under nonequilibrium preferential enrichment conditions. Chem. Commun. 2012, 48, 2791–2793. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Takahashi, H.; Coquerel, G. Twenty-Five Years’ History, Mechanism, and Generality of Preferential Enrichment as a Complexity Phenomenon. In Advances in Organic Crystal Chemistry; Sakamoto, M., Uekusa, H., Eds.; Springer: Singapore, 2020; pp. 405–432. [Google Scholar] [CrossRef]
- Coquerel, G.; Hoquante, M. Spontaneous and Controlled Macroscopic Chiral Symmetry Breaking by Means of Crystallization. Symmetry 2020, 12, 1796–1813. [Google Scholar] [CrossRef]
- Horiguchi, M.; Okuhara, S.; Shimano, E.; Fujimoto, D.; Takahashi, H.; Tsue, H.; Tamura, R. Mechanistic flexibility of solvent-assisted solid-to-solid polymorphic transition causing preferential enrichment: Significant contribution of π/π and CH/π interactions as well as hydrogen bonds. Cryst. Growth Des. 2007, 7, 1643–1652. [Google Scholar] [CrossRef]
- Takahashi, H.; Tamura, R.; Yabunaka, S.; Ushio, T. Crystal structure of a new racemate showing Preferential Enrichment: Evidence for the existence as a racemic mixed crystal composed of the two enantiomers. Mendeleev. Commun. 2003, 13, 119–121. [Google Scholar] [CrossRef]
- Tamura, R.; Mizuta, M.; Yabunaka, S.; Fujimoto, D.; Ariga, S.; Okuhara, S.; Ikuma, N.; Takahashi, H.; Tsue, H. Induction and Inhibition of Preferential Enrichment by Controlling the Mode of the Polymorphic Transition with Seed Crystals. Chem. Eur. J. 2006, 12, 3515–3527. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwama, S.; Clevers, S.; Veesler, S.; Coquerel, G.; Tsue, H.; Tamura, R. In situ observation of polymorphic transition during crystallization of organic compounds showing preferential enrichment by means of temperature-controlled video-microscopy and time-resolved X-ray powder diffraction. Cryst. Growth Des. 2017, 17, 671–676. [Google Scholar] [CrossRef]
- Brandel, C.; Petit, S.; Cartigny, Y.; Coquerel, G. Structural Aspects of Solid Solutions of Enantiomers. Curr. Pharm. Des. 2016, 22, 4929–4941. [Google Scholar] [CrossRef]
- De Saint-Jores, C.; Brandel, C.; Gharbi, N.; Sanselme, M.; Cardinael, P.; Coquerel, G. Limitations of Preferential Enrichment: A Case Study on Tryptophan Ethyl Ester Hydrochloride. Chem. Eng. Technol. 2019, 42, 1500–1504. [Google Scholar] [CrossRef]
- Iwama, S.; Kuyama, K.; Mori, Y.; Manoj, K.; Gonnade, R.G.; Suzuki, K.; Hughes, C.E.; Williams, P.A.; Harris, K.D.M.; Veesler, S.; et al. Highly efficient chiral resolution of DL-arginine by cocrystal formation followed by recrystallization under preferential-enrichment conditions. Chem. Eur. J. 2014, 24, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Iwama, S.; Coquerel, G.; Tamura, R. A kinetic/thermodynamic origin of regular chiral fluctuation or symmetry breaking unique to preferential enrichment. Chem. Eur. J. 2016, 22, 11660–11666. [Google Scholar] [CrossRef]
- Manoj, K.; Takahashi, H.; Iwama, S.; Gonnade, R.; Tsue, H.; Tamura, R. Crystal structure analysis of highly efficient chiral resolution of (RS)-arginine-fumaric acid cocrystal under preferential enrichment conditions. J. Mol. Struct. 2021, 1245, 131073. [Google Scholar] [CrossRef]
- Gonnade, R.G.; Iwama, S.; Mori, Y.; Takahashi, H.; Tsue, H.; Tamura, R. Observation of Efficient Preferential Enrichment Phenomenon for a Cocrystal of (DL)-Phenylalanine and Fumaric Acid under Nonequilibrium Crystallization Conditions. Cryst. Growth Des. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. How Do Crystals Nucleate and Grow: Ostwald’s Rule of Stages and beyond. In Thermal Physics and Thermal Analysis; Šesták, J., Hubík, P., Mareš, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 11, pp. 195–211. [Google Scholar] [CrossRef]
- Ricci, J.E. The Phase Rule and Heterogeneous Equilibrium; Dover Publications: Mineola, NY, USA, 1966. [Google Scholar]
- Harfouche, L.; Clevers, S.; Coquerel, G.; Rietveld, I. Nucleation behaviour of racemic and enantiopure Histidine. CrystEngComm 2021, 23, 8379–8385. [Google Scholar] [CrossRef]
- Zlokazov, M.V.; Pivnitsky, K.K. Lamellar conglomerate. Mend. Comm. 2020, 30, 1–6. [Google Scholar] [CrossRef]
- Brandel, C.; Cartigny, Y.; Coquerel, G.; ter Horst, J.-H.; Petit, S. Prenucleation Self-Assembly and Chiral Discrimination Mechanisms during Solution Crystallisation of Racemic Diprophylline. Chem. Eur. J. 2016, 22, 16103–16112. [Google Scholar] [CrossRef] [PubMed]
- Coquerel, G.; Sanselme, M.; Lafontaine, A. Method of measuring scattering of X-rays, its applications and implementation device. EP2694953A1, 2 April 2012. [Google Scholar]


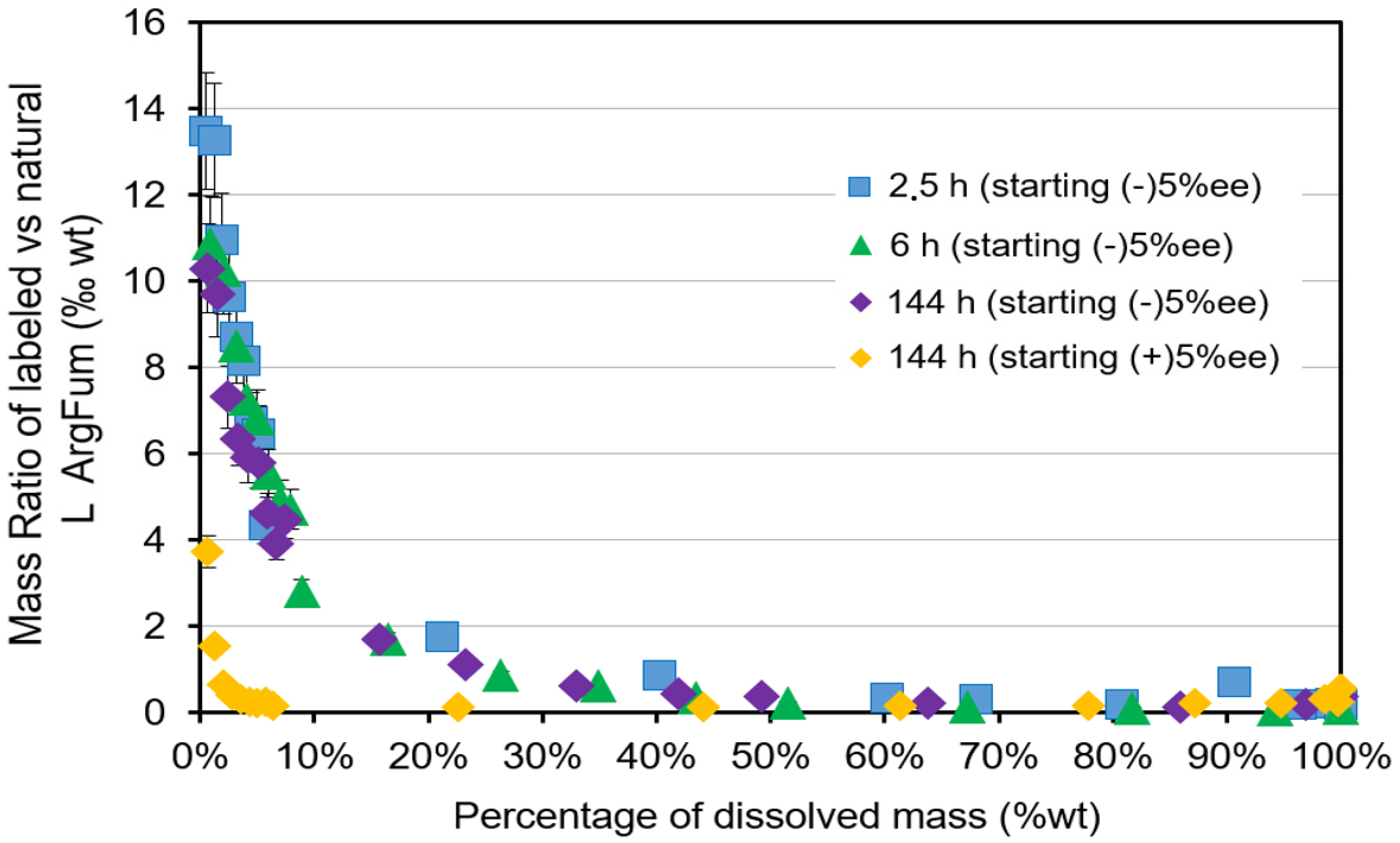
| Composition of the Liquid Phase | |||
| mL (mg) | mD (mg) | mtotal (mg) | ee (%) |
| 63 ± 2 | 1.0 ± 0.1 | 64 ± 2 | +96 ± 1 |
| Composition of the Solid Phase | |||
| mL (mg) | mD (mg) | mtotal (mg) | ee (%) |
| 353 ± 9 | 380 ± 10 | 733 ± 19 | −3.7± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Saint Jores, C.; Brandel, C.; Vaccaro, M.; Gharbi, N.; Schmitz-Afonso, I.; Cardinael, P.; Tamura, R.; Coquerel, G. Reinvestigating the Preferential Enrichment of DL-Arginine Fumarate: New Thoughts on the Mechanism of This Far from Equilibrium Crystallization Phenomenon. Molecules 2022, 27, 8652. https://doi.org/10.3390/molecules27248652
De Saint Jores C, Brandel C, Vaccaro M, Gharbi N, Schmitz-Afonso I, Cardinael P, Tamura R, Coquerel G. Reinvestigating the Preferential Enrichment of DL-Arginine Fumarate: New Thoughts on the Mechanism of This Far from Equilibrium Crystallization Phenomenon. Molecules. 2022; 27(24):8652. https://doi.org/10.3390/molecules27248652
Chicago/Turabian StyleDe Saint Jores, Clément, Clément Brandel, Marie Vaccaro, Najla Gharbi, Isabelle Schmitz-Afonso, Pascal Cardinael, Rui Tamura, and Gérard Coquerel. 2022. "Reinvestigating the Preferential Enrichment of DL-Arginine Fumarate: New Thoughts on the Mechanism of This Far from Equilibrium Crystallization Phenomenon" Molecules 27, no. 24: 8652. https://doi.org/10.3390/molecules27248652
APA StyleDe Saint Jores, C., Brandel, C., Vaccaro, M., Gharbi, N., Schmitz-Afonso, I., Cardinael, P., Tamura, R., & Coquerel, G. (2022). Reinvestigating the Preferential Enrichment of DL-Arginine Fumarate: New Thoughts on the Mechanism of This Far from Equilibrium Crystallization Phenomenon. Molecules, 27(24), 8652. https://doi.org/10.3390/molecules27248652







