Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Purification of Montmorillonite
2.3. Intercalation of Ionic Liquids into Montmorillonite: Full Characterization
2.4. Agarose Film as a Holder of the New Green Sorbent
2.5. Headspace SPME Configuration
2.6. GC-ECD Instrument
2.7. Experimental Design for Headspace Extraction
2.8. Design for Desorption Time with Methanol
2.9. Spiked Aqueous and Real Samples
3. Results and Discussion
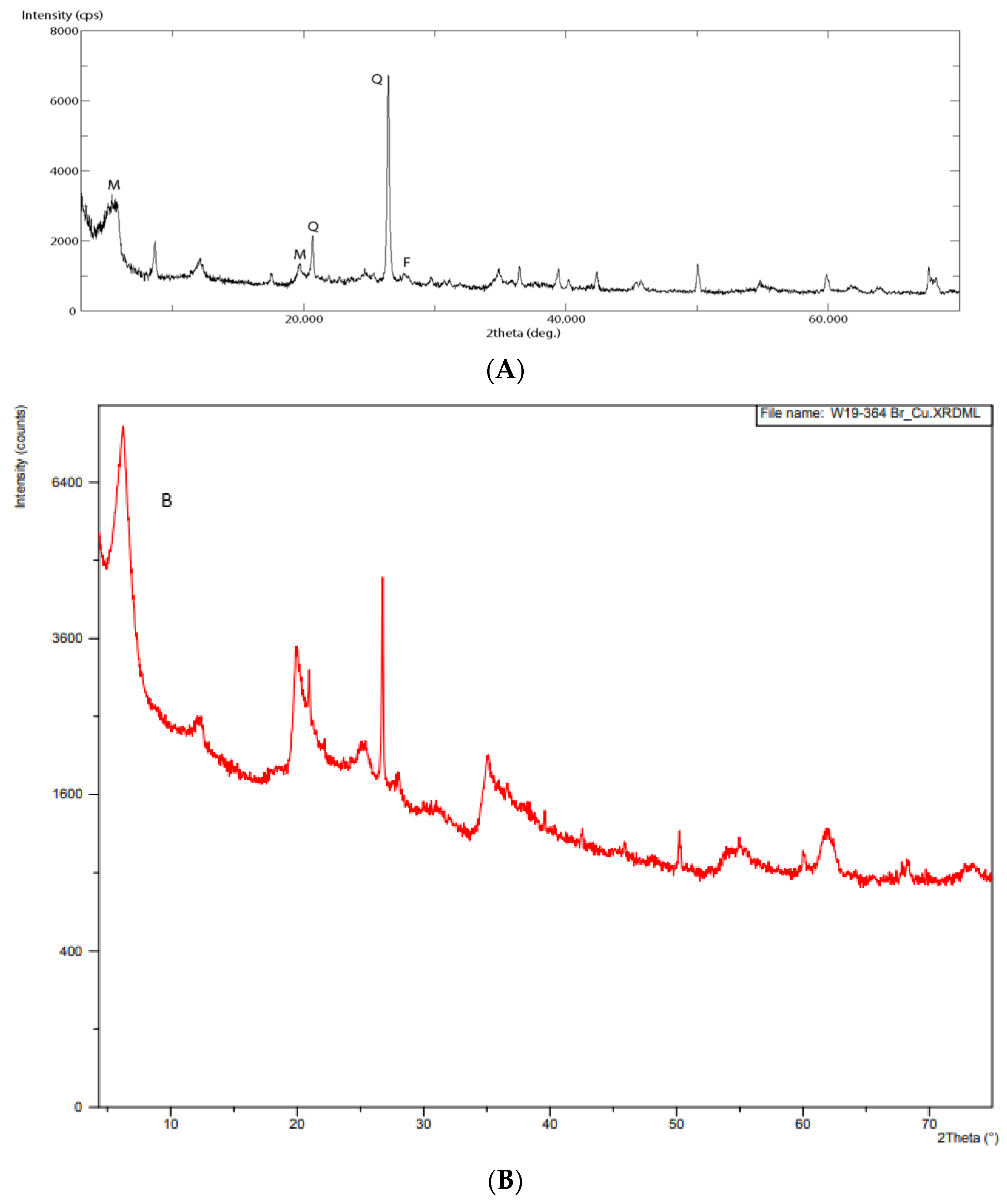
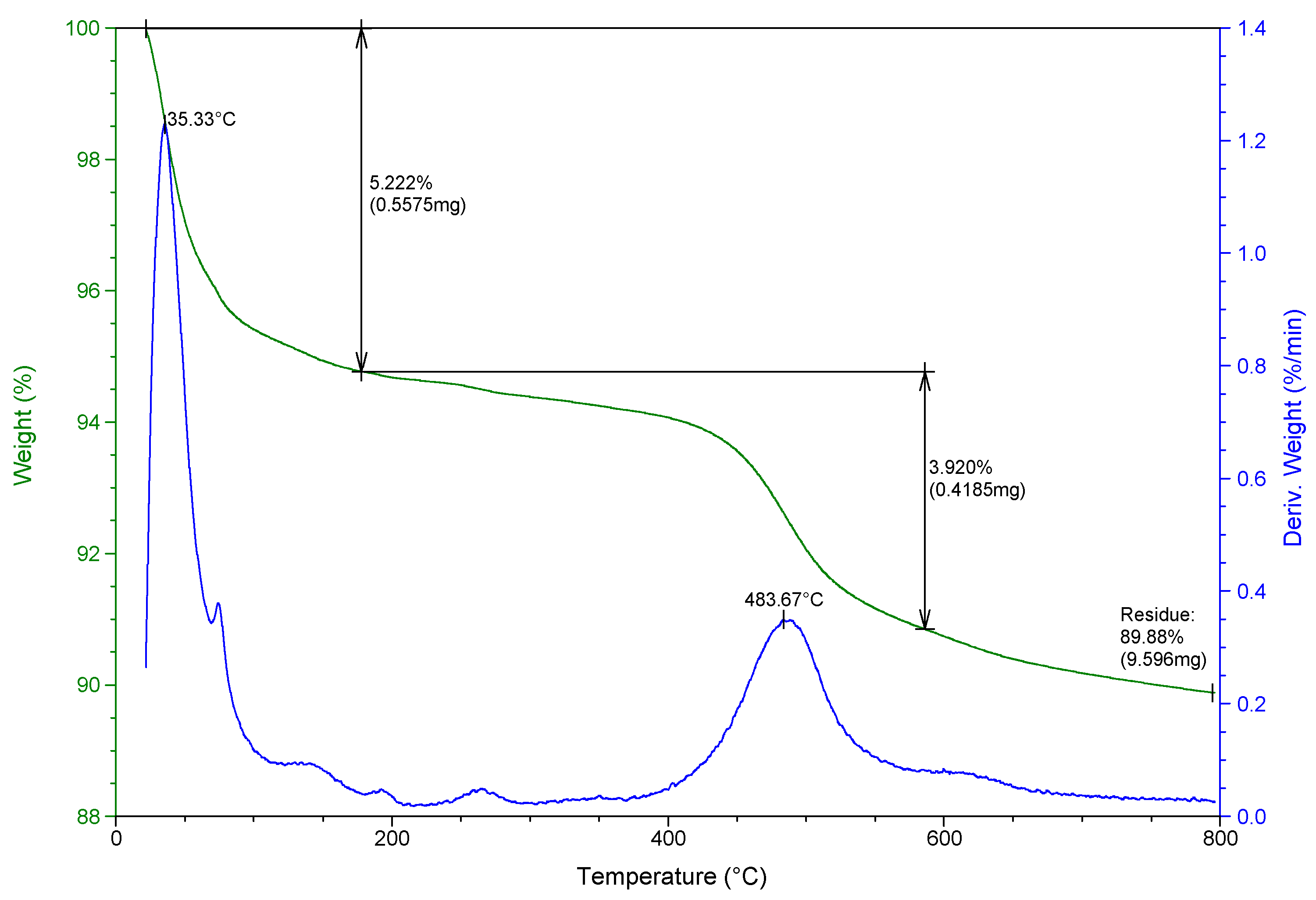
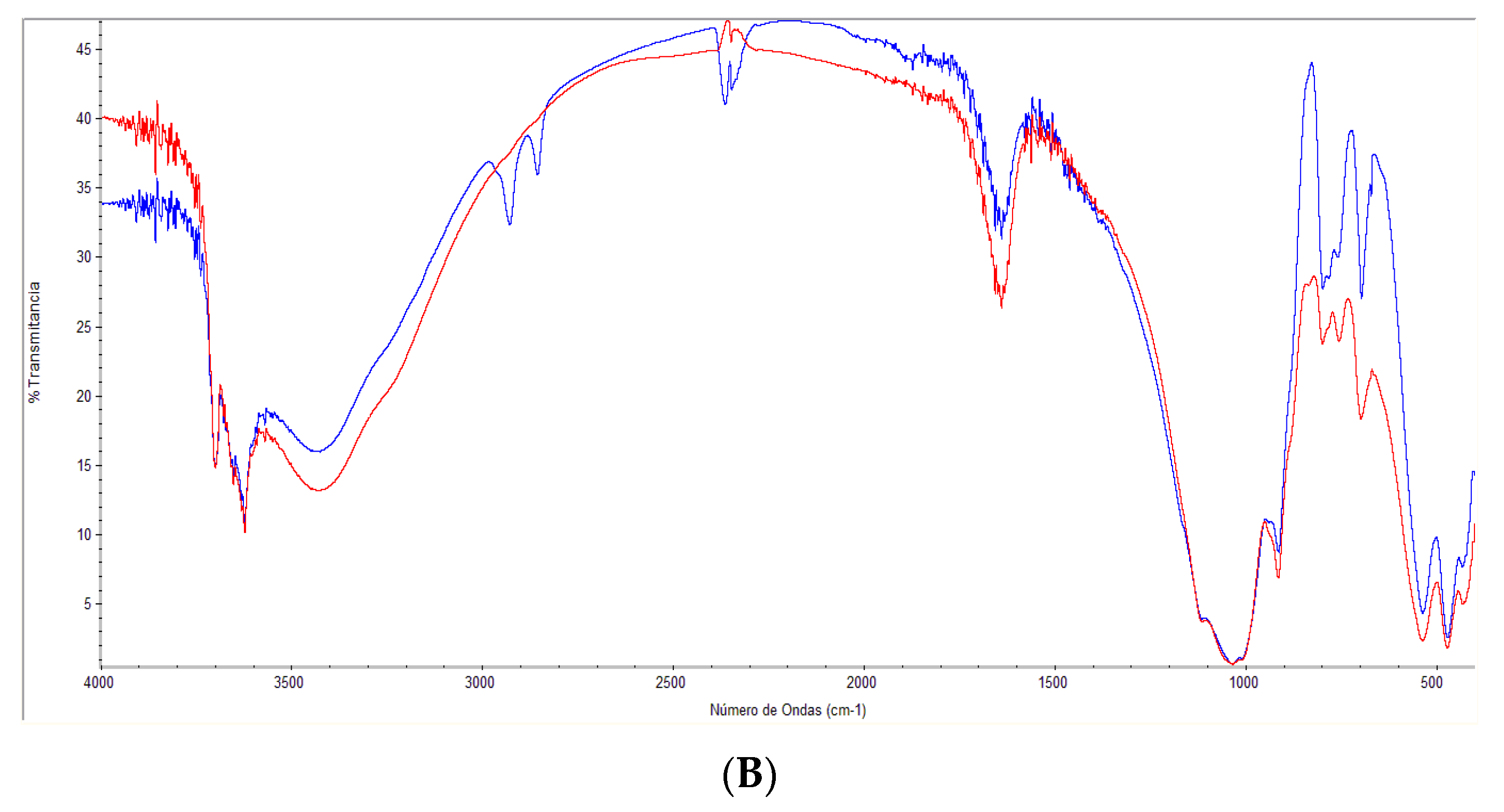
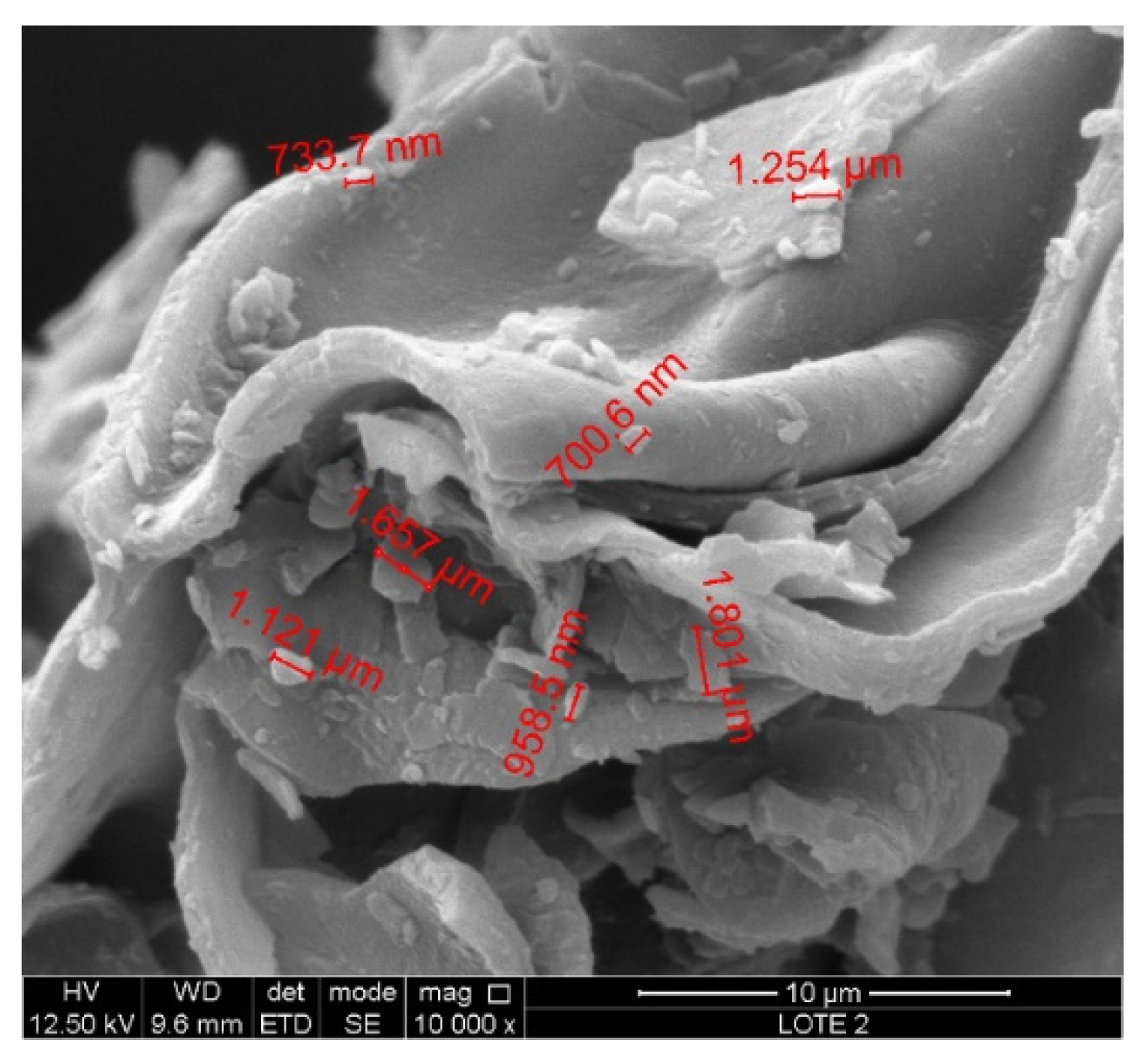
3.1. Characterization of Raw and Modified Bentonite Clay
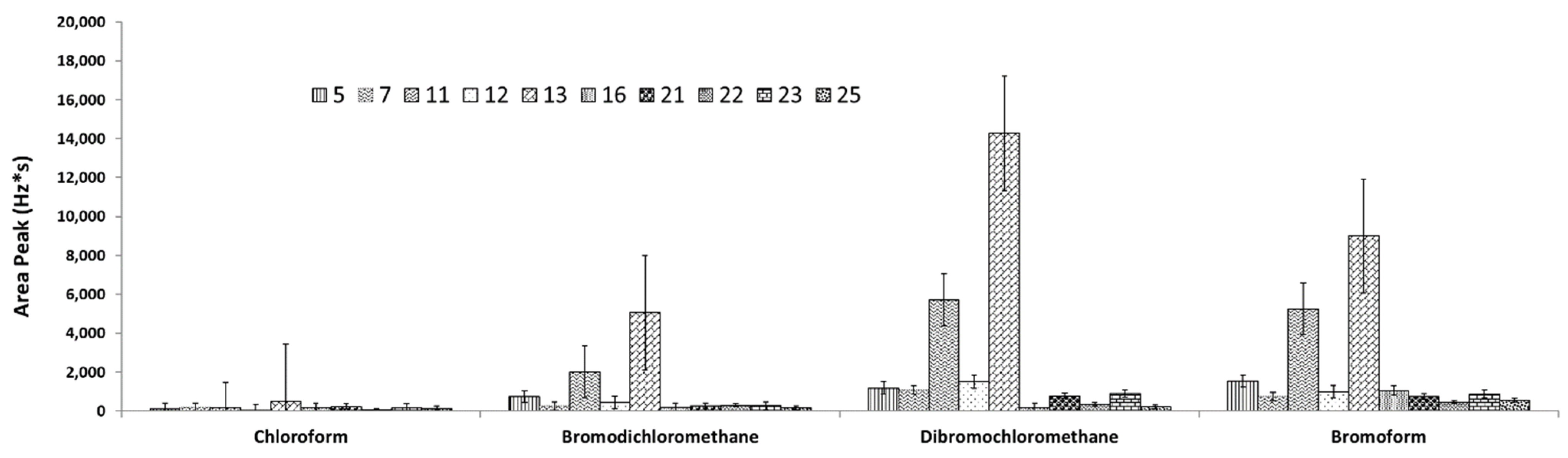
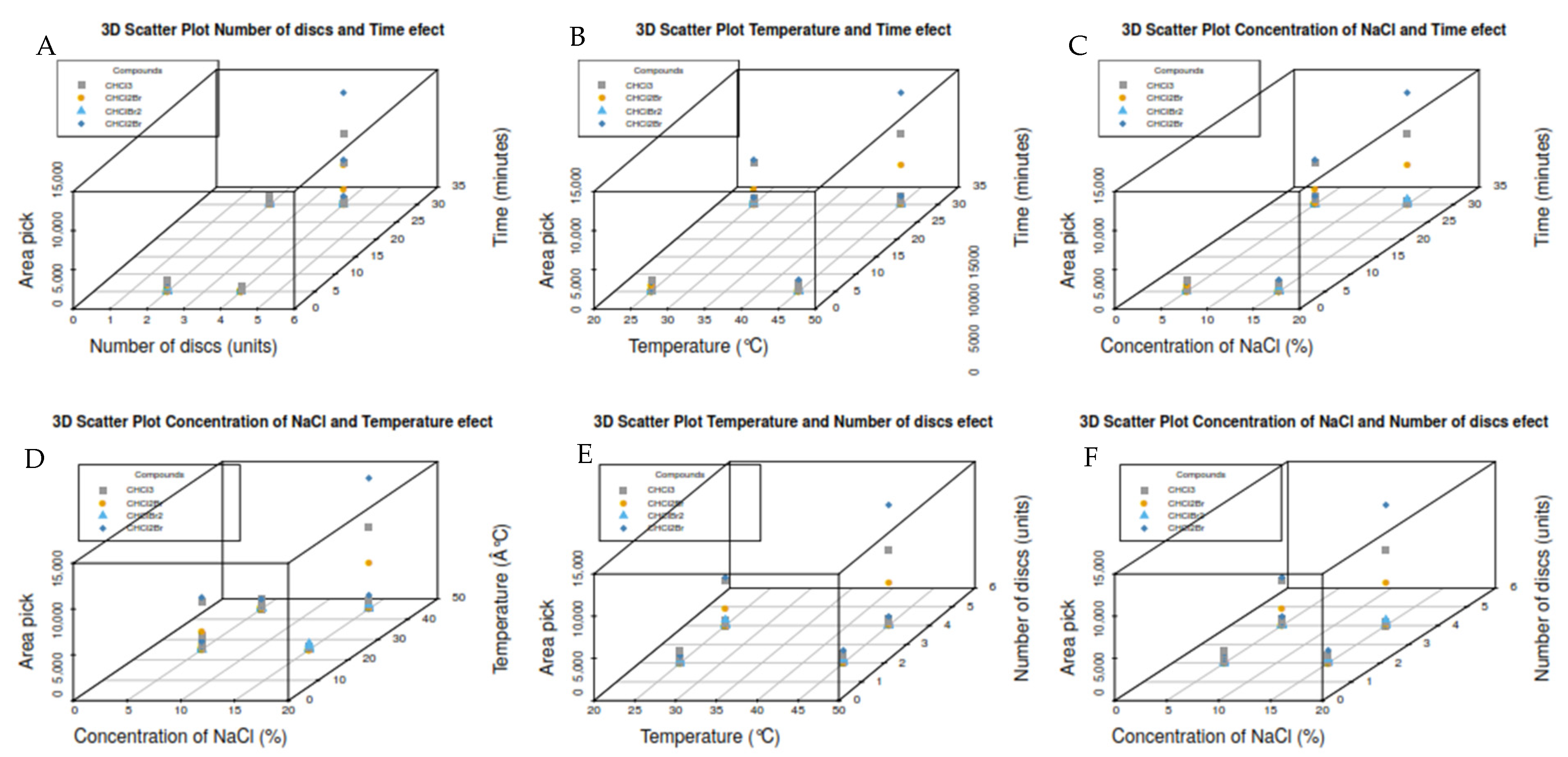
3.2. Design of Experiments (DOE) for Headspace Extraction (Screening)
3.3. Design of Experiments DOE for Desorption Time with Methanol
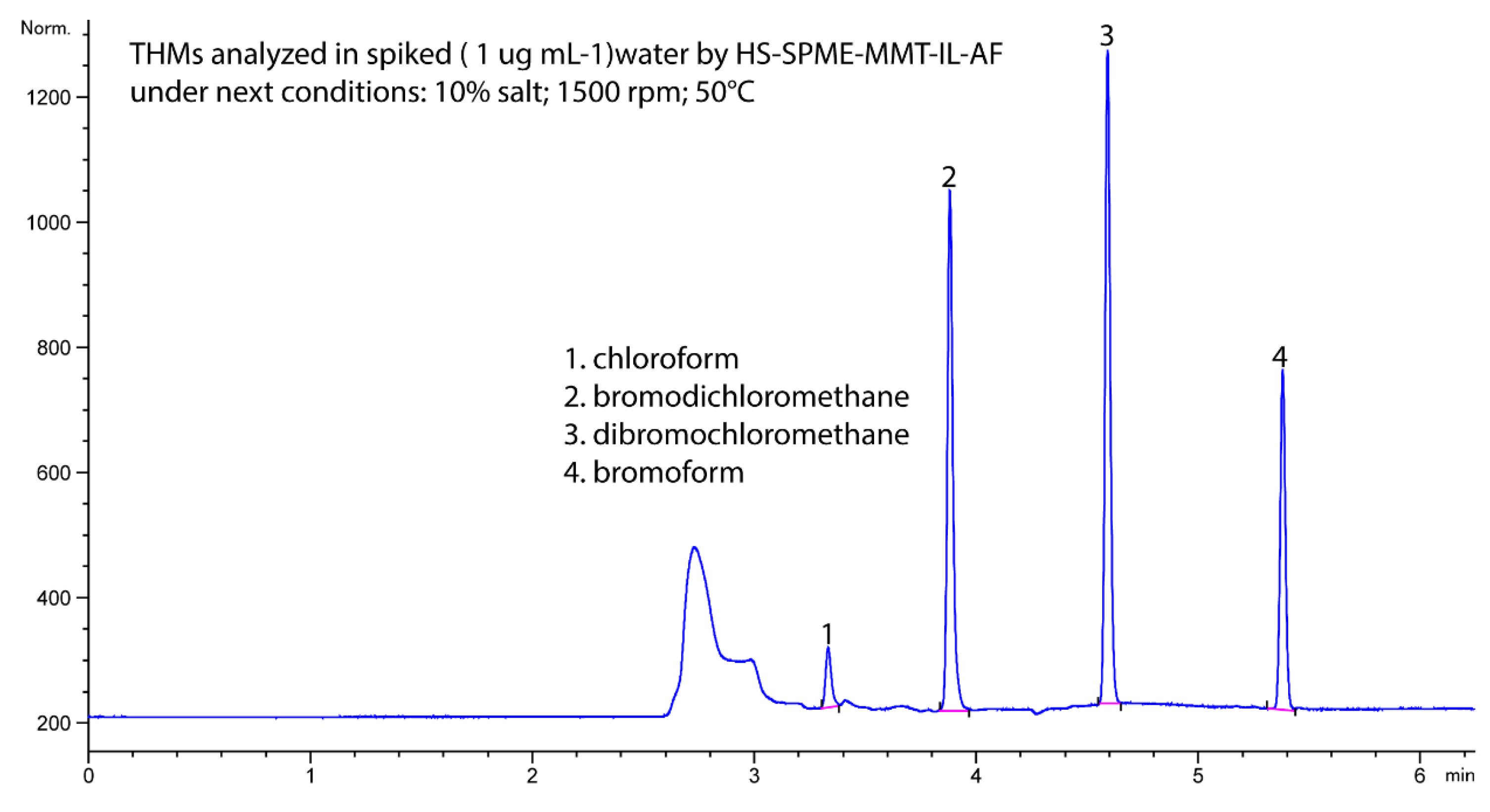
3.4. Analytical Characteristics of the Developed Method
3.5. Analysis of Water Samples
3.6. By Comparing with Other THMs Analysis Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunez, Z.R.; Wright, J.M.; Blount, B.C.; Silva, L.K.; Jones, E.; Chan, R.L.; Pegram, R.A.; Singer, P.C.; Savitz, D.A. Comparison of Trihalomethanes in Tap Water and Blood: A Case Study in the United States. Environ. Health Perspect. 2012, 120, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Andrés, J.; Peperzak, L. Operational and environmental factors affecting disinfection byproducts formation in ballast water treatment systems. Chemosphere 2019, 232, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Farré, M.J.; Knight, N. Comparing three Australian natural organic matter isolates to the Suwannee river standard: Reactivity, disinfection by-product yield, and removal by drinking water treatments. Sci. Total Environ. 2019, 685, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Clayton, G.E.; Thorn, R.M.S.; Reynolds, D.M. Comparison of Trihalomethane Formation Using Chlorine-Based Disinfectants within a Model System; Applications Within Point-of-Use Drinking Water Treatment. Front. Environ. Sci. 2019, 7, 35. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Parkinson, D.-R.; Barter, D.; Gaultois, R. Comparison of Spot and Time Weighted Averaging (TWA) Sampling with SPME-GC/MS Methods for Trihalomethane (THM) Analysis. Separations 2016, 3, 5. [Google Scholar] [CrossRef]
- Gouveia, P.; Felgueiras, F.; Mourão, Z.; Fernandes, E.D.O.; Moreira, A.; Gabriel, M.F. Predicting health risk from exposure to trihalomethanes in an Olympic-size indoor swimming pool among elite swimmers and coaches. J. Toxicol. Environ. Health Part A Curr. Issues 2019, 82, 577–590. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Detellier, C. Clay Minerals—Ionic Liquids, Nanoarchitectures, and Applications. Adv. Funct. Mater. 2018, 28, 1703845. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis Thomas. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Fiscal-Ladino, J.A.; Obando-Ceballos, M.; Rosero-Moreano, M.; Montaño, D.F.; Cardona, W.; Giraldo, L.F.; Richter, P. Ionic liquids intercalated in montmorillonite as the sorptive phase for the extraction of low-polarity organic compounds from water by rotating-disc sorptive extraction. Anal. Chim. Acta 2017, 953, 23–31. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Treviño, M.J.S.; Zarazúa, S.; Płotka-Wasylka, J. Nanosorbents as Materials for Extraction Processes of Environmental Contaminants and Others. Molecules 2022, 27, 1067. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.; Fiscal, J.A.; Ceballos, S.; De La Ossa, A.; Taborda, G.; Nerín, C.; Rosero-Moreano, M. Hollow-fiber solvent bar microextraction with gas chromatography and electron capture detection determination of disinfection byproducts in water samples. J. Sep. Sci. 2015, 38, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.; Sanagi, M.M.; Ibrahim, W.A.W.; Hasan, M.N. Multi-walled carbon nanotube-impregnated agarose film microextraction of polycyclic aromatic hydrocarbons in green tea beverage. Talanta 2013, 106, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hara, E. Marco Grassi o-DGT for Analysis of Emerging Contaminants from Water Samples. Ph.D. Thesis, University Federal of Paraná, Curitiba, Brazil, 2017. [Google Scholar]
- Rosero-Moreano, M. New Trends in Chemical Analysis of Disinfection By-Products. In Disinfection; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Rosero-Moreano, M.; Aguirre, M.; Pezo, D.; Taborda, G.; Dussán, C.; Nerin, C. Solventless Microextraction Techniques for Determination of Trihalomethanes by Gas Chromatography in Drinking Water. Water Air Soil Pollut. 2012, 223, 667–678. [Google Scholar] [CrossRef]
- Aguirre-González, M.; Taborda-Ocampo, G.; Dussan-Lubert, C.; Nerin, C.; Rosero-Moreano, M. Optimization of the HS-SPME technique by using response surface methodology for evaluating chlorine disinfection by-products by GC in drinking water. J. Braz. Chem. Soc. 2011, 22, 2330–2336. [Google Scholar] [CrossRef][Green Version]
- Kinani, A.; Kinani, S.; Richard, B.; Lorthioy, M.; Bouchonnet, S. Formation and determination of organohalogen by-products in water—Part I. Discussing the parameters influencing the formation of organohalogen by-products and the relevance of estimating their concentration using the AOX (adsorbable organic halide) meth. TrAC-Trends Anal. Chem. 2016, 85, 273–280. [Google Scholar] [CrossRef]
- Alexandrou, L.D.; Meehan, B.J.; Morrison, P.D.; Jones, O.A.H. A New Method for the Fast Analysis of Trihalomethanes in Tap and Recycled Waters Using Headspace Gas Chromatography with Micro-Electron Capture Detection. Int. J. Environ. Res. Public Health 2017, 14, 527. [Google Scholar] [CrossRef]
- Laaks, J.; Jochmann, M.A.; Schilling, B.; Schmidt, T.C. In-Tube Extraction of Volatile Organic Compounds from Aqueous Samples: An Economical Alternative to Purge and Trap Enrichment. Anal. Chem. 2010, 82, 7641–7648. [Google Scholar] [CrossRef] [PubMed]




| Factor | Low Level | High Level |
|---|---|---|
| 1 Salt concentration (% w/v) | 5 | 10 |
| 2 Distance between discs (mm) | 2 | 5 |
| 3 Extraction temperature (°C) | 25 | 45 |
| 4 Number of discs | 2 | 4 |
| 5 Extraction time (min) | 5 | 30 |
| Exp. | Factors (Units) | Area Peak | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (%) | 2 (mm) | 3 (°C) | 4 (# discs) | 5 (min) | CHCl3 | CHCl2Br | CHClBr2 | CHBr3 | |
| 1 | 5 | 5 | 45 | 2 | 30 | 167 | 148 | 84 | 204 |
| 2 | 15 | 5 | 45 | 4 | 5 | 16 | 24 | 36 | 204 |
| 3 | 5 | 5 | 45 | 4 | 5 | 38 | 25 | 57 | 60 |
| 4 | 15 | 5 | 25 | 2 | 30 | 412 | 39 | 254 | 328 |
| 5 | 5 | 2 | 25 | 2 | 5 | 96 | 740 | 1184 | 1528 |
| 6 | 5 | 2 | 25 | 4 | 5 | 193 | 79 | 371 | 342 |
| 7 | 5 | 2 | 45 | 4 | 30 | 113 | 255 | 1075 | 732 |
| 8 | 5 | 2 | 45 | 2 | 5 | 54 | 115 | 477 | 340 |
| 9 | 15 | 5 | 45 | 4 | 30 | 358 | 18 | 61 | 102 |
| 10 | 15 | 2 | 25 | 2 | 30 | 147 | 18 | 51 | 176 |
| 11 | 5 | 5 | 25 | 4 | 30 | 22 | 2006 | 5709 | 5252 |
| 12 | 15 | 5 | 45 | 2 | 5 | 497 | 440 | 1507 | 992 |
| 13 | 15 | 2 | 45 | 4 | 30 | 3 | 5062 | 14,281 | 9000 |
| 14 | 15 | 2 | 25 | 2 | 5 | 13 | 14 | 14 | 37 |
| 15 | 15 | 5 | 25 | 4 | 30 | 171 | 5 | 54 | 254 |
| 16 | 5 | 2 | 45 | 2 | 30 | 260 | 191 | 172 | 1058 |
| 17 | 15 | 2 | 45 | 4 | 5 | 1 | 269 | 156 | 376 |
| 18 | 15 | 5 | 25 | 2 | 5 | 182 | 91 | 10 | |
| 19 | 5 | 5 | 25 | 4 | 5 | 17 | 52 | 157 | 625 |
| 20 | 15 | 5 | 25 | 4 | 5 | 235 | 0 | 0 | 0 |
| 21 | 5 | 5 | 25 | 2 | 30 | 54 | 250 | 787 | 745 |
| 22 | 15 | 5 | 45 | 2 | 30 | 166 | 293 | 348 | 441 |
| 23 | 5 | 2 | 25 | 2 | 30 | 61 | 271 | 892 | 878 |
| 24 | 15 | 2 | 45 | 2 | 5 | 144 | 229 | 119 | 380 |
| 25 | 5 | 2 | 45 | 4 | 5 | 70 | 151 | 228 | 549 |
| 26 | 15 | 2 | 45 | 2 | 30 | 98 | 207 | 70 | 229 |
| 27 | 5 | 5 | 25 | 2 | 5 | 0 | 181 | 109 | 229 |
| 28 | 5 | 5 | 45 | 2 | 5 | 82 | 0 | 0 | 0 |
| 29 | 5 | 5 | 45 | 4 | 30 | 89 | 142 | 86 | 262 |
| 30 | 15 | 2 | 25 | 4 | 30 | 684 | 141 | 358 | 395 |
| 31 | 15 | 2 | 25 | 4 | 5 | 30 | 0 | 0 | 0 |
| 32 | 5 | 2 | 25 | 4 | 30 | 260 | 21 | 29 | 52 |
| THMs | Assay | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 0 min. | 10 min. | 30 min. | 60 min. | |
| Chloroform | 497 | 355 | 637 | 20 |
| 327 | 0 | 14 | 41 | |
| Bromodichloromethane | 5062 | 2556 | 174 | 239 |
| 9179 | 2338 | 297 | 318 | |
| Dibromochloromethane | 14,281 | 9572 | 1088 | 1723 |
| 16,713 | 12,582 | 3548 | 295 | |
| Bromoform | 9000 | 7657 | 3230 | 1804 |
| 8119 | 9212 | 7350 | 120 | |
| Compound | LOD | LOQ | Linear Range | R2 | RSD | Relative Recovery | Enrichment |
|---|---|---|---|---|---|---|---|
| [ng mL−1] | [ng mL−1] | [ng mL−1] | [%] | [%] | Factor * | ||
| Chloroform | 1.7 | 5.6 | 10–1000 | 0.997 | 8.3 | 87 ± 9 | 87 |
| Bromodichloromethane | 1.9 | 6.2 | 10–1000 | 0.997 | 7.7 | 89 ± 7 | 89 |
| Chlorodibromomethane | 2.7 | 8.9 | 10–1000 | 0.997 | 5.7 | 86 ± 3 | 86 |
| Bromoform | 3.7 | 9.9 | 10–1000 | 0.999 | 5.8 | 95 ± 2 | 95 |
| Extraction | Instrument | LOD | LDR | Relative Recovery | RSD | Ref. |
|---|---|---|---|---|---|---|
| Technique | ng mL−1 | ng mL−1 | % | % | ||
| HF-SBME | GC-µECD | 0.017–0.037 | 10–900 | 74–91 | 5.7–10.3 | [15] |
| HF-LPME | GC-ECD | 0.018–0.049 | 0.88–337.5 | 80.3–104.2 | 1.8–3.7 | [19] |
| HS | GC-MS | 0.023–0.102 | 1.04–230.8 | 86.3–90.0 | 6.8–7.8 | |
| HS-SPME | GC-µECD | 0.057–0.319 | 5–200. | 74.7–120.9 | 1.8–11.0 | [20] |
| SBSE | GC-HRMS | N.R. | N.R. | [21] | ||
| HS | GC-µECD | 0.09–0.14 | 0.1–100 | 2.4–4.3 | [22] | |
| ITEX | GC-MS | 01–10 | 90–103 | <10 | [23] | |
| (pg/mL) | ||||||
| HS-SPME-MMT-IL-AF | GC-µECD | 1.7–3.7 | 10–1000 | 87–95 | <8 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Duque, G.; Lozada-Castro, J.J.; Hara, E.L.Y.; Grassi, M.T.; Rosero-Moreano, M.; Ríos-Acevedo, J.J. Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode. Molecules 2022, 27, 8653. https://doi.org/10.3390/molecules27248653
Sánchez-Duque G, Lozada-Castro JJ, Hara ELY, Grassi MT, Rosero-Moreano M, Ríos-Acevedo JJ. Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode. Molecules. 2022; 27(24):8653. https://doi.org/10.3390/molecules27248653
Chicago/Turabian StyleSánchez-Duque, Gustavo, Juan José Lozada-Castro, Emerson Luis Yoshio Hara, Marco Tadeu Grassi, Milton Rosero-Moreano, and Jhon Jairo Ríos-Acevedo. 2022. "Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode" Molecules 27, no. 24: 8653. https://doi.org/10.3390/molecules27248653
APA StyleSánchez-Duque, G., Lozada-Castro, J. J., Hara, E. L. Y., Grassi, M. T., Rosero-Moreano, M., & Ríos-Acevedo, J. J. (2022). Alternative Ecosorbent for the Determination of Trihalomethanes in Aqueous Samples in SPME Mode. Molecules, 27(24), 8653. https://doi.org/10.3390/molecules27248653







