Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms
Abstract
1. Introduction
2. Results
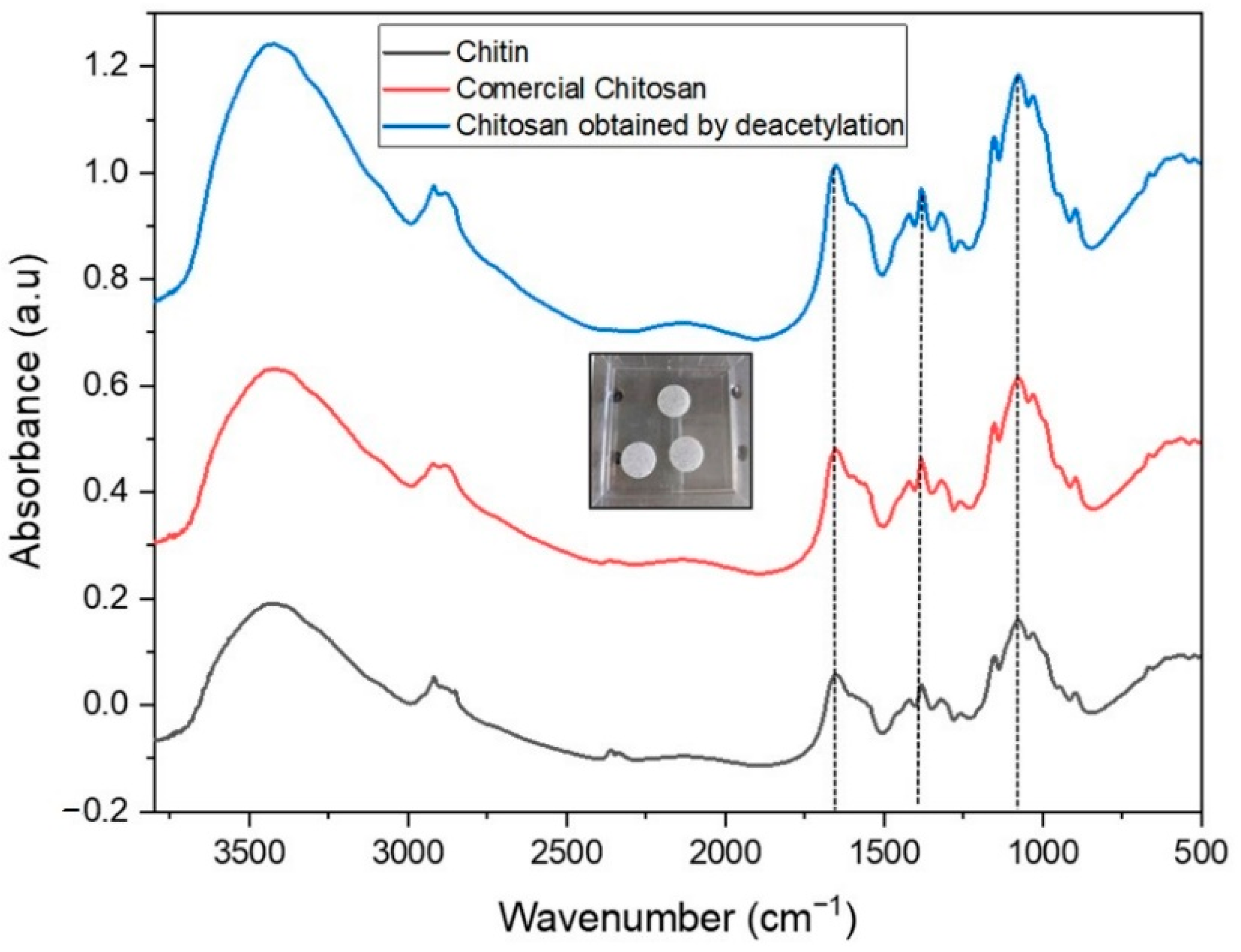
2.1. IR Characterization of Chitin and Chitosan
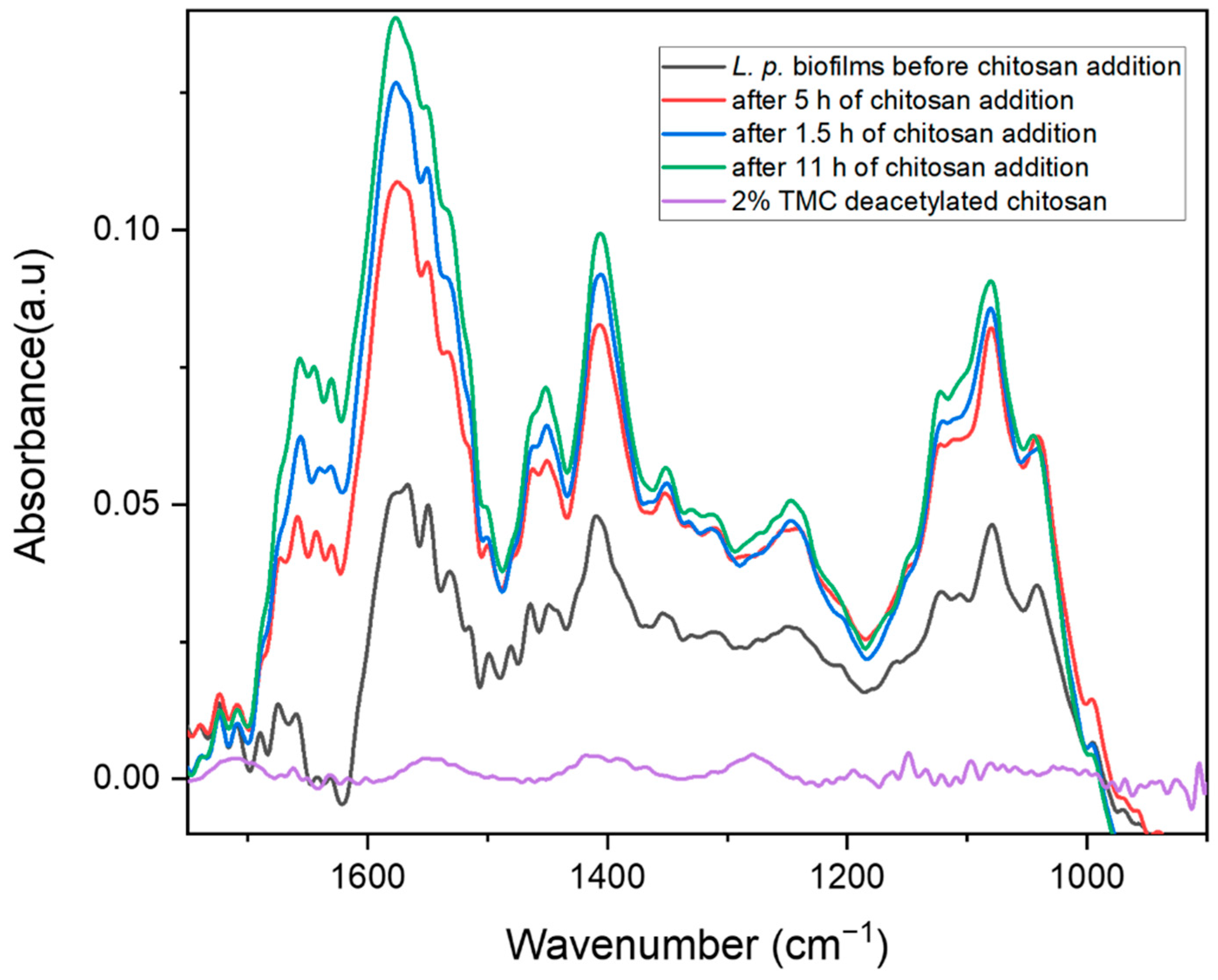
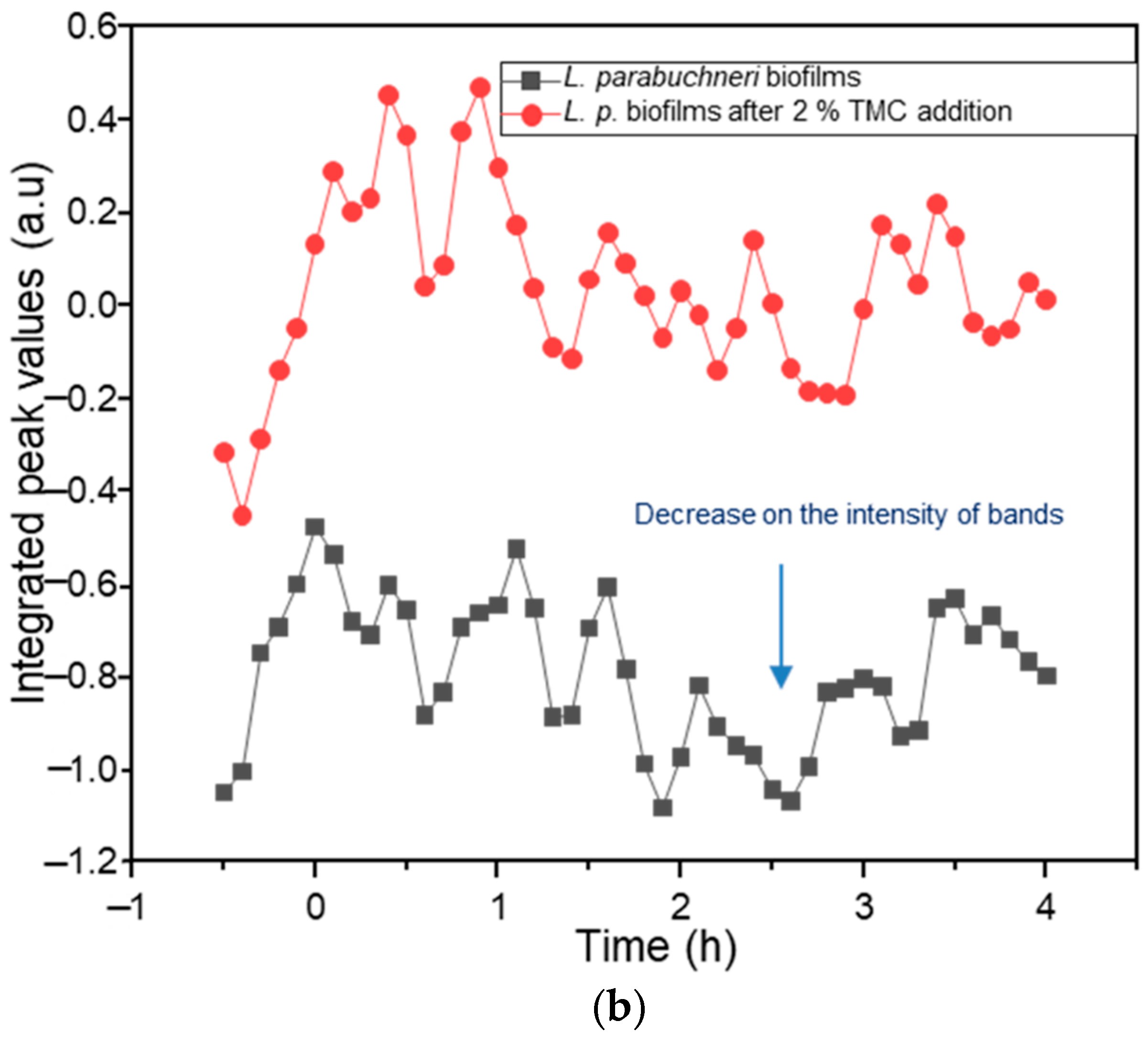
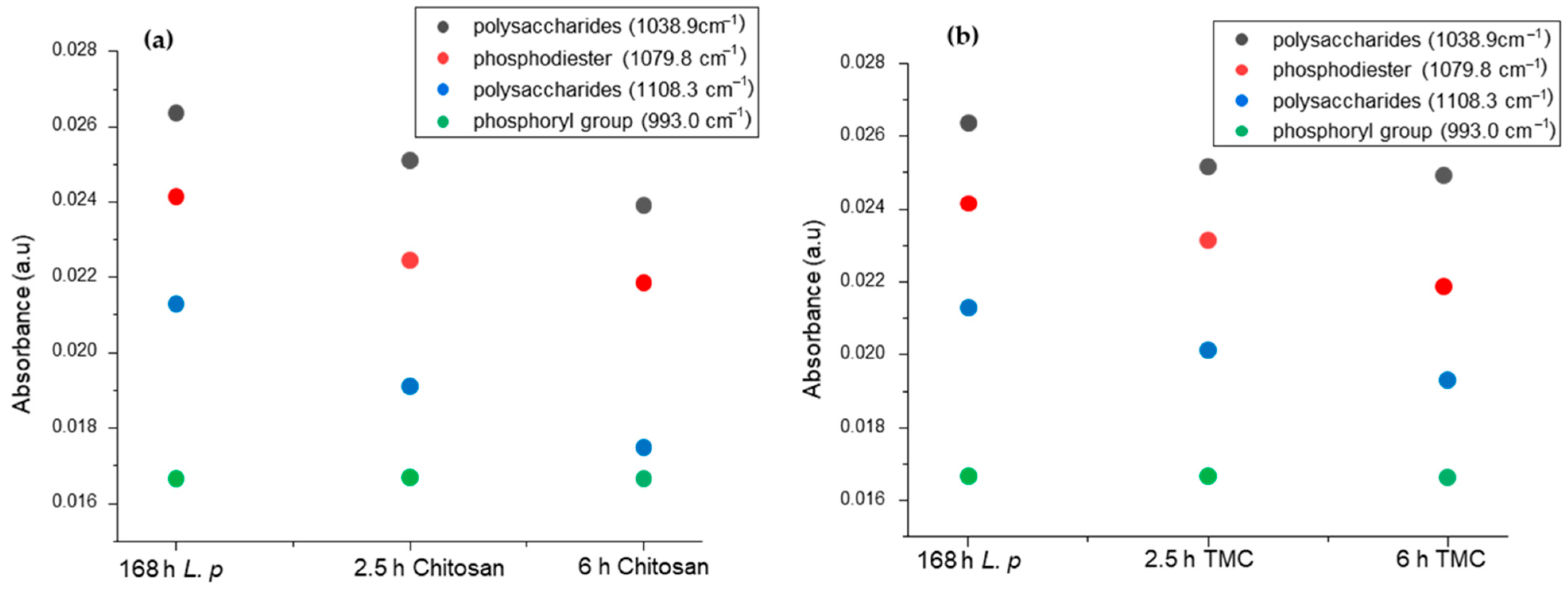
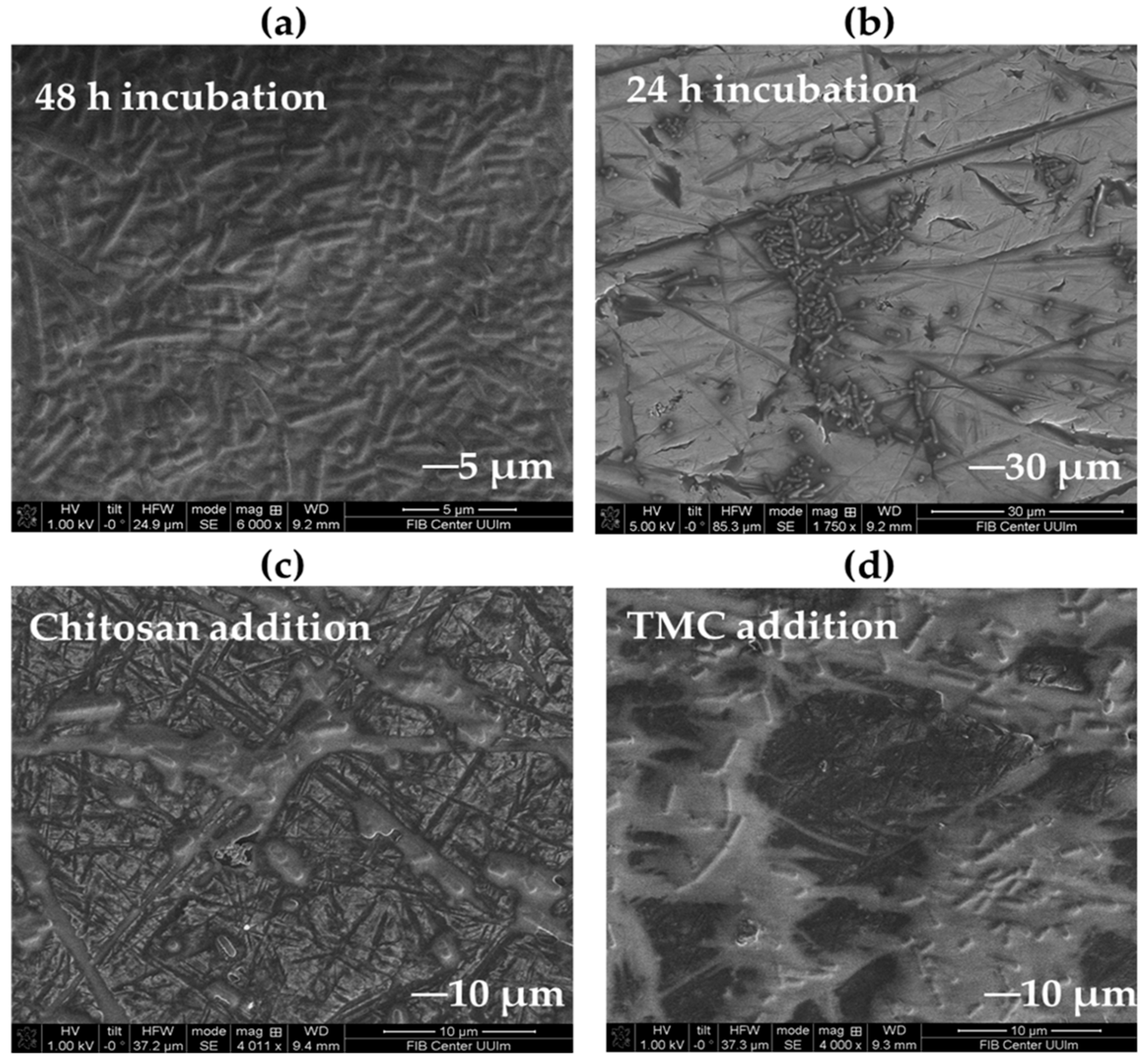
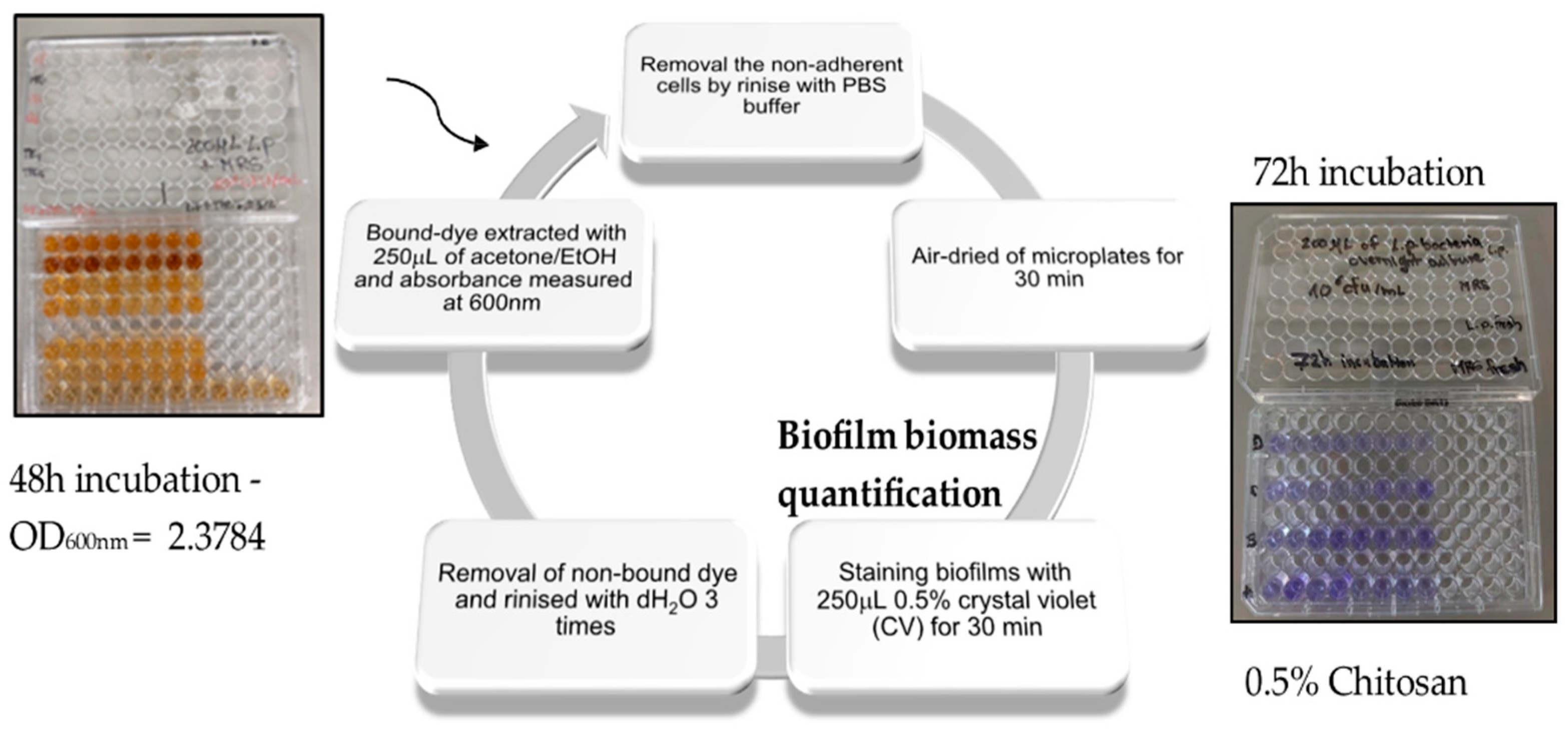
2.2. Biofilm Inhibition by Chitosan
2.3. Biofilm Inhibition by Methylated Chitosan Derivative (TMC)
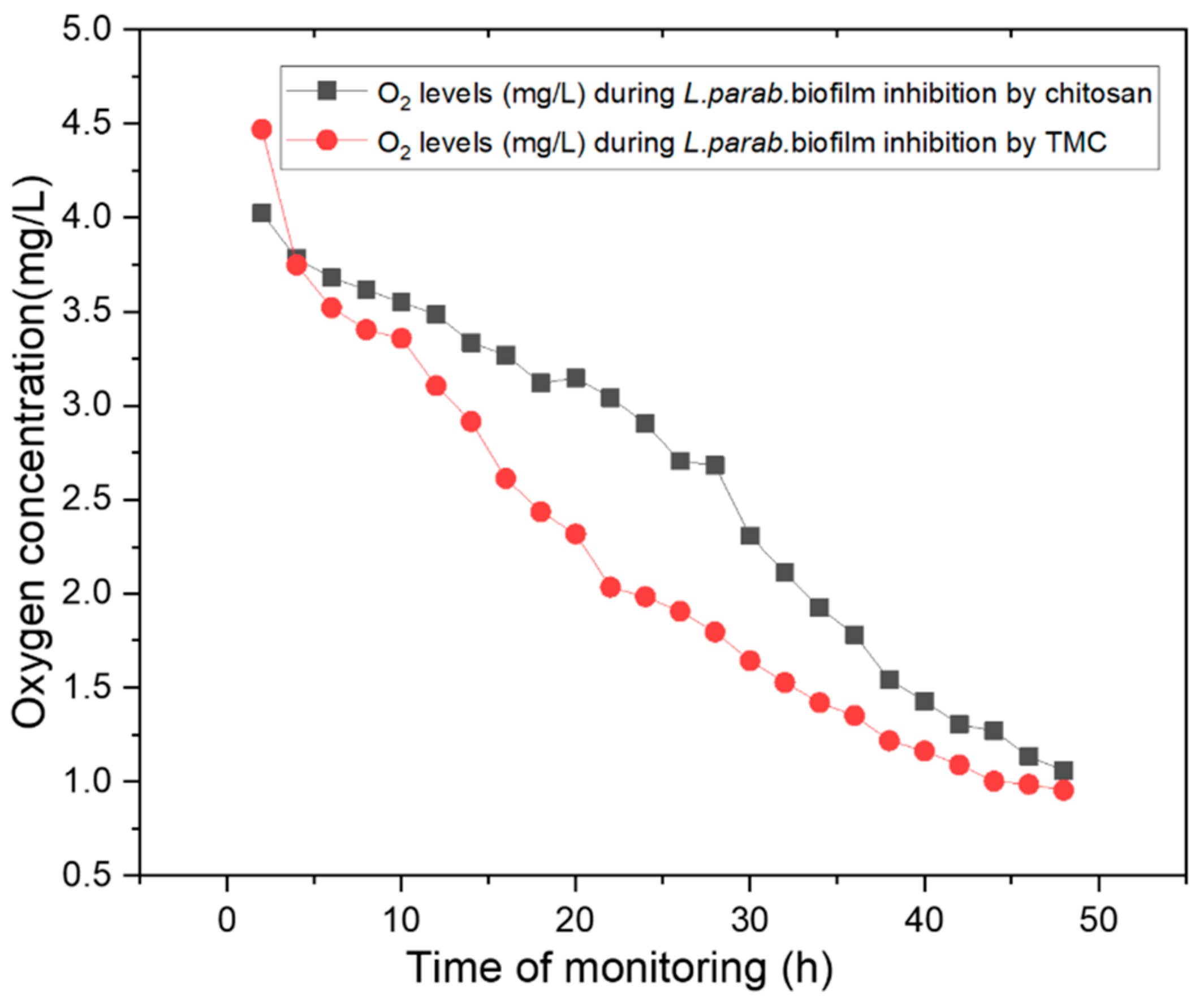
2.4. Monitoring Oxygen Concentrations during Biofilm Inhibition
2.5. Modeling EPS Production during Biofilm Formation and Inhibition
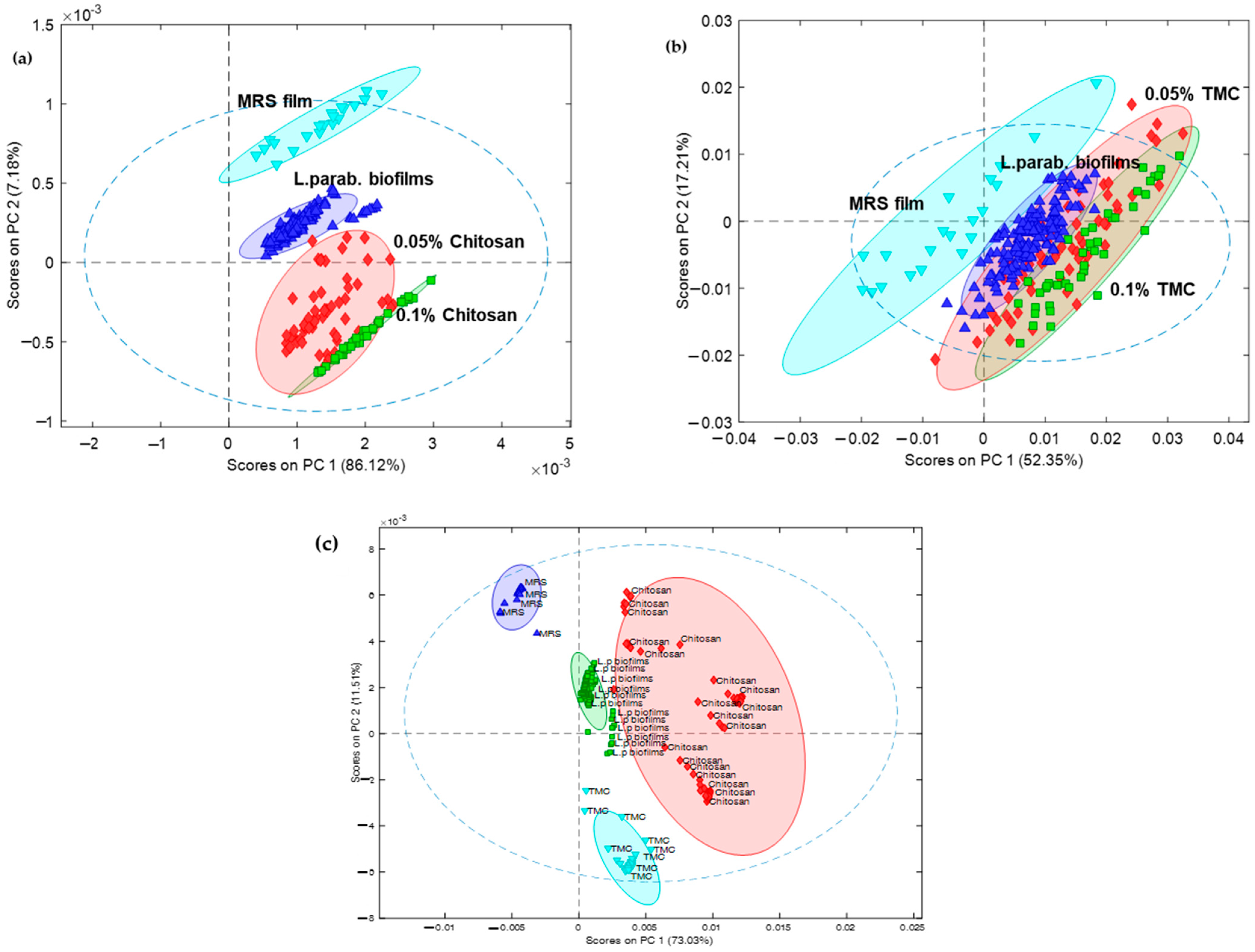
2.6. Multivariate Analysis of the Obtained IR Spectra
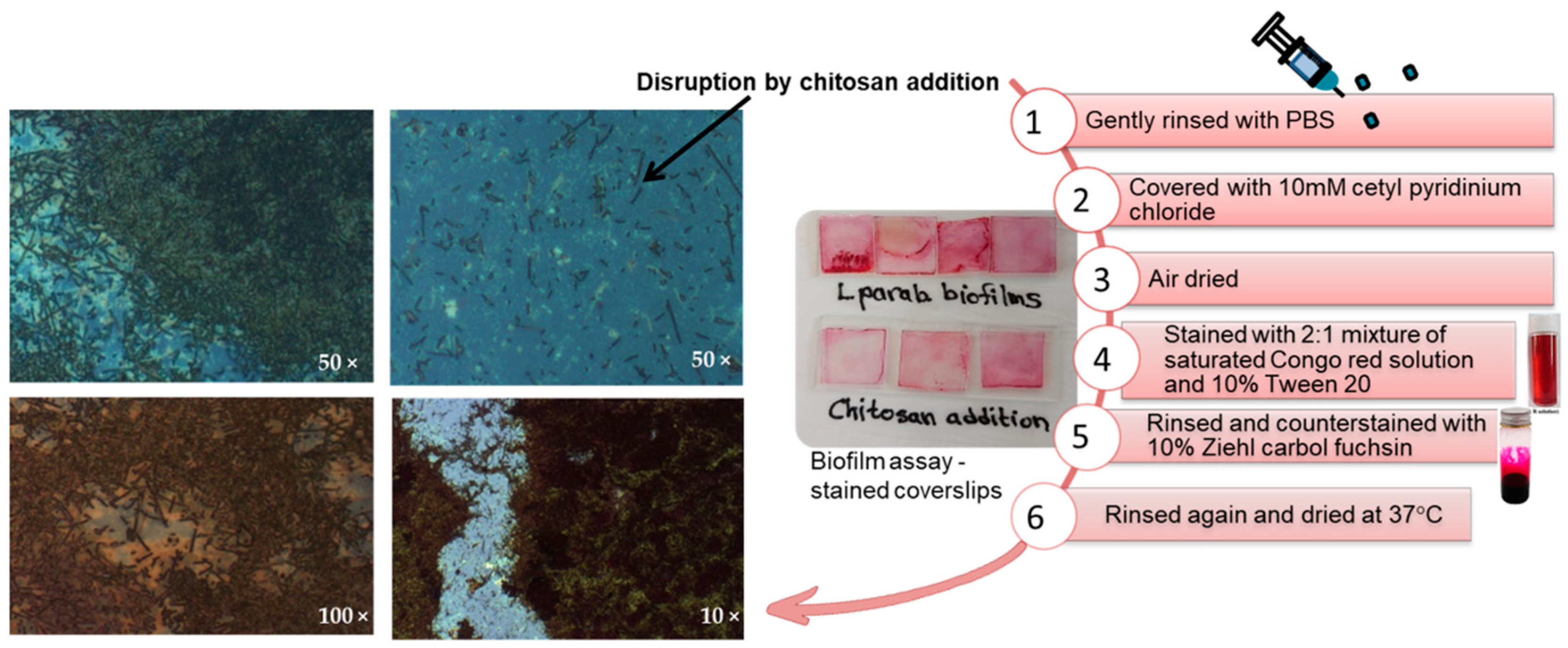
2.7. Microscopic Studies of Biofilm Disruption
2.8. Viability Assay—An Antibiofilm Study
3. Materials and Methods
3.1. Bacterial Cultivation
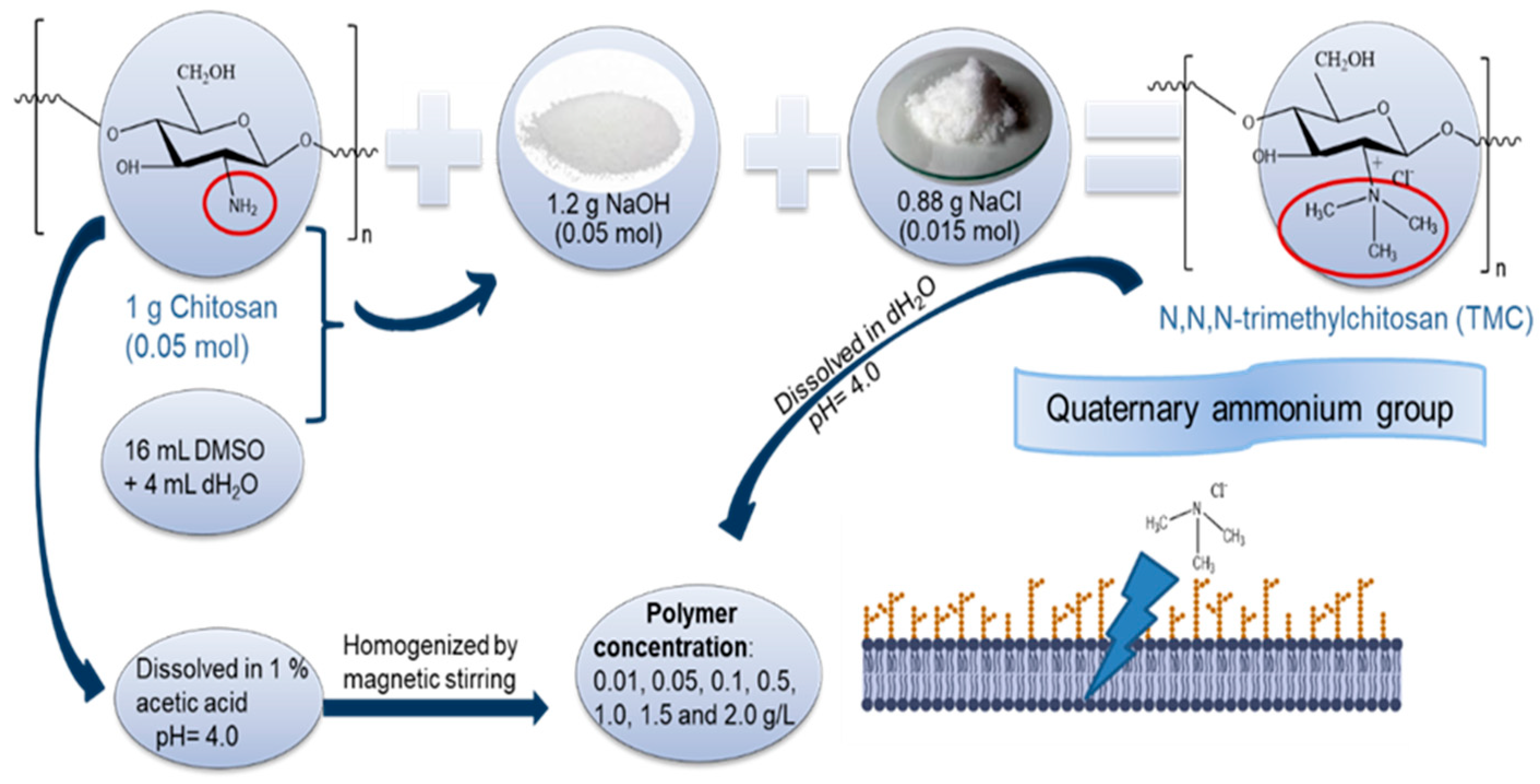
3.2. Stock Solution and Methylation Process
3.3. Vial Cell Assay
3.4. Biofilms Grown on Glass Coverslips
3.5. Biofilms Grown on Stainless Steel
3.6. IR-ATR Flow System for Biofilm Monitoring
3.7. Integration of IR Spectroscopy and Fluorescence Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of Plant Phenolic Compounds on Biofilm Formation by Pseudomonas aeruginosa. Apmis 2013, 121, 1073–1081. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Anand, S.K. Significance of Microbial Biofilms in Food Industry: A Review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm Formation by Lactic Acid Bacteria and Resistance to Environmental Stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef]
- Somers, E.B.; Johnson, M.E.; Wong, A.C.L. Biofilm Formation and Contamination of Cheese by Nonstarter Lactic Acid Bacteria in the Dairy Environment. J. Dairy Sci. 2001, 84, 1926–1936. [Google Scholar] [CrossRef]
- Berthoud, H.; Wüthrich, D.; Bruggmann, R.; Wechsler, D.; Fröhlich-Wyder, M.T.; Irmler, S. Development of New Methods for the Quantitative Detection and Typing of Lactobacillus parabuchneri in Dairy Products. Int. Dairy J. 2017, 70, 65–71. [Google Scholar] [CrossRef]
- Ascone, P.; Maurer, J.; Haldemann, J.; Irmler, S.; Berthoud, H.; Portmann, R.; Fröhlich-Wyder, M.T.; Wechsler, D. Prevalence and Diversity of Histamine-Forming Lactobacillus parabuchneri Strains in Raw Milk and Cheese—A Case Study. Int. Dairy J. 2017, 70, 26–33. [Google Scholar] [CrossRef]
- Shkodra, B.; Abera, B.D.; Cantarella, G.; Douaki, A.; Avancini, E.; Petti, L.; Lugli, P. Flexible and Printed Electrochemical Immunosensor Coated with Oxygen Plasma Treated SWCNTs for Histamine Detection. Biosensors 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; Del Rio, B.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Biofilm-Forming Capacity in Biogenic Amine-Producing Bacteria Isolated from Dairy Products. Front. Microbiol. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Holzapfel, W.H. Improved Screening Procedure for Biogenic Amine Production by Lactic Acid Bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Wyder, M.T.; Guggisberg, D.; Badertscher, R.; Wechsler, D.; Wittwer, A.; Irmler, S. The Effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the Eye Formation of Semi-Hard Cheese. Int. Dairy J. 2013, 33, 120–128. [Google Scholar] [CrossRef]
- Bajrami, D.; Fischer, S.; Barth, H.; Sarquis, M.A.; Ladero, V.M. In Situ Monitoring of Lentilactobacillus parabuchneri Bio Fi Lm Formation via Real-Time Infrared Spectroscopy. npj Biofilms Microbiomes 2022, 8, 92. [Google Scholar] [CrossRef]
- Ladero, V.; Fernández, M.; Alvarez, M.A. Effect of Post-Ripening Processing on the Histamine and Histamine-Producing Bacteria Contents of Different Cheeses. Int. Dairy J. 2009, 19, 759–762. [Google Scholar] [CrossRef]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Roig-Sagués, A.X.; Trujillo-Mesa, A.J.; Vidal-Carou, M.C. Evaluation of Biogenic Amines and Microbial Counts throughout the Ripening of Goat Cheeses from Pasteurized and Raw Milk. J. Dairy Res. 2004, 71, 245–252. [Google Scholar] [CrossRef]
- Kukushkina, E.A.; Mateos, H.; Altun, N.; Sportelli, M.C.; Gonzalez, P.; Picca, R.A.; Cioffi, N. Highly Stable Core-Shell Nanocolloids: Synergy between Nano-Silver and Natural Polymers to Prevent Biofilm Formation. Antibiotics 2022, 11, 1396. [Google Scholar] [CrossRef]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Bullock, G.; Blazer, V.; Tsukuda, S.; Summerfelt, S. Toxicity of Acidified Chitosan for Cultured Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2000, 185, 273–280. [Google Scholar] [CrossRef]
- Borchard, G.; Som, C.; Zinn, M.; Ostafe, V.; Borges, O.; Perale, G.; Wick, P. Editorial: Polymeric Nano-Biomaterials for Medical Applications: Advancements in Developing and Implementation Considering Safety-by-Design Concepts. Front. Bioeng. Biotechnol. 2020, 8, 599950. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.; Pezzoli, D.; Chevallier, P.; Souza, C.; Candiani, G. Antibacterial Coatings Based on Chitosan for Pharmaceutical and Biomedical Applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Matica, A.; Menghiu, G.; Ostafe, V. Antibacterial Properties of Chitin and Chitosans. Former Ann. West Univ. Timisoara-Ser. Chem. 2017, 26, 39–54. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Sadeghi, A.M.M.; Amini, M.; Avadi, M.R.; Siedi, F.; Rafiee-Tehrani, M.; Junginger, H.E. Synthesis, Characterization, and Antibacterial Effects of Trimethylated and Triethylated 6-NH2-6-Deoxy Chitosan. J. Bioact. Compat. Polym. 2008, 23, 262–275. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, J.; Li, T.; Qiao, C.; Li, Y. Synthesis and Antibacterial Activities of Quaternary Ammonium Salt of Gelatin. J. Macromol. Sci. Part B Phys. 2014, 53, 133–141. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in Antimicrobial Activities of Chitin, Chitosan and Its Oligosaccharides: A Systematic Study Needs for Food Applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Wang, W.; Liu, Q. Preparation and Antibacterial Activity of a Water-Soluble Chitosan Derivative. Carbohydr. Polym. 2002, 50, 35–40. [Google Scholar] [CrossRef]
- Ristić, T.; Lasič, S.; Kosalec, I.; Bračič, M.; Fras-Zemljič, L. The Effect of Chitosan Nanoparticles onto Lactobacillus Cells. React. Funct. Polym. 2015, 97, 56–62. [Google Scholar] [CrossRef]
- Pan, C.; Rezaei, H.; Soor, A. Chitosan Disrupts Membrane Permeability of Lactic Acid Bacteria. J. Exp. Microbiol. Inmunol. 2011, 15, 7–14. [Google Scholar]
- Tanganini, I.C.; Shirahigue, L.D.; Altenhofen da Silva, M.; Francisco, K.R.; Ceccato-Antonini, S.R. Bioprocessing of Shrimp Wastes to Obtain Chitosan and Its Antimicrobial Potential in the Context of Ethanolic Fermentation against Bacterial Contamination. 3 Biotech 2020, 10, 135. [Google Scholar] [CrossRef]
- Hmed, A.A.; Sofy, A.R.; Sharaf, A.E.-M.M.A.; El-Dougdoug, K.A. Effectiveness of Chitosan as Naturally-Derived Antimicrobial to Fulfill the Needs of Today’s Consumers Looking for Food without Hazards of Chemical Preservatives. J. Microbiol. Res. 2017, 7, 55–67. [Google Scholar] [CrossRef]
- Savard, T.; Beaulieu, C.; Boucher, I.; Champagne, C.P. Antimicrobial Action of Hydrolyzed Chitosan against Spoilage Yeasts and Lactic Acid Bacteria of Fermented Vegetables. J. Food Prot. 2002, 65, 828–833. [Google Scholar] [CrossRef]
- de Britto, D.; Assis, O.B.G. A Novel Method for Obtaining a Quaternary Salt of Chitosan. Carbohydr. Polym. 2007, 69, 305–310. [Google Scholar] [CrossRef]
- Agarwal, M.; Nagar, D.P.; Srivastava, N.; Agarwal, M.K. Chitosan Nanoparticles Based Drug Delivery: An Update. Int. J. Adv. Multidiscip. Res. 2015, 2, 1–13. [Google Scholar]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Niculescu, A.G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Tantala, J.; Thumanu, K.; Rachtanapun, C. An Assessment of Antibacterial Mode of Action of Chitosan on Listeria Innocua Cells Using Real-Time HATR-FTIR Spectroscopy. Int. J. Biol. Macromol. 2019, 135, 386–393. [Google Scholar] [CrossRef]
- Anicuta, S.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier Transform Infrared (Ftir) Spectroscopy for Characterization of Antimicrobial Films Containing Chitosan. An. Univ. Oradea Fasc. Ecotoxicol. Zooteh. Tehnol. Ind. Aliment. 2010, 2010, 1234–1240. [Google Scholar]
- Quilès, F.; Saadi, S.; Francius, G.; Bacharouche, J.; Humbert, F. In Situ and Real Time Investigation of the Evolution of a Pseudomonas fluorescens Nascent Biofilm in the Presence of an Antimicrobial Peptide. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yunda, E.; Alem, H.; Francius, G.; Gago, R.; Quilès, F. Chemical Functionalization of the Zinc Selenide Surface and Its Impact on Lactobacillus rhamnosus GG Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 14933–14945. [Google Scholar] [CrossRef] [PubMed]
- Yunda, E.; Quilès, F. In Situ Spectroscopic Analysis of Lactobacillus rhamnosus GG Flow on an Abiotic Surface Reveals a Role for Nutrients in Biofilm Development. Biofouling 2019, 35, 494–507. [Google Scholar] [CrossRef]
- Humbert, F.; Quilès, F. In-Situ Study of Early Stages of Biofilm Formation under Different Environmental Stresses by ATR-FTIR Spectroscopy. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 1, 889–895. [Google Scholar]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banat, I.M.; Banpurkar, A.G. Inhibition of Pathogenic Bacterial Biofilms on PDMS Based Implants by L. acidophilus Derived Biosurfactant. BMC Microbiol. 2019, 19, 39. [Google Scholar] [CrossRef]
- Gonçalves, R.C.; da Silva, D.P.; Signini, R.; Naves, P.L.F. Inhibition of Bacterial Biofilms by Carboxymethyl Chitosan Combined with Silver, Zinc and Copper Salts. Int. J. Biol. Macromol. 2017, 105, 385–392. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and Bioactivities of the Exopolysaccharide from a Probiotic Strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Lorite, G.S.; de Souza, A.A.; Neubauer, D.; Mizaikoff, B.; Kranz, C.; Cotta, M.A. On the Role of Extracellular Polymeric Substances during Early Stages of Xylella fastidiosa Biofilm Formation. Colloids Surf. B Biointerfaces 2013, 102, 519–525. [Google Scholar] [CrossRef]
- Stenclova, P.; Freisinger, S.; Barth, H.; Kromka, A.; Mizaikoff, B. Cyclic Changes in the Amide Bands Within Escherichia coli Biofilms Monitored Using Real-Time Infrared Attenuated Total Reflection Spectroscopy (IR-ATR). Appl. Spectrosc. 2019, 73, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, G.; Sportelli, M.C.; Heinzmann, A.; Picca, R.A.; Valentini, A.; Barth, H.; Mizaikoff, B.; Cioffi, N.; Kranz, C. Silver-Fluoropolymer (Ag-CFX) Films: Kinetic Study of Silver Release, and Spectroscopic-Microscopic Insight into the Inhibition of P. fluorescens Biofilm Formation. Anal. Chim. Acta 2022, 1212, 339892. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N. Inhibiting P. fluorescens Biofilms with Fluoropolymer-Embedded Silver Nanoparticles: An in-Situ Spectroscopic Study. Sci. Rep. 2017, 7, 11870. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Garcia, J.G.; Williams, R.; Elmassry, M.; West, A.; Hamood, A.; Hurtado, D.; Gudenkauf, B.; Ventolini, G.; Schlabritz-Loutsevitch, N. Lactobacilli spp.: Real-Time Evaluation of Biofilm Growth. BMC Microbiol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the Bacterial Cell Envelope by Antimicrobial Peptides Gramicidin S and PGLa as Revealed by Transmission and Scanning Electron Microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Acemi, A. Monitoring the Effects of Chitosan on the Profile of Certain Cell Wall and Membrane Biomolecules in the Leaves of Eruca vesicaria ssp. sativa through FT-IR Spectroscopy. Plant Physiol. Biochem. 2022, 173, 25–32. [Google Scholar] [CrossRef]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-Friendly Extraction and Characterization of Chitin and Chitosan from the Shrimp Shell Waste via Microwave Irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An Infrared Investigation in Relation with Chitin and Chitosan Characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Xie, T.; Liao, Z.; Lei, H.; Fang, X.; Wang, J.; Zhong, Q. Antibacterial Activity of Food-Grade Chitosan against Vibrio parahaemolyticus Biofilms. Microb. Pathog. 2017, 110, 291–297. [Google Scholar] [CrossRef]
- Ibañez-Peinado, D.; Ubeda-Manzanaro, M.; Martínez, A.; Rodrigo, D. Antimicrobial Effect of Insect Chitosan on Salmonella Typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes Survival. PLoS ONE 2020, 15, e0244153. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Y.; Terzi, G. In Vitro Effects of Chitosan on the Survival of Listeria Monocytogenes. Erciyes Üniversitesi Vet. Fakültesi Derg. 2016, 13, 11–18. [Google Scholar]
- Khurana, R.; Fink, A.L. Do Parallel β-Helix Proteins Have a Unique Fourier Transform Infrared Spectrum? Biophys. J. 2000, 78, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the Antimicrobial Activity of Chitosan and Its Quaternized Derivative on E. coli and S. aureus Growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Shalan, A.E.; Sabaa, M.W.; Mohamed, R.R. One-Pot Green Synthesis of Antimicrobial Chitosan Derivative Nanocomposites to Control Foodborne Pathogens. RSC Adv. 2022, 12, 1095–1104. [Google Scholar] [CrossRef]
- Dobbs, G.T.; Mizaikoff, B. Shining New Light on Old Principles: Localization of Evanescent Field Interactions at Infrared-Attenuated Total Reflection Sensing Interfaces. Appl. Spectrosc. 2006, 60, 573–583. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Yi, Z.; Zeng, F.; Wu, W.; Qiao, Y.; Zhao, X.; Cheng, X.; Tian, Y. Hydrogel-Based Fluorescent Dual pH and Oxygen Sensors Loaded in 96-Well Plates for High-Throughput Cell Metabolism Studies. Sensors 2018, 18, 564. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.B.; Radhakrishnan, T.K. Fiber-Optic Sensors for the Estimation of Oxygen Gradients within Biofilms on Metals. Opt. Lasers Eng. 2008, 46, 321–327. [Google Scholar] [CrossRef]
- Pedraz, L.; Blanco-Cabra, N.; Torrents, E. Gradual Adaptation of Facultative Anaerobic Pathogens to Microaerobic and Anaerobic Conditions. FASEB J. 2020, 34, 2912–2928. [Google Scholar] [CrossRef]
- Scheicher, S.R.; Kainz, B.; Köstler, S.; Suppan, M.; Bizzarri, A.; Pum, D.; Sleytr, U.B.; Ribitsch, V. Optical Oxygen Sensors Based on Pt(II) Porphyrin Dye Immobilized on S-Layer Protein Matrices. Biosens. Bioelectron. 2009, 25, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Bettenbrock, K.; Bai, H.; Ederer, M.; Green, J.; Hellingwerf, K.J.; Holcombe, M.; Kunz, S.; Rolfe, M.D.; Sanguinetti, G.; Sawodny, O.; et al. Towards a Systems Level Understanding of the Oxygen Response of Escherichia coli. Adv. Microb. Physiol. 2014, 64, 65–114. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Ocone, A.; Stapleton, M.R.; Hall, S.; Trotter, E.W.; Poole, R.K.; Sanguinetti, G.; Green, J. Systems Analysis of Transcription Factor Activities in Environments with Stable and Dynamic Oxygen Concentrations. Open Biol. 2012, 2, 120091. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shumway, B.R.; Gao, W.; Youngbull, C.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. Influence of Matrices on Oxygen Sensing of Three Sensing Films with Chemically Conjugated Platinum Porphyrin Probes and Preliminary Application for Monitoring of Oxygen Consumption of Escherichia coli (E. coli). Sens. Actuators B Chem. 2010, 150, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen Limitation Contributes to Antibiotic Tolerance of Pseudomonas aeruginosa in Biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Wyder, M.T.; Bisig, W.; Guggisberg, D.; Irmler, S.; Jakob, E.; Wechsler, D. Influence of Low pH on the Metabolic Activity of Lactobacillus buchneri and Lactobacillus parabuchneri Strains in Tilsit-Type Model Cheese. Dairy Sci. Technol. 2015, 95, 569–585. [Google Scholar] [CrossRef]
- Botello-Morte, L.; Moniente, M.; Gil-Ramírez, Y.; Virto, R.; García-Gonzalo, D.; Pagán, R. Identification by Means of Molecular Tools of the Microbiota Responsible for the Formation of Histamine Accumulated in Commercial Cheeses in Spain. Food Control 2022, 133, 108595. [Google Scholar] [CrossRef]
- Stoodley, P.; DeBeer, D.; Lappin-Scott, H.M. Influence of Electric Fields and pH on Biofilm Structure as Related to the Bioelectric Effect. Antimicrob. Agents Chemother. 1997, 41, 1876–1879. [Google Scholar] [CrossRef]
- Ji, Q.X.; Chen, X.G.; Zhao, Q.S.; Liu, C.S.; Cheng, X.J.; Wang, L.C. Injectable Thermosensitive Hydrogel Based on Chitosan and Quaternized Chitosan and the Biomedical Properties. J. Mater. Sci. Mater. Med. 2009, 20, 1603–1610. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Lu, H.; Liu, Z.; Wu, Y.; Mao, J.; Gong, S. Effects of the Combined Application of Trimethylated Chitosan and Carbodiimide on the Biostability and Antibacterial Activity of Dentin Collagen Matrix. Polymers 2022, 14, 3166. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K. De Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Tran, V.N.; Dasagrandhi, C.; Truong, V.G.; Kim, Y.M.; Kang, H.W. Antibacterial Activity of Staphylococcus aureus Biofilm under Combined Exposure of Glutaraldehyde, near-Infrared Light, and 405-Nm Laser. PLoS ONE 2018, 13, e0202821. [Google Scholar] [CrossRef] [PubMed]
- Lorite, G.S.; Rodrigues, C.M.; de Souza, A.A.; Kranz, C.; Mizaikoff, B.; Cotta, M.A. The Role of Conditioning Film Formation and Surface Chemical Changes on Xylella fastidiosa Adhesion and Biofilm Evolution. J. Colloid Interface Sci. 2011, 359, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Saxena, A.; Song, B.; Ward, B.B.; Beveridge, T.J.; Myneni, S.C.B. Elucidation of Functional Groups on Gram-Positive and Gram-Negative Bacterial Surfaces Using Infrared Spectroscopy. Langmuir 2004, 20, 11433–11442. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.J.; Mukome, F.N.D.; Zhang, X. ATR-FTIR Spectroscopic Evidence for Biomolecular Phosphorus and Carboxyl Groups Facilitating Bacterial Adhesion to Iron Oxides. Colloids Surf. B Biointerfaces 2014, 119, 38–46. [Google Scholar] [CrossRef]
- Quilès, F.; Humbert, F.; Delille, A. Analysis of Changes in Attenuated Total Reflection FTIR Fingerprints of Pseudomonas fluorescens from Planktonic State to Nascent Biofilm State. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2010, 75, 610–616. [Google Scholar] [CrossRef]
- Brangule, A.; Skadiņš, I.; Reinis, A.; Gross, K.A.; Kroča, J. In Vitro Characterization Perspectives Using Fourier Transform Infrared Photoacoustic Spectroscopy (FTIR PAS) and Diffuse Reflectance Infrared Spectroscopy (DRIFT). In Proceedings of the Key Engineering Materials, Kuantan, Malaysia, 1–2 August 2017; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2017; Volume 758, pp. 273–277. [Google Scholar] [CrossRef]
- Neugebauer, U.; Schmid, U.; Baumann, K.; Ziebuhr, W.; Kozitskaya, S.; Deckert, V.; Schmitt, M.; Popp, J. Towards a Detailed Understanding of Bacterial Metabolism—Spectroscopic Characterization of Stophylococcus epidermidis. ChemPhysChem 2007, 8, 124–137. [Google Scholar] [CrossRef]
- Jung, G.B.; Nam, S.W.; Choi, S.; Lee, G.-J.; Park, H.-K. Evaluation of Antibiotic Effects on Pseudomonas aeruginosa Biofilm Using Raman Spectroscopy and Multivariate Analysis. Biomed. Opt. Express 2014, 5, 3238. [Google Scholar] [CrossRef]
- Obaid, N.A. Application of Infra-Red Spectroscopy to the Evaluation of Biofilm Formation and Pathogenesis of Nontypeable Haemophilus Influenzae. PhD Thesis, University of Tasmania, Hobart, Australia, 2015. [Google Scholar]
- Jollife, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Mohsin, G.F.; Schmitt, F.J.; Kanzler, C.; Hoehl, A.; Hornemann, A. PCA-Based Identification and Differentiation of FTIR Data from Model Melanoidins with Specific Molecular Compositions. Food Chem. 2019, 281, 106–113. [Google Scholar] [CrossRef]
- Suci, P.A.; Siedlecki, K.J.; Palmer, R.J.; White, D.C.; Geesey, G.G. Combined Light Microscopy and Attenuated Total Reflection Fourier Transform Infrared Spectroscopy for Integration of Biofilm Structure, Distribution, and Chemistry at Solid-Liquid Interfaces. Appl. Environ. Microbiol. 1997, 63, 4600–4603. [Google Scholar] [CrossRef]
- Larimer, C.; Suter, J.D.; Bonheyo, G.; Addleman, R.S. In Situ Non-Destructive Measurement of Biofilm Thickness and Topology in an Interferometric Optical Microscope. J. Biophotonics 2016, 9, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Allan, J.A.; Theodoropoulos, C.; Ross, T.; Beagley, K.W.; Knox, C.L. Hormone-Dependent Bacterial Growth, Persistence and Biofilm Formation—A Pilot Study Investigating Human Follicular Fluid Collected during IVF Cycles. PLoS ONE 2012, 7, e0049965. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; del Rio, B.; Sanchez-Llana, E.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Lactobacillus parabuchneri Produces Histamine in Refrigerated Cheese at a Temperature-Dependent Rate. Int. J. Food Sci. Technol. 2018, 53, 2342–2348. [Google Scholar] [CrossRef]
- Gamby, J.; Pailleret, A.; Clodic, C.B.; Pradier, C.M.; Tribollet, B. In Situ Detection and Characterization of Potable Water Biofilms on Materials by Microscopic, Spectroscopic and Electrochemistry Methods. Electrochim. Acta 2008, 54, 66–73. [Google Scholar] [CrossRef]
- Lorite, G.S.; Janissen, R.; Clerici, J.H.; Rodrigues, C.M.; Tomaz, J.P.; Mizaikoff, B.; Kranz, C.; de Souza, A.A.; Cotta, M.A. Surface Physicochemical Properties at the Micro and Nano Length Scales: Role on Bacterial Adhesion and Xylella fastidiosa Biofilm Development. PLoS ONE 2013, 8, e0075247. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Wang, G.; Xu, X.; Zhou, G. In Situ Characterization and Analysis of Salmonella Biofilm Formation under Meat Processing Environments Using a Combined Microscopic and Spectroscopic Approach. Int. J. Food Microbiol. 2013, 167, 293–302. [Google Scholar] [CrossRef]
- Pintado, M. Antimicrobial Effect of Chitosan against Periodontal Pathogens Biofilms. SOJ Microbiol. Infect. Dis. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and Insights into Chitosan Antimicrobial Activity against Anaerobic Oral Pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef]
- Fernández Ramírez, M.D.; Smid, E.J.; Abee, T.; Nierop Groot, M.N. Characterisation of Biofilms Formed by Lactobacillus plantarum WCFS1 and Food Spoilage Isolates. Int. J. Food Microbiol. 2015, 207, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberg, S. Cell Death in Pseudomonas aeruginosa Biofilm Development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.H.; de Jonge, M.I. For the Greater Good: Programmed Cell Death in Bacterial Communities. Microbiol. Res. 2018, 207, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Cvitkovitch, D.G.; Lévesque, C.M. Cell Death in Streptococcus mutans Biofilms: A Link between CSP and Extracellular DNA. FEMS Microbiol. Lett. 2009, 299, 261–266. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—Ninth Edition. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2015; Volume 32, p. 18.
- Allison, D.G.; Sutherland, I.W. The Role of Exopolysaccharides in Adhesion of Freshwater Bacteria. J. Gen. Microbiol. 1987, 133, 1319–1327. [Google Scholar] [CrossRef][Green Version]
- McDonagh, C.; Kolle, C.; McEvoy, A.K.; Dowling, D.L.; Cafolla, A.A.; Cullen, S.J.; MacCraith, B.D. Phase Fluorometric Dissolved Oxygen Sensor. Sens. Actuators B Chem. 2001, 74, 124–130. [Google Scholar] [CrossRef]


| Peak Attribution | x0 [cm−1] | w Range [cm−1] |
|---|---|---|
| P-OH/C-C ( [84,86] | 1108.3 | 5–45 |
| ν P = O () [50,84,85] | 1079.8 | 5–30 |
| ν O–H/δ C–O () [86] | 1038.9 | 5–35 |
| Ν phosphoryl group () [50] | 993.0 | 5–50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajrami, D.; Fischer, S.; Barth, H.; Hossain, S.I.; Cioffi, N.; Mizaikoff, B. Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms. Molecules 2022, 27, 8647. https://doi.org/10.3390/molecules27248647
Bajrami D, Fischer S, Barth H, Hossain SI, Cioffi N, Mizaikoff B. Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms. Molecules. 2022; 27(24):8647. https://doi.org/10.3390/molecules27248647
Chicago/Turabian StyleBajrami, Diellza, Stephan Fischer, Holger Barth, Syed Imdadul Hossain, Nicola Cioffi, and Boris Mizaikoff. 2022. "Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms" Molecules 27, no. 24: 8647. https://doi.org/10.3390/molecules27248647
APA StyleBajrami, D., Fischer, S., Barth, H., Hossain, S. I., Cioffi, N., & Mizaikoff, B. (2022). Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms. Molecules, 27(24), 8647. https://doi.org/10.3390/molecules27248647











