Anti-Biofouling Electrochemical Sensor Based on the Binary Nanocomposite of Silica Nanochannel Array and Graphene for Doxorubicin Detection in Human Serum and Urine Samples
Abstract
1. Introduction
2. Results and Discussion
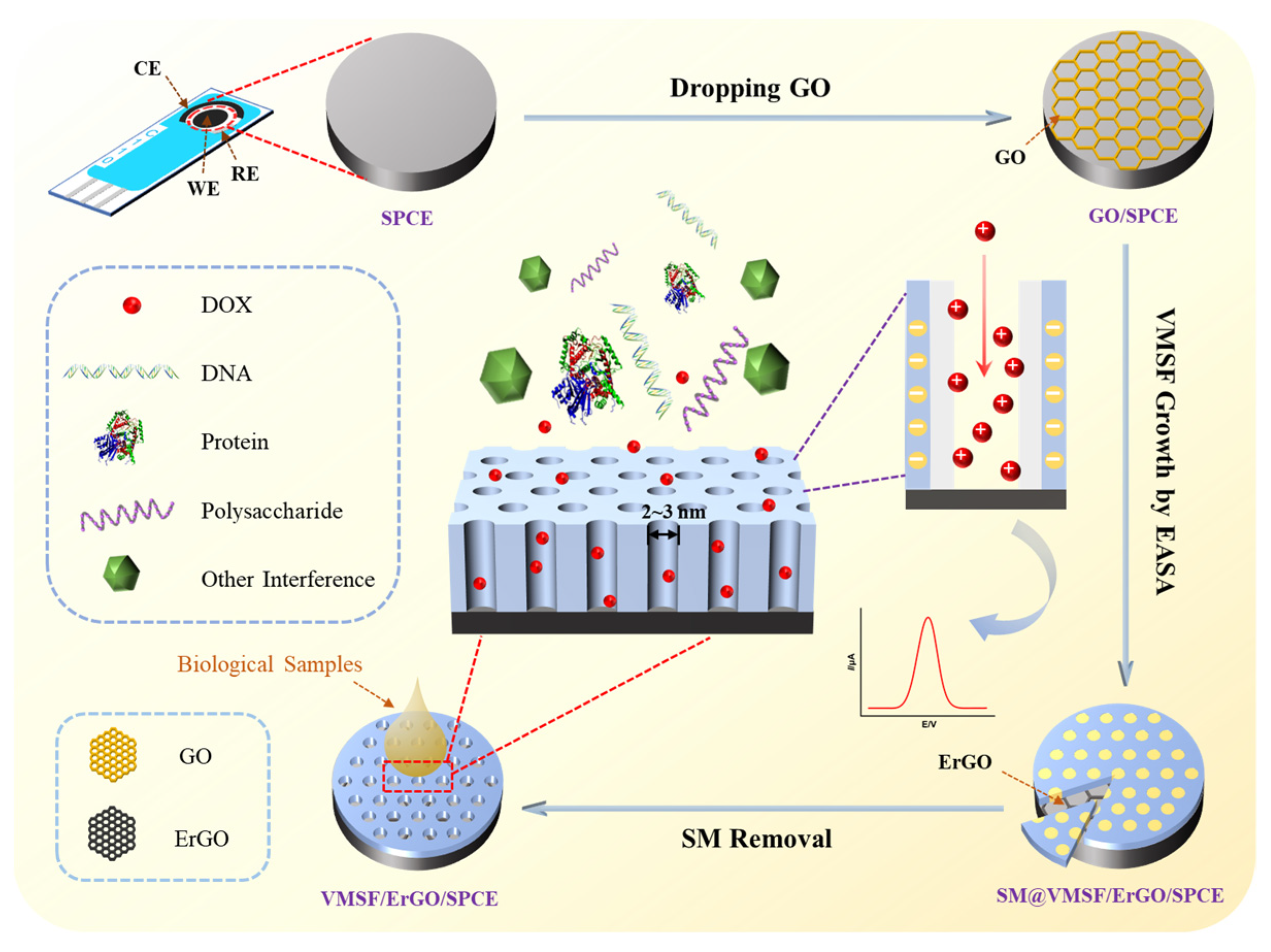
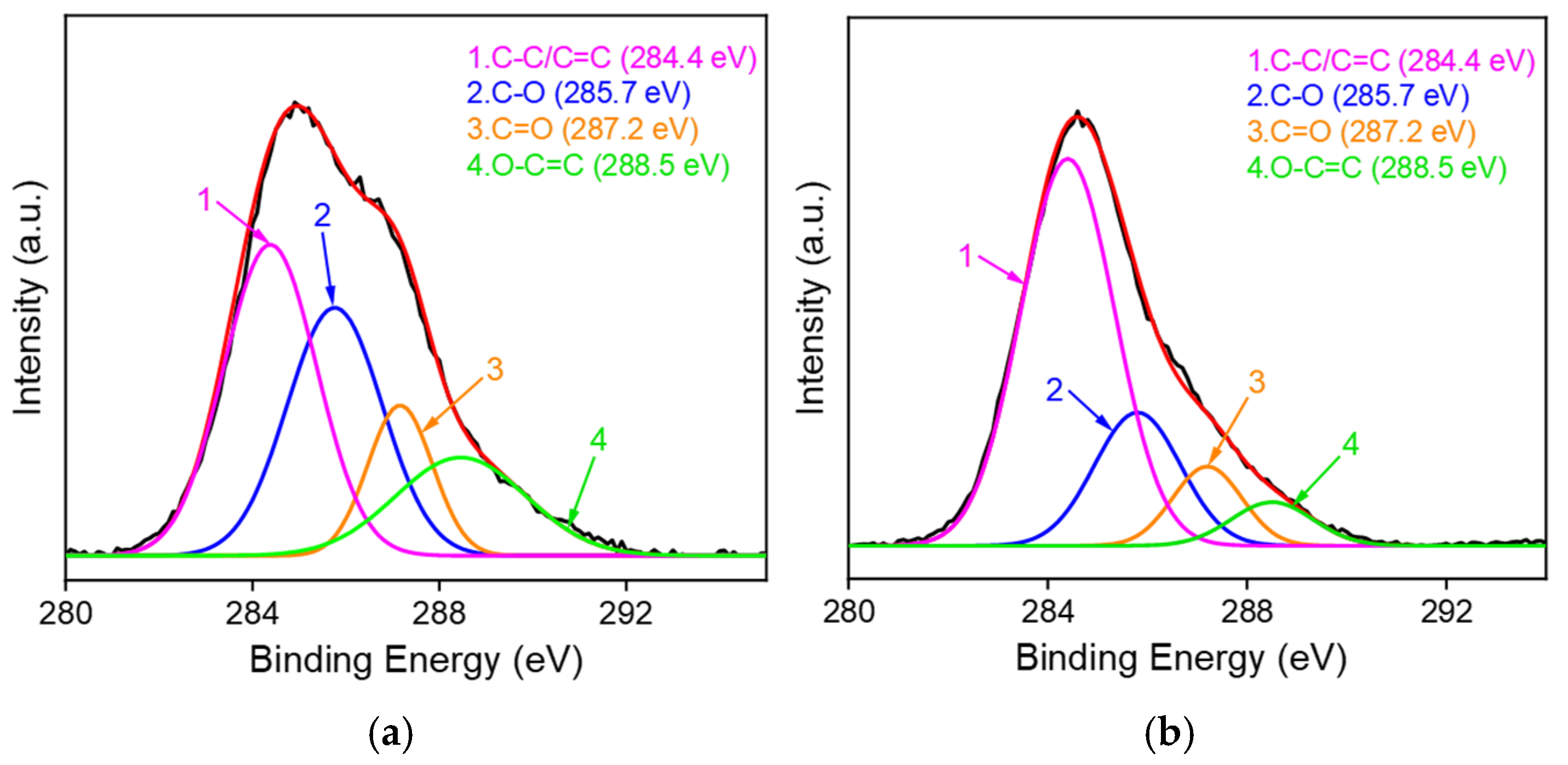
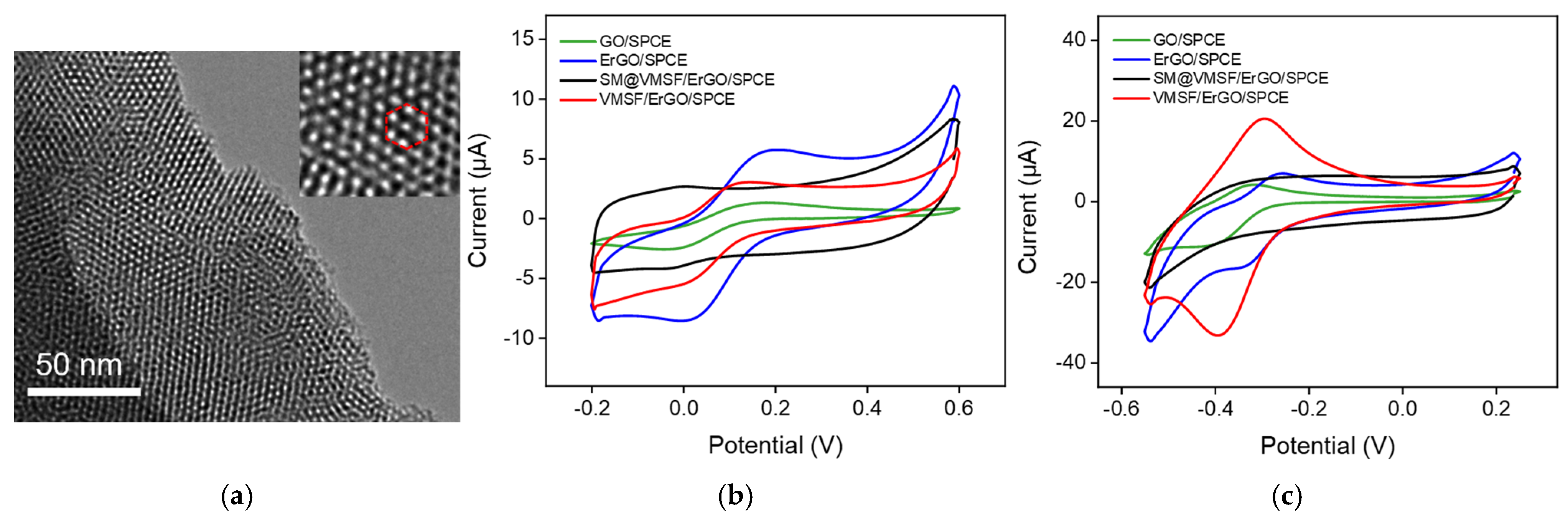
2.1. Fabrication and Characterizations of VMSF/ErGO/SPCE
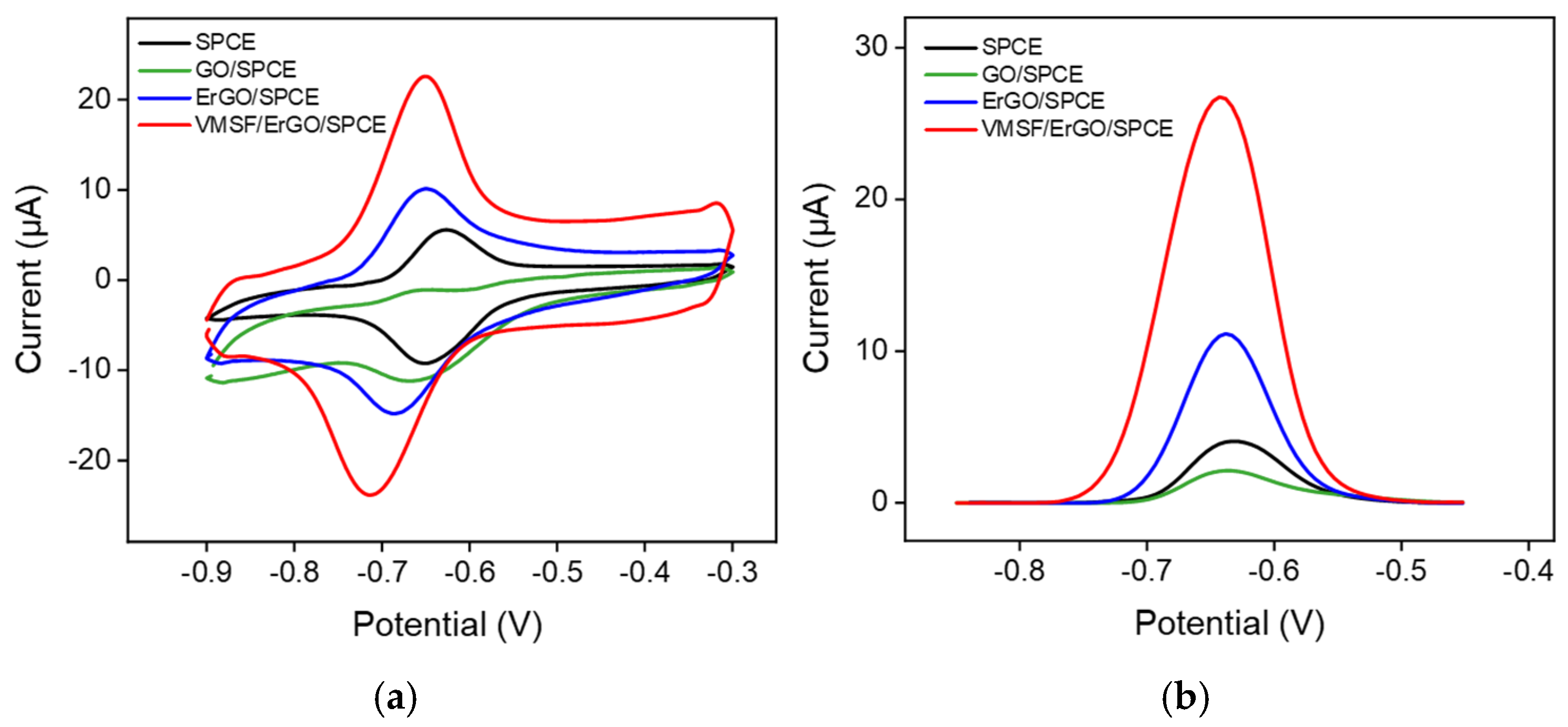
2.2. Electrochemical Behavior of DOX at the VMSF/ErGO/SPCE
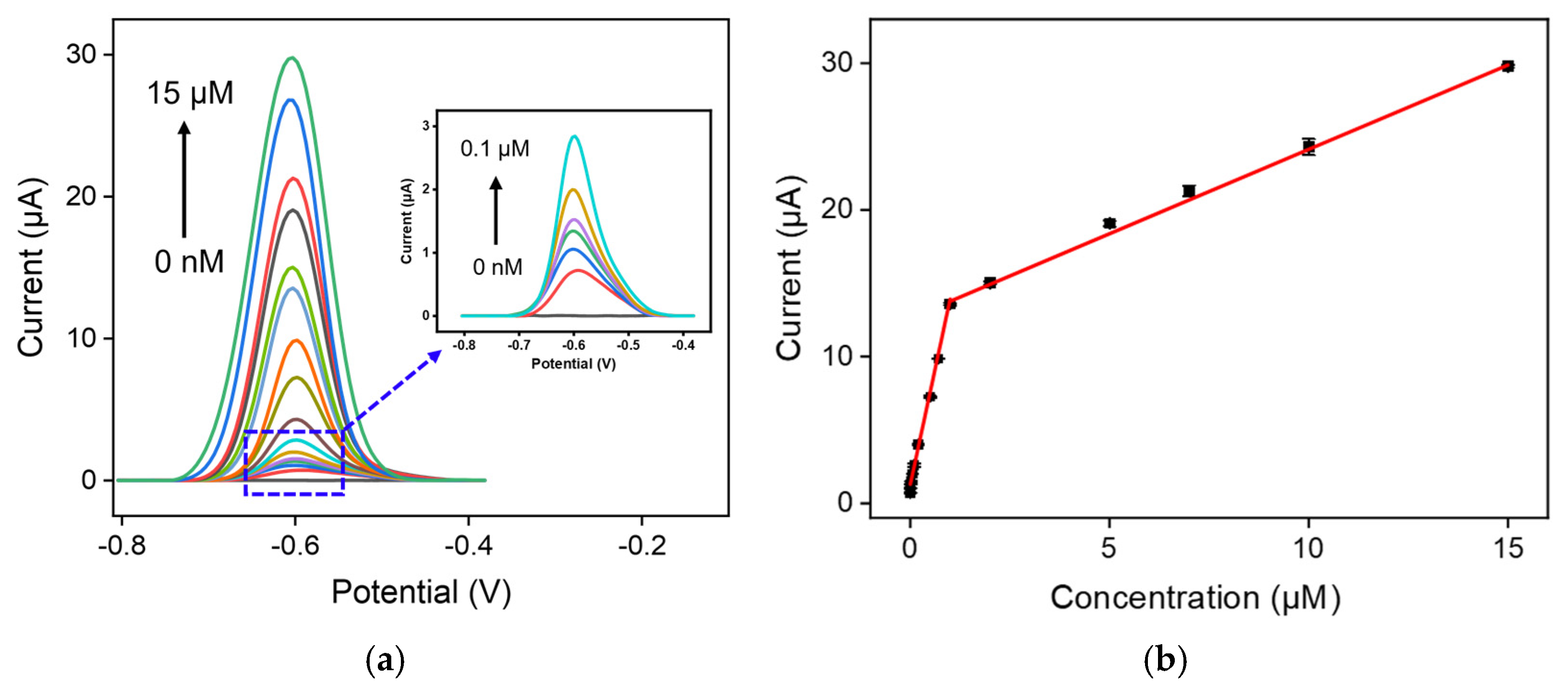
2.3. Analytical Performance of VMSF/ErGO/SPCE towards DOX
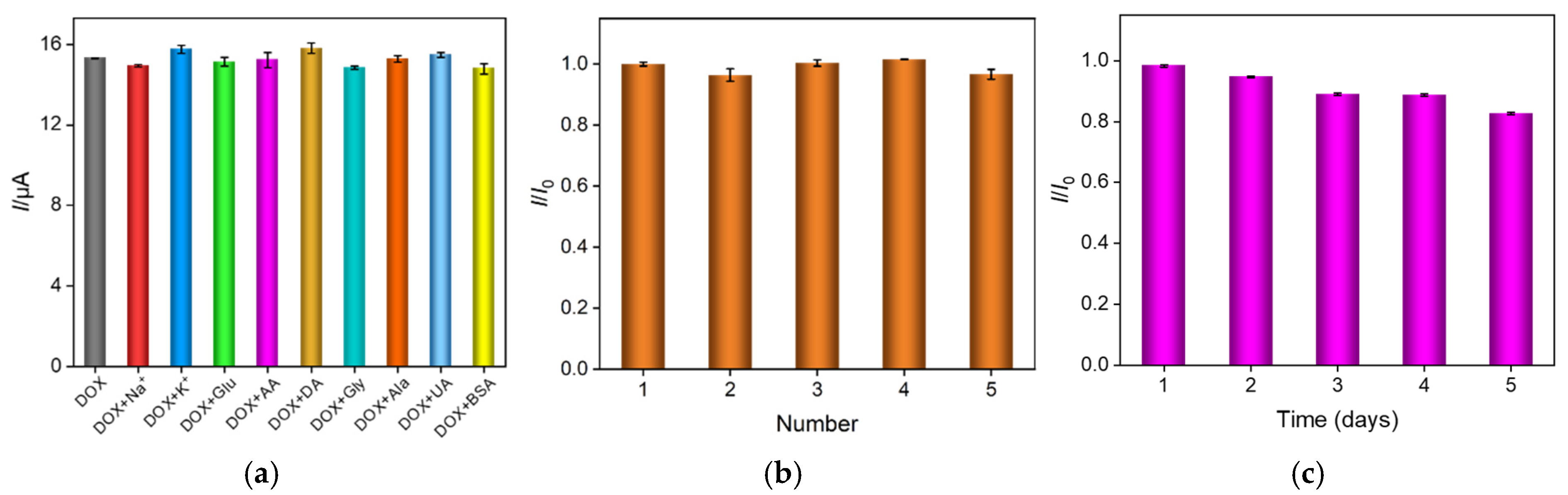
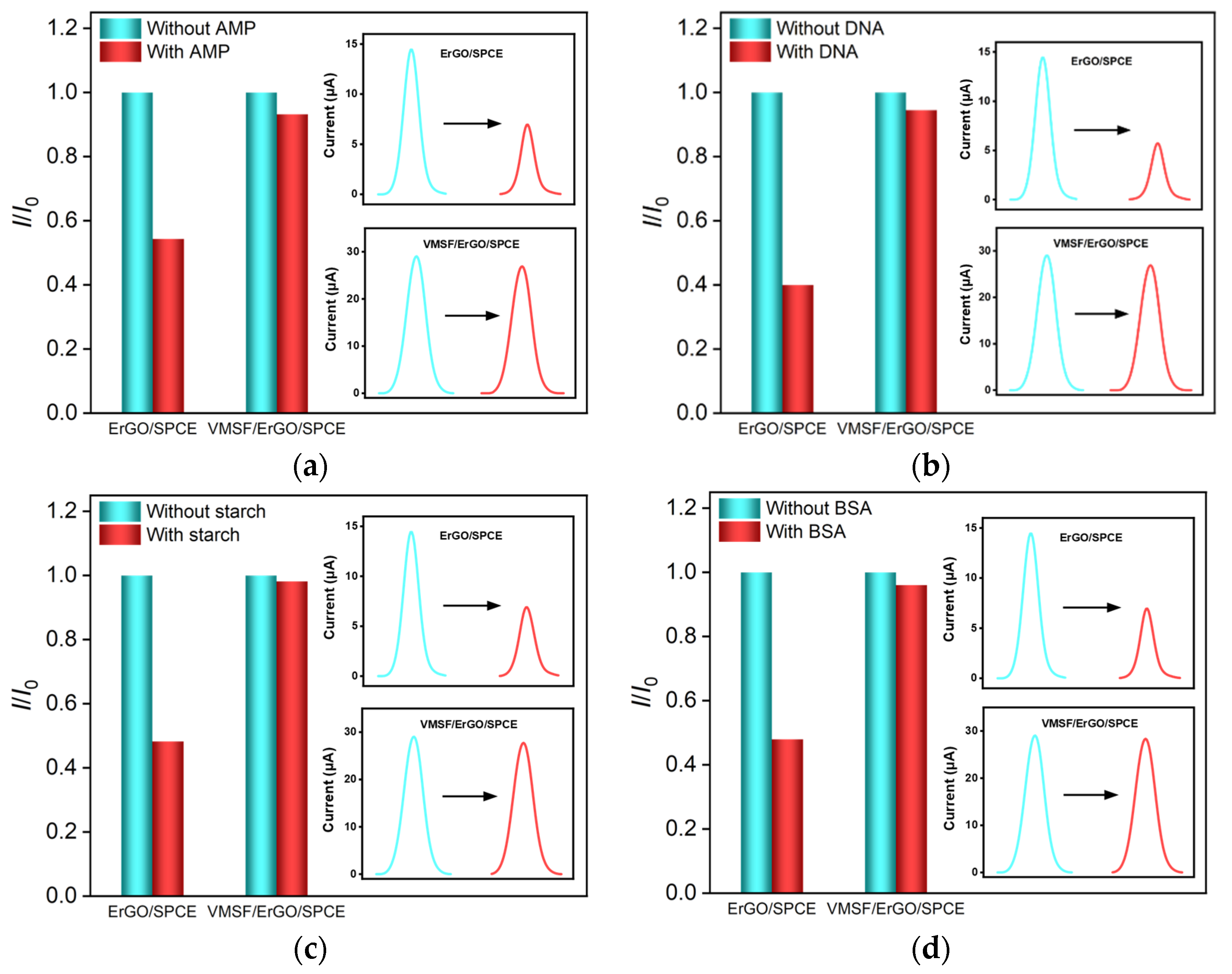
2.4. Anti-Interference, Reproducibility and Stability of VMSF/ErGO/SPCE
2.5. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instruments and Equipment
3.3. Preparation of VMSF/ErGO/SPCE
3.4. Electrochemical Detection of DOX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhang, P.; Zhao, Q.; Zhang, Y.; Cao, L.; Luan, Y. Doxorubicin-loaded polypeptide nanorods based on electrostatic interactions for cancer therapy. J. Colloid Interface Sci. 2016, 464, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Liu, M.; Iwahata, H.; Rogers, H.B.; Woodruff, T.K. Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation. Toxicol. Sci. 2017, 157, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.M.; Jiang, W.; Mudalige, T. Direct quantification of unencapsulated doxorubicin in liposomal doxorubicin formulations using capillary electrophoresis. Int. J. Pharm. 2018, 549, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, X.; Qian, Y.; Liu, J.; Li, L.; Liu, J.; Chen, J. Direct and sensitive detection of sulfide ions based on one-step synthesis of ionic liquid functionalized fluorescent carbon nanoribbons. RSC Adv. 2019, 9, 37484–37490. [Google Scholar] [CrossRef]
- Roszkowska, A.; Tascon, M.; Bojko, B.; Gorynski, K.; Dos Santos, P.R.; Cypel, M.; Pawliszyn, J. Equilibrium ex vivo calibration of homogenized tissue for in vivo SPME quantitation of doxorubicin in lung tissue. Talanta 2018, 183, 304–310. [Google Scholar] [CrossRef]
- Panikar, S.S.; Banu, N.; Escobar, E.R.; Garcia, G.R.; Cervantes-Martinez, J.; Villegas, T.C.; Salas, P.; De la Rosa, E. Stealth modified bottom up SERS substrates for label-free therapeutic drug monitoring of doxorubicin in blood serum. Talanta 2020, 218, 121138. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Norouzi, H.; Mahmoudi, M.; Goicoechea, H.C.; Jalalvand, A.R. Fabrication of a novel impedimetric biosensor for label free detection of DNA damage induced by doxorubicin. Int. J. Biol. Macromol. 2019, 124, 963–971. [Google Scholar] [CrossRef]
- Hashemzadeh, N.; Hasanzadeh, M.; Shadjou, N.; Eivazi-Ziaei, J.; Khoubnasabjafari, M.; Jouyban, A. Graphene quantum dot modified glassy carbon electrode for the determination of doxorubicin hydrochloride in human plasma. J. Pharm. Anal. 2016, 6, 235–241. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef]
- Wang, M.; Lin, J.; Gong, J.; Ma, M.; Tang, H.; Liu, J.; Yan, F. Rapid and sensitive determination of doxorubicin in human whole blood by vertically-ordered mesoporous silica film modified electrochemically pretreated glassy carbon electrodes. RSC Adv. 2021, 11, 9021–9028. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Jin, Q.; Zhou, H.; Sailjoi, A.; Liu, J.; Tang, W. Fast one-step fabrication of a vertically-ordered mesoporous silica-nanochannel film on graphene for direct and sensitive detection of doxorubicin in human whole blood. J. Mater. Chem. C 2020, 8, 7113–7119. [Google Scholar] [CrossRef]
- de la Escosura-Muniz, A.; Merkoci, A. A nanochannel/nanoparticle-based filtering and sensing platform for direct detection of a cancer biomarker in blood. Small 2011, 7, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Prime, K.; Whitesides, G. Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Daggumati, P.; Matharu, Z.; Wang, L.; Seker, E. Biofouling-Resilient Nanoporous Gold Electrodes for DNA Sensing. Anal. Chem. 2015, 87, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, D.; Wang, W.; Liu, J.; Thng, S.T.G.; Chen, P. A silk-microneedle patch to detect glucose in the interstitial fluid of skin or plant tissue. Sens. Actuators B Chem. 2022, 372, 132626. [Google Scholar] [CrossRef]
- Zhou, P.; Yao, L.; Chen, K.; Su, B. Silica Nanochannel Membranes for Electrochemical Analysis and Molecular Sieving: A Comprehensive Review. Crit. Rev. Anal. Chem. 2020, 50, 424–444. [Google Scholar] [CrossRef]
- Li, G.; Belwal, T.; Luo, Z.; Li, Y.; Li, L.; Xu, Y.; Lin, X. Direct detection of Pb2+ and Cd2+ in juice and beverage samples using PDMS modified nanochannels electrochemical sensors. Food Chem. 2021, 356, 129632. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-ordered mesoporous silica films for electrochemical detection of Hg(II) ion in pharmaceuticals and soil samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; He, Y.; Ding, L.; Su, B. Highly Ordered Binary Assembly of Silica Mesochannels and Surfactant Micelles for Extraction and Electrochemical Analysis of Trace Nitroaromatic Explosives and Pesticides. Anal. Chem. 2015, 87, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Dong, G.; Zhu, S.; Yan, F.; Liu, J. Direct and Sensitive Electrochemical Detection of Bisphenol A in Complex Environmental Samples Using a Simple and Convenient Nanochannel-Modified Electrode. Front. Chem. 2022, 10, 900282. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Su, R.; Yu, G.; Liu, L.; Yan, F. Highly sensitive electrochemical detection of paraquat in environmental water samples using a vertically ordered mesoporous silica film and a nanocarbon composite. Nanomaterials 2022, 12, 3632. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Ma, X.; Jin, Q.; Tong, Y.; Tang, H.; Lin, X.; Liu, J. Phenylboronic acid-functionalized vertically ordered mesoporous silica films for selective electrochemical determination of fluoride ion in tap water. Microchim. Acta 2020, 187, 470. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liao, W.; Zhou, H.; Tong, Y.; Yan, F.; Tang, H.; Liu, J. Highly sensitive detection of rutin in pharmaceuticals and human serum using ITO electrodes modified with vertically-ordered mesoporous silica-graphene nanocomposite films. J. Mater. Chem. B 2020, 8, 10630–10636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef]
- Liang, R.; Jiang, J.; Zheng, Y.; Sailjoi, A.; Chen, J.; Liu, J.; Li, H. Vertically oriented mesoporous silica film modified fluorine-doped tin oxide electrode for enhanced electrochemiluminescence detection of lidocaine in serum. RSC Adv. 2021, 11, 34669–34675. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Zhou, H.; Liu, J.; Li, L.; Liu, J.; Yan, F.; Luo, T. Direct electrochemical detection of 4-aminophenol in pharmaceuticals using ITO electrodes modified with vertically-ordered mesoporous silica-nanochannel films. J. Electroanal. Chem. 2020, 878, 114568. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, T.; Luo, T.; Luo, X.; Yan, F.; Tang, W.; Liu, J. Bipolar silica nanochannel array confined electrochemiluminescence for ultrasensitive detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2022, 215, 114563. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive immunosensor for prostate-specific antigen based on enhanced electrochemiluminescence by vertically ordered mesoporous silica-nanochannel film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Luo, X.; Wu, W.; Liu, J. Fabrication of a Disposable Electrochemical Immunosensor Based on Nanochannel Array Modified Electrodes and Gated Electrochemical Signals for Sensitive Determination of C-Reactive Protein. Nanomaterials 2022, 12, 3981. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Zhang, R.; Yan, F. Dual-mode electrochemiluminescence and electrochemical sensor for alpha-fetoprotein detection in human serum based on vertically ordered mesoporous silica films. Front. Chem. 2022, 10, 1023998. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced Surfactant Self-Assembly Driven to Vertical Growth of Oriented Mesoporous Films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, T.; Zhou, H.; Yan, F.; Liu, Y. Silica nanochannels boosting Ru(bpy)32+-mediated electrochemical sensor for the detection of guanine in beer and pharmaceutical samples. Front. Nutr. 2022, 9, 987442. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, e1808283. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Han, Y.; Zhu, X.; Gong, J.; Luo, T.; Zhao, C.; Liu, J.; Liu, J.; Li, X. Sensitive Detection of Sulfide Ion Based on Fluorescent Ionic Liquid-Graphene Quantum Dots Nanocomposite. Front. Chem. 2021, 9, 658045. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar]
- Gong, J.; Zhang, Z.; Zeng, Z.; Wang, W.; Kong, L.; Liu, J.; Chen, P. Graphene quantum dots assisted exfoliation of atomically-thin 2D materials and as-formed 0D/2D van der Waals heterojunction for HER. Carbon 2021, 184, 554–561. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, V.; Singh, T.A.; Pabbathi, A.; Das, J. Fabrication of novel carbon dots/cerium oxide nanocomposites for highly sensitive electrochemical detection of doxorubicin. Diam. Relat. Mater. 2022, 125, 109037. [Google Scholar] [CrossRef]
- Ehsani, M.; Soleymani, J.; Mohammadalizadeh, P.; Hasanzadeh, M.; Jouyban, A.; Khoubnasabjafari, M.; Vaez-Gharamaleki, Y. Low potential detection of doxorubicin using a sensitive electrochemical sensor based on glassy carbon electrode modified with silver nanoparticles-supported poly(chitosan): A new platform in pharmaceutical analysis. Microchem. J. 2021, 165, 106101. [Google Scholar] [CrossRef]
- Rong, S.; Zou, L.; Meng, L.; Yang, X.; Dai, J.; Wu, M.; Qiu, R.; Tian, Y.; Feng, X.; Ren, X.; et al. Dual function metal-organic frameworks based ratiometric electrochemical sensor for detection of doxorubicin. Anal. Chim. Acta 2022, 1196, 339545. [Google Scholar] [CrossRef]
- Er, E.; Erk, N. Construction of a sensitive electrochemical sensor based on 1T-MoS2 nanosheets decorated with shape-controlled gold nanostructures for the voltammetric determination of doxorubicin. Microchim. Acta 2020, 187, 223. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Z.; Jin, H.; Gui, R. Sulfur nanoparticle-encapsulated MOF and boron nanosheet-ferrocene complex modified electrode platform for ratiometric electrochemical sensing of adriamycin and real-time monitoring of drug release. Microchem. J. 2022, 177, 107319. [Google Scholar] [CrossRef]
- Jahandari, S.; Taher, M.A.; Karimi-Maleh, H.; Mansouri, G. Simultaneous voltammetric determination of glutathione, doxorubicin and tyrosine based on the electrocatalytic effect of a nickel(II) complex and of Pt:Co nanoparticles as a conductive mediator. Microchim. Acta 2019, 186, 493. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.H.; Norouzi, Z. A new nanostructure consisting of nitrogen-doped carbon nanoonions for an electrochemical sensor to the determination of doxorubicin. Microchem. J. 2020, 157, 105098. [Google Scholar] [CrossRef]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for anti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J. Electroanal. Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Yan, F.; Luo, T.; Jin, Q.; Zhou, H.; Sailjoi, A.; Dong, G.; Liu, J.; Tang, W. Tailoring molecular permeability of vertically-ordered mesoporous silica-nanochannel films on graphene for selectively enhanced determination of dihydroxybenzene isomers in environmental water samples. J. Hazard. Mater. 2021, 410, 124636. [Google Scholar] [CrossRef] [PubMed]
| Electrode | Method | Linear Range (μM) | Sensitivity (μA μM−1) | LOD (nM) | Ref. |
|---|---|---|---|---|---|
| CDs/CeO2/SPCE | CV | 0.2–20 | 1.39 | 90 | [47] |
| AgNPs-CS-GCE | SWV | 0.103–8.6 | 0.861 | 103 | [48] |
| MB@MWCNTs/UiO-66-NH2/GCE | CV | 0.1–75 | 0.0183 | 51 | [49] |
| AuNRDs/1T-MoS2/SPE | DPV | 0.01–9.5 | 0.895 | 2.5 | [50] |
| SNPs@MOF/BNSs-Fc/GCE | SWV | 0.01–10 | 0.641 | 2 | [51] |
| BPPDNi/Pt:CO-NPs/CPE | SWV | 0.5–300 | 0.0677 | 100 | [52] |
| N-CNOs/GCE | DPV | 2 × 10−4–10 | 2.49 | 0.06 | [53] |
| VMSF/ErGO/GCE | DPV | 0.001–20 | 7.815 | 0.77 | [11] |
| VMSF/ErGO/SPCE | DPV | 0.002–1 1–15 | 12.6 1.15 | 1 | This work |
| Sample | Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Human serum | 0.0100 | 0.0103 | 3.1 | 103 |
| 0.100 | 0.102 | 1.8 | 102 | |
| 1.00 | 0.999 | 1.7 | 99.9 | |
| 5.00 | 4.96 | 1.0 | 99.2 | |
| Urine | 0.0100 | 0.0101 | 3.0 | 101 |
| 0.100 | 0.104 | 2.8 | 104 | |
| 1.00 | 0.998 | 2.7 | 99.8 | |
| 5.00 | 5.21 | 1.6 | 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-Biofouling Electrochemical Sensor Based on the Binary Nanocomposite of Silica Nanochannel Array and Graphene for Doxorubicin Detection in Human Serum and Urine Samples. Molecules 2022, 27, 8640. https://doi.org/10.3390/molecules27248640
Lv N, Qiu X, Han Q, Xi F, Wang Y, Chen J. Anti-Biofouling Electrochemical Sensor Based on the Binary Nanocomposite of Silica Nanochannel Array and Graphene for Doxorubicin Detection in Human Serum and Urine Samples. Molecules. 2022; 27(24):8640. https://doi.org/10.3390/molecules27248640
Chicago/Turabian StyleLv, Ning, Xun Qiu, Qianqian Han, Fengna Xi, Yina Wang, and Jun Chen. 2022. "Anti-Biofouling Electrochemical Sensor Based on the Binary Nanocomposite of Silica Nanochannel Array and Graphene for Doxorubicin Detection in Human Serum and Urine Samples" Molecules 27, no. 24: 8640. https://doi.org/10.3390/molecules27248640
APA StyleLv, N., Qiu, X., Han, Q., Xi, F., Wang, Y., & Chen, J. (2022). Anti-Biofouling Electrochemical Sensor Based on the Binary Nanocomposite of Silica Nanochannel Array and Graphene for Doxorubicin Detection in Human Serum and Urine Samples. Molecules, 27(24), 8640. https://doi.org/10.3390/molecules27248640







