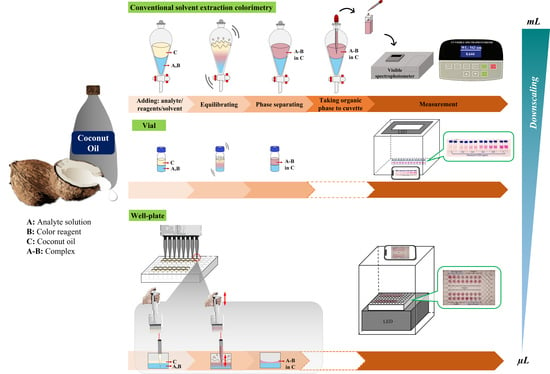
Green Downscaling of Solvent Extractive Determination Employing Coconut Oil as Natural Solvent with Smartphone Colorimetric Detection: Demonstrating the Concept via Cu(II) Assay Using 1,5-Diphenylcarbazide
Abstract
1. Introduction
2. Results and Discussion
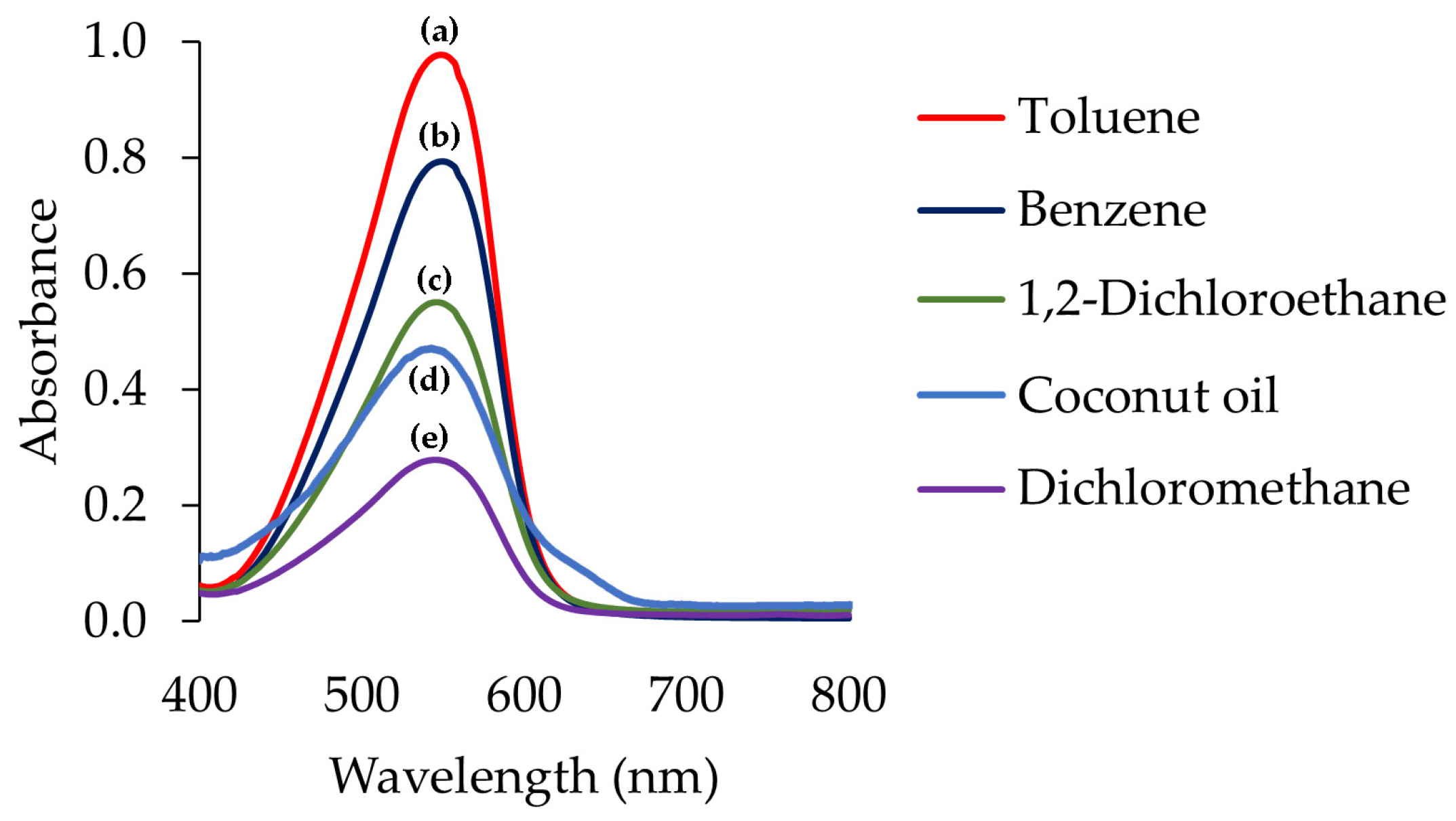
2.1. Reinvestigation for Cu(II) Extraction toward the Reaction between Cu(II) and DPC
2.2. Downscaling Using a Small Vial
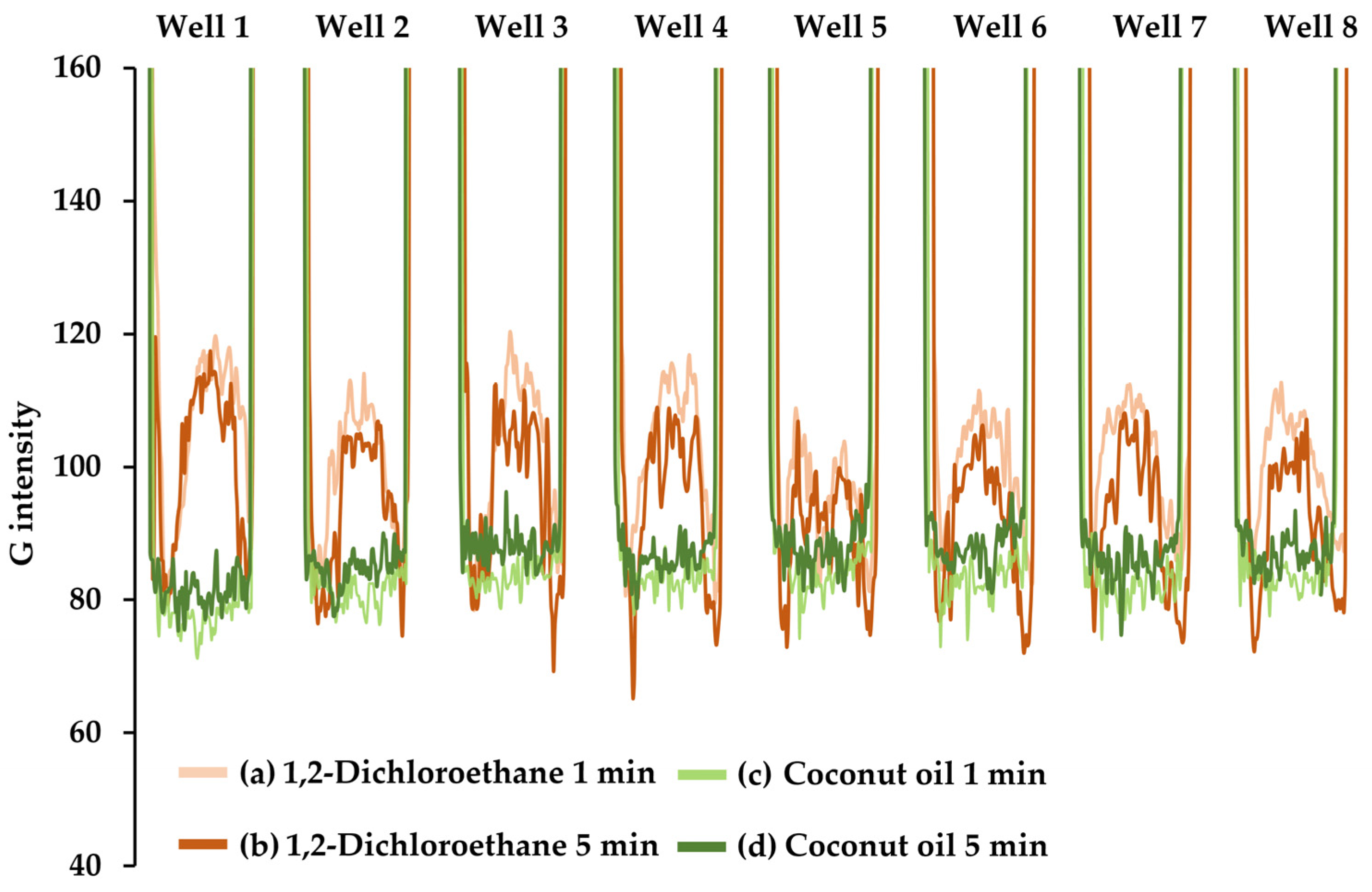
2.3. Downscaling Using a Well Plate
2.4. Analytical Performance
2.4.1. Analytical Characteristics of the Downscaling Extraction Using Vial
2.4.2. Analytical Characteristics of the Downscaling Extraction Using Well Plate
2.5. Applications
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Reinvestigation of Cu(II)-DPC Extracted into Organic Solvents
3.3. Downscaling Solvent Extraction Determination of Cu(II) Using DPC
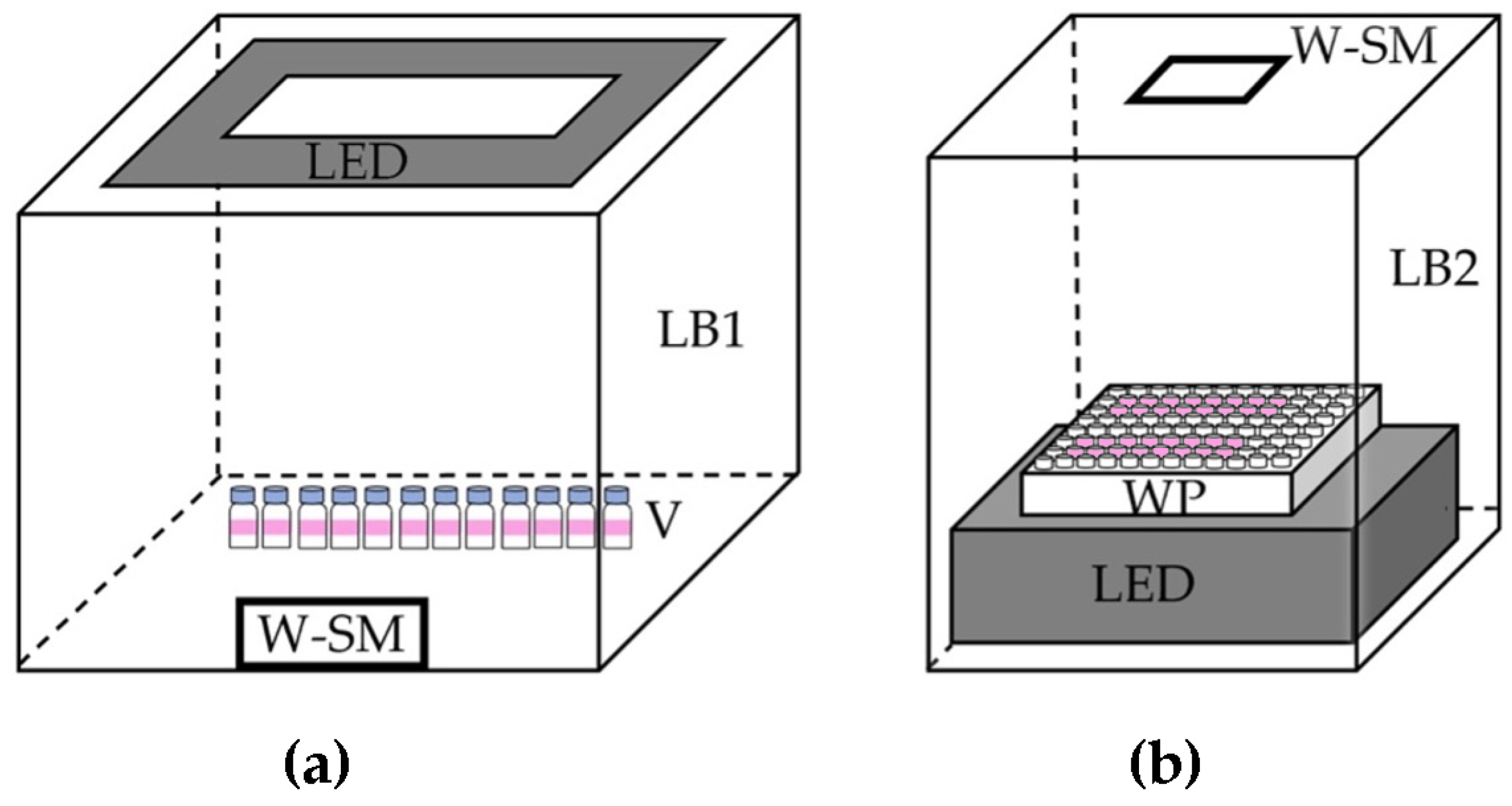
3.3.1. Using a Small Vial
3.3.2. Using a Well Plate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltzman, B.E. Colorimetric microdetermination of cadmium with dithizone. Anal. Chem. 1953, 25, 493–496. [Google Scholar] [CrossRef]
- Stoner, R.E.; Dasler, W. Spectrophotometric determination of copper following extraction with 1,5-diphenylcarbohydrazide in benzene. Anal. Chem. 1960, 32, 1207–1208. [Google Scholar] [CrossRef]
- Dinsel, D.L.; Sweet, T.R. Separation and determination of iron(II) and iron(III) with anthranilic acid using solvent extraction and spectrophotometry. Anal. Chem. 1963, 35, 2077–2081. [Google Scholar] [CrossRef]
- Dagnall, R.M.; West, T.S.; Young, P. Determination of lead with 4-(2-pyridylazo)-resorcinol—I: Spectrophotometry and solvent extraction. Talanta 1965, 12, 583–588. [Google Scholar] [CrossRef]
- Chennuri, S.L.; Haldar, B.C. Solvent extraction of zinc(II) with ethylthioacetoacetate into ethyl acetate. J. Radioanal. Nucl. Chem. 1984, 84, 197–200. [Google Scholar] [CrossRef]
- Sato, S. Differential determination of antimony(III) and antimony(V) by solvent extraction-spectrophotometry with mandelic acid and malachite green, based on the difference in reaction rates. Talanta 1985, 32, 341–344. [Google Scholar] [CrossRef]
- Terra, L.H.S.Á.; da Cunha Areias, M.C.; Gaubeur, I.; Encarnación, M.; Suárez-iha, V. Solvent extraction-spectrophotometric determination of nickel(II) in natural waters using di-2-pyridyl ketone benzoylhydrazone. Spectrosc. Lett. 1999, 32, 257–271. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Yavir, K.; Konieczna, K.; Marcinkowski, Ł.; Kloskowski, A. Ionic liquids in the microextraction techniques: The influence of ILs structure and properties. Trends Anal. Chem. 2020, 130, 115994. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Mohamed, H.M.; Kurowska-Susdorf, A.; Dewani, R.; Fares, M.Y.; Andruch, V. Green analytical chemistry as an integral part of sustainable education development. Curr. Opin. Green Sustain. Chem. 2021, 31, 100508. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green techniques in green analytical chemistry. Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Abdul Halim, H.S.a.; Selamat, J.; Mirhosseini, S.H.; Hussain, N. Sensory preference and bloom stability of chocolate containing cocoa butter substitute from coconut oil. J. Saudi Soc. Agric. Sci. 2019, 18, 443–448. [Google Scholar] [CrossRef]
- Rizzo, G.; Masic, U.; Harrold, J.A.; Norton, J.E.; Halford, J.C.G. Coconut and sunflower oil ratios in ice cream influence subsequent food selection and intake. Physiol. Behav. 2016, 164, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fabiano-Tixier, A.S.; Ginies, C.; Chemat, F. Direct green extraction of volatile aroma compounds using vegetable oils as solvents: Theoretical and experimental solubility study. LWT-Food Sci. Technol. 2014, 59, 724–731. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Krishnan, V.; Praveen, S.; Hebbar, K.B. Dietary prospects of coconut oil for the prevention and treatment of Alzheimer’s disease (ad): A review of recent evidences. Trends Food Sci. Technol. 2021, 112, 201–211. [Google Scholar] [CrossRef]
- Woolley, J.; Gibbons, T.; Patel, K.; Sacco, R. The effect of oil pulling with coconut oil to improve dental hygiene and oral health: A systematic review. Heliyon 2020, 6, e04789. [Google Scholar] [CrossRef]
- Sandupama, P.; Munasinghe, D.; Jayasinghe, M. Coconut oil as a therapeutic treatment for Alzheimer’s disease: A review. J. Future Foods 2022, 2, 41–52. [Google Scholar] [CrossRef]
- Mahbub, K.; Octaviani, I.D.; Astuti, I.Y.; Sisunandar, S.; Dhiani, B.A. Oil from kopyor coconut (Cocos nucifera var. Kopyor) for cosmetic application. Ind. Crops Prod. 2022, 186, 115221. [Google Scholar] [CrossRef]
- Pham, T.L.-B.; Thi, T.T.; Nguyen, H.T.-T.; Lao, T.D.; Binh, N.T.; Nguyen, Q.D. Anti-aging effects of a serum based on coconut oil combined with deer antler stem cell extract on a mouse model of skin aging. Cells 2022, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Kamairudin, N.; Gani, S.S.A.; Masoumi, H.R.F.; Hashim, P. Optimization of natural lipstick formulation based on pitaya (Hylocereus polyrhizus) seed oil using D-optimal mixture experimental design. Molecules 2014, 19, 16672–16683. [Google Scholar] [CrossRef] [PubMed]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Nadjet, B.; Bakoz, P.Y.; Adbellah, G.; Boudjema, H. Vegetable oils extraction of polycyclic aromatic hydrocarbons from an Algerian quagmire. Am. J. Appl. Chem. 2014, 2, 6–9. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Khoshmaram, L.; Mohammadi, M. Combination of a smart phone based low-cost portable colorimeter with air-assisted liquid-liquid microextraction for speciation and determination of chromium (III) and (VI). Microchem. J. 2021, 164, 105991. [Google Scholar] [CrossRef]
- Shishehbore, M.R.; Nasirizadeh, N.; Haji, S.A.M.; Tabatabaee, M. Spectrophotometric determination of trace copper after preconcentration with 1,5- diphenylcarbazone on microcrystalline naphthalene. Can. J. Anal. Sci. Spectrosc. 2005, 50, 130–134. [Google Scholar]
- Crespo, G.A.; Andrade, F.J.; Iñón, F.A.; Tudino, M.B. Kinetic method for the determination of trace amounts of copper(II) in water matrices by its catalytic effect on the oxidation of 1,5-diphenylcarbazide. Anal. Chim. Acta 2005, 539, 317–325. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Essex, UK, 2010; pp. 110–126. [Google Scholar]
- Eller, Z.; Varga, Z.; Hancsók, J. Advanced production process of jet fuel components from technical grade coconut oil with special hydrocracking. Fuel 2016, 182, 713–720. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration and Spectrophotometric Inorganic Analysis, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; p. 6. [Google Scholar]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2015, 41, 409–421. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Alamu, O.J.; Dehinbo, O.; Sulaiman, A.M. Production and testing of coconut oil biodiesel fuel and its blend. Leonardo J. Sci. 2010, 16, 95–104. [Google Scholar]
- Dahim, M.; Al-Mattarneh, H.; Ismail, R. Simple capacitor dielectric sensors for determination of water content in transformer oil. Int. J. Eng. Res. Technol. 2018, 7, 157–160. [Google Scholar]
- Safety Data Sheet of Benzene. Available online: https://www.airgas.com/msds/001062.pdf (accessed on 1 November 2022).
- Safety Data Sheet of Toluene. Available online: https://www.airgas.com/msds/001063.pdf (accessed on 1 November 2022).
- Safety Data Sheet of Dichloromethane. Available online: https://pdf4pro.com/fullscreen/dichloromethane-material-safety-data-56924.html (accessed on 1 November 2022).
- Safety Data Sheet of 1,2-Dichloroethane. Available online: https://www.airgas.com/msds/001068.pdf (accessed on 1 November 2022).
- Safety Data Sheet of Coconut Oil. Available online: https://www.mccsd.net/cms/lib/NY02208580/Centricity/Shared/Material%20Safety%20Data%20Sheets%20_MSDS_/MSDS%20Sheets_Coconut_Oil_205_00.pdf (accessed on 1 November 2022).
- Khan, A.I. A GC-FID Method for the Comparison of Acid-and Base-Catalyzed Derivatization of Fatty Acids to FAMEs in Three Edible Oils; Thermo Fisher Scientific: Runcorn, UK, 2013; p. 20733. [Google Scholar]
| Solvent | Calibration Equation | |
|---|---|---|
| Conventional Spectrophotometry | Image Processing | |
| 1,2-Dichloroethane | Absorbance = 0.58 [Cu(II)] + 0.08, (R2 = 0.98) | G intensity = −134 [Cu(II)] + 234, (R2 = 0.97) |
| Coconut oil | − | G intensity = −142 [Cu(II)] + 222, (R2 = 0.98) |
| Samples | Vial Procedure | Well Plate Procedure | ||||
| Visible Spectrophotometer | Smartphone Camera | Smartphone Camera | ||||
| Cu Found (ppm) | %Recovery | Cu Found (ppm) | %Recovery | Cu Found (ppm) | %Recovery | |
| 1 | 0.41± 0.02 | 103 ± 3.70 | 0.41 ± 0.02 | 103 ± 4.88 | 0.34 ± 0.01 | 85 ± 1.49 |
| 2 | 0.34 ± 0.00 | 85 ± 0.00 | 0.44 ± 0.00 | 110 ± 0.00 | 0.40 ± 0.00 | 103 ± 1.05 |
| 3 | 0.40 ± 0.01 | 100 ± 1.27 | 0.45 ± 0.01 | 113 ± 1.12 | 0.40 ± 0.03 | 100 ± 7.41 |
| 4 | 0.37 ± 0.01 | 93 ± 1.37 | 0.40 ± 0.01 | 100 ± 2.50 | 0.45 ± 0.00 | 113 ± 0.97 |
| 5 | 0.37 ± 0.01 | 93 ± 1.37 | 0.43 ± 0.02 | 108 ± 3.53 | 0.40 ± 0.01 | 100 ± 3.23 |
| 6 | 0.41 ± 0.01 | 103 ± 2.44 | 0.44 ± 0.01 | 110 ± 1.15 | 0.34 ± 0.01 | 85 ± 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesonkan, K.; Yeerum, C.; Kiwfo, K.; Grudpan, K.; Vongboot, M. Green Downscaling of Solvent Extractive Determination Employing Coconut Oil as Natural Solvent with Smartphone Colorimetric Detection: Demonstrating the Concept via Cu(II) Assay Using 1,5-Diphenylcarbazide. Molecules 2022, 27, 8622. https://doi.org/10.3390/molecules27238622
Kesonkan K, Yeerum C, Kiwfo K, Grudpan K, Vongboot M. Green Downscaling of Solvent Extractive Determination Employing Coconut Oil as Natural Solvent with Smartphone Colorimetric Detection: Demonstrating the Concept via Cu(II) Assay Using 1,5-Diphenylcarbazide. Molecules. 2022; 27(23):8622. https://doi.org/10.3390/molecules27238622
Chicago/Turabian StyleKesonkan, Kullapon, Chonnipa Yeerum, Kanokwan Kiwfo, Kate Grudpan, and Monnapat Vongboot. 2022. "Green Downscaling of Solvent Extractive Determination Employing Coconut Oil as Natural Solvent with Smartphone Colorimetric Detection: Demonstrating the Concept via Cu(II) Assay Using 1,5-Diphenylcarbazide" Molecules 27, no. 23: 8622. https://doi.org/10.3390/molecules27238622
APA StyleKesonkan, K., Yeerum, C., Kiwfo, K., Grudpan, K., & Vongboot, M. (2022). Green Downscaling of Solvent Extractive Determination Employing Coconut Oil as Natural Solvent with Smartphone Colorimetric Detection: Demonstrating the Concept via Cu(II) Assay Using 1,5-Diphenylcarbazide. Molecules, 27(23), 8622. https://doi.org/10.3390/molecules27238622