Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace
Abstract
1. Introduction
2. Results and Discussion
2.1. Central Composite Rotatable Design Analysis
2.2. Statistical Analysis
2.3. Diagnostics of the Adequacy of the Models
2.4. Percentage Contribution of Process Variables
2.5. Assessment of the Influence of Process Variables
2.5.1. Assessment of the Influence of Individual Process Variables on the Total Phenolic Content of Olive Mill Pomace Extracts
2.5.2. Assessment of the Influence of Combined Process Variables on the Total Phenolic Content of Olive Mill Pomace Extracts
2.6. Optimization of Process Variables and Validation of the Optimized Conditions
2.7. Characterization of the Optimized Olive Mill Pomace Extracts
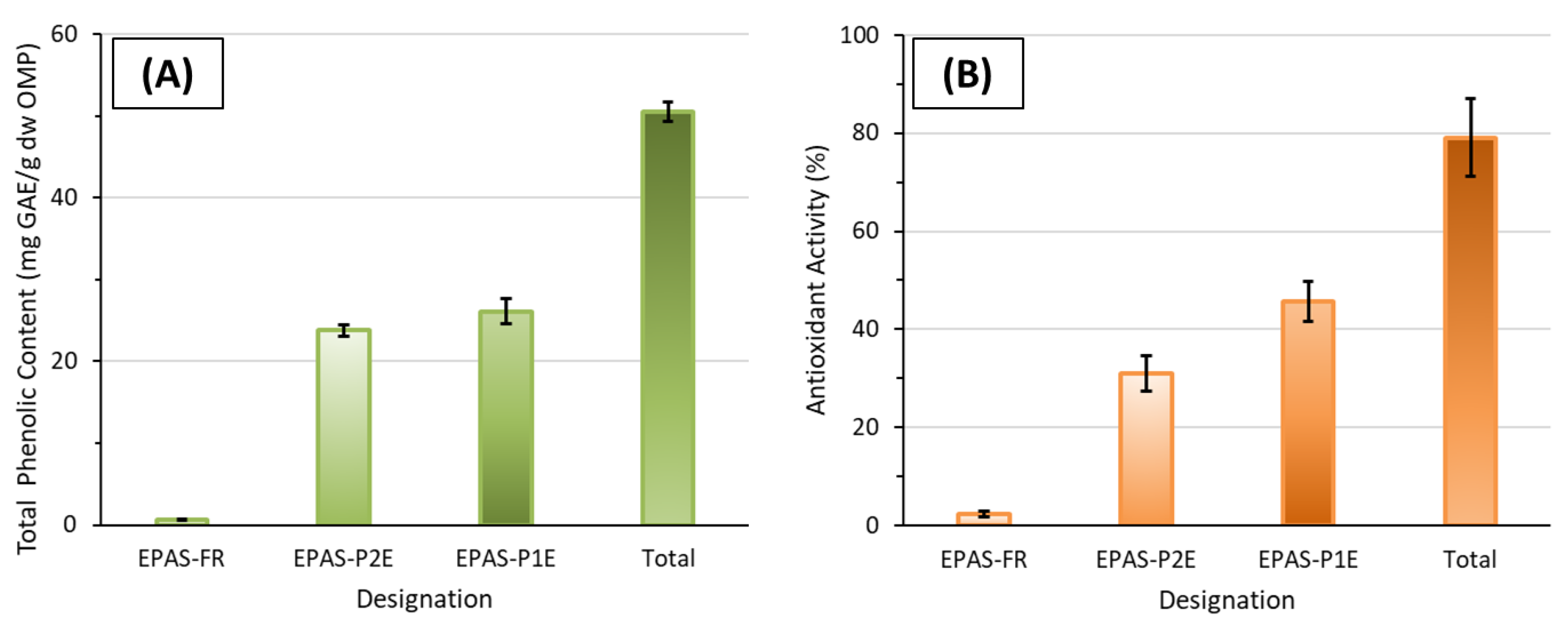
2.7.1. Contribution of Alternative Extract Fractions on the Total Phenolic Content and the Total Antioxidant Activity
2.7.2. RP-HPLC Analysis of Phenolic Antioxidants
2.7.3. Effect of Acidic Hydrolysis Pre-Treatment on the Total Phenolic Content and the Total Antioxidant Activity
3. Materials and Methods
3.1. Extraction of Phenolic Antioxidants
3.2. Characterization of the Extracts
3.2.1. Determination of the Total Phenolic Content and Antioxidant Activity
3.2.2. Analysis of Individual Phenolic Compounds by Reserved-Phase High-Performance Liquid Chromatography (RP-HPLC)
3.3. Design of Experiments for the Optimization of the Extraction Procedure
3.3.1. Experimental Design
3.3.2. Statistical Analysis
3.3.3. Contributions of Process Variables
3.3.4. Determination of the Optimized Extraction Conditions
3.3.5. Verification of the Predicted Optimized Extraction Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [PubMed]
- Paulo, F.; Santos, L. Deriving valorization of phenolic compounds from olive oil by-products for food applications through microencapsulation approaches: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 920–945. [Google Scholar] [PubMed]
- Peeters, K.; Miklavčič Višnjevec, A.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules 2021, 26, 6946. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Martínez-Carballo, E.; Cambeiro-Pérez, N.; Rial-Otero, R.; Figueiredo-González, M.; Cancho-Grande, B. Applicability of an In-Vitro Digestion Model to Assess the Bioaccessibility of Phenolic Compounds from Olive-Related Products. Molecules 2021, 26, 6667. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Paulo, F.; Tavares, L.; Santos, L. Extraction and encapsulation of bioactive compounds from olive mill pomace: Influence of loading content on the physicochemical and structural properties of microparticles. J. Food Meas. Charact. 2022, 16, 3077–3094. [Google Scholar] [CrossRef]
- Abdul Qadir, M.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of Phenolic Compounds and Antioxidant and Antimicrobial Activities of Some Common Herbs. Int. J. Anal. Chem. 2017, 2017, 3475738. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. [Google Scholar]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natmoeural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT Food Sci. Technol. 2017, 79, 640–646. [Google Scholar]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar]
- Uribe, E.; Pasten, A.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Ortiz, J.; Di Scala, K. Comparison of Chemical Composition, Bioactive Compounds and Antioxidant Activity of Three Olive-Waste Cakes. J. Food Biochem. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Kharia, A.A.; Singhai, A.K. Screening of Most Effective Variables for Development of Gastroretentive Mucoadhesive Nanoparticles by Taguchi Design. ISRN Nanomater. 2013, 2013, 348095. [Google Scholar] [CrossRef][Green Version]
- Yetilmezsoy, K.; Demirel, S.; Vanderbei, R.J. Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: Box–Behnken experimental design. J. Hazard. Mater. 2009, 171, 551–562. [Google Scholar]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New Phenolic Compounds Hydrothermally Extracted from the Olive Oil Byproduct Alperujo and Their Antioxidative Activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Böhmer-Maas, B.W.; Otero, D.M.; Zambiazi, R.C.; Aranha, B.C. Optimization of the extraction of phenolic compounds from olive pomace using response surface methodology. Rev. Ceres 2020, 67, 181–190. [Google Scholar]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef]
- Del Boccio, P.; Di Deo, A.; De Curtis, A.; Celli, N.; Iacoviello, L.; Rotilio, D. Liquid chromatography–tandem mass spectrometry analysis of oleuropein and its metabolite hydroxytyrosol in rat plasma and urine after oral administration. J. Chromatogr. B 2003, 785, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Encapsulation of the Antioxidant Tyrosol and Characterization of Loaded Microparticles: An Integrative Approach on the Study of the Polymer-Carriers and Loading Contents. Food Bioprocess Technol. 2020, 13, 764–785. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010. [Google Scholar]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque de Castro, M.D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography–Tandem Mass Spectrometry with a Quadrupole–Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar]
- Japón-Luján, R.; Luque de Castro, M.D. Static−Dynamic Superheated Liquid Extraction of Hydroxytyrosol and Other Biophenols from Alperujo (a Semisolid Residue of the Olive Oil Industry). J. Agric. Food Chem. 2007, 55, 3629–3634. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, Separation, and Identification of Phenolic Compounds in Virgin Olive Oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548–562. [Google Scholar]
- Prakash Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Khataee, A.R.; Fathinia, M.; Aber, S.; Zarei, M. Optimization of photocatalytic treatment of dye solution on supported TiO2 nanoparticles by central composite design: Intermediates identification. J. Hazard. Mater. 2010, 181, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; El Hamri, R.; Taitai, A. Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using Box–Behnken design and desirability function. Appl. Surf. Sci. 2012, 258, 4402–4410. [Google Scholar]


 represent design points;
represent design points;  represent 95% confident interval band).
represent 95% confident interval band).
 represent design points;
represent design points;  represent 95% confident interval band).
represent 95% confident interval band).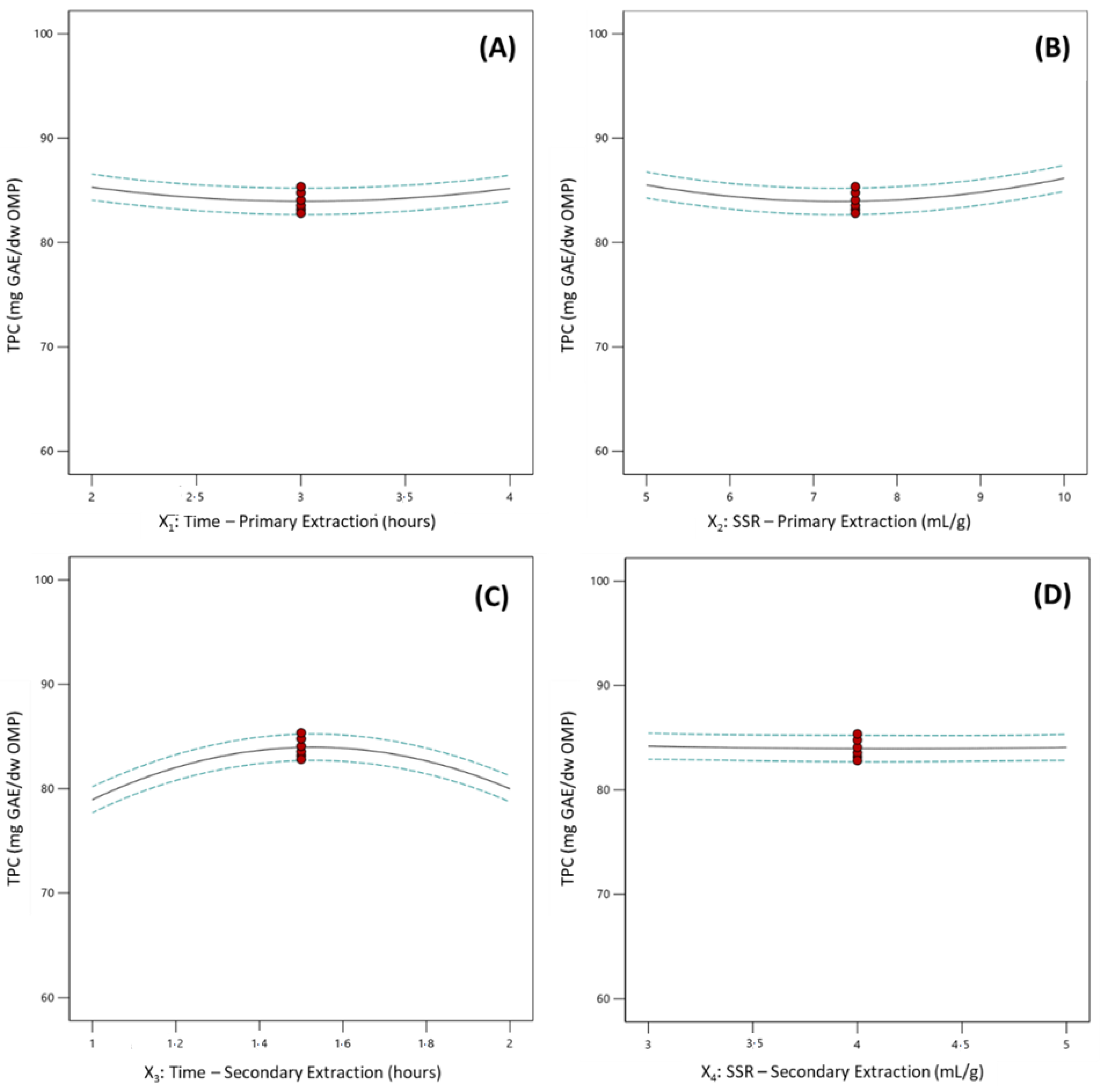
 represent a design point). (A) X1: Time—Primary Extraction (hours) versus X2: solvent-to-sample ratio (SSR)—Primary Extraction (mL/g); (B) X1 versus X3: Time—Secondary Extraction (hours); (C) X1 versus X4: Time—Secondary Extraction (hours); (D) X2 versus X3; (E) X3 versus X4; (F) X3 versus X4.
represent a design point). (A) X1: Time—Primary Extraction (hours) versus X2: solvent-to-sample ratio (SSR)—Primary Extraction (mL/g); (B) X1 versus X3: Time—Secondary Extraction (hours); (C) X1 versus X4: Time—Secondary Extraction (hours); (D) X2 versus X3; (E) X3 versus X4; (F) X3 versus X4.
 represent a design point). (A) X1: Time—Primary Extraction (hours) versus X2: solvent-to-sample ratio (SSR)—Primary Extraction (mL/g); (B) X1 versus X3: Time—Secondary Extraction (hours); (C) X1 versus X4: Time—Secondary Extraction (hours); (D) X2 versus X3; (E) X3 versus X4; (F) X3 versus X4.
represent a design point). (A) X1: Time—Primary Extraction (hours) versus X2: solvent-to-sample ratio (SSR)—Primary Extraction (mL/g); (B) X1 versus X3: Time—Secondary Extraction (hours); (C) X1 versus X4: Time—Secondary Extraction (hours); (D) X2 versus X3; (E) X3 versus X4; (F) X3 versus X4.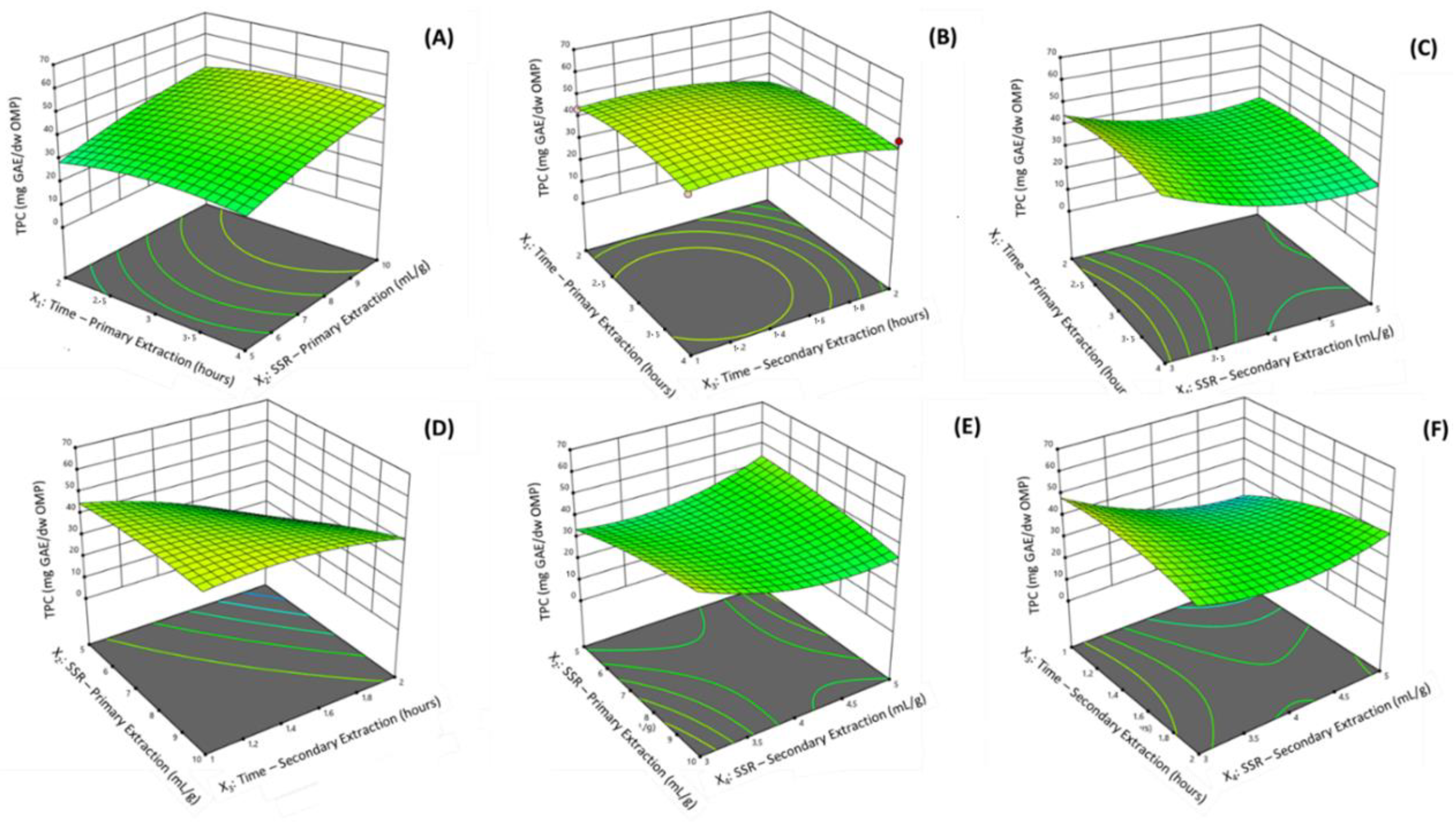
 represent design points;
represent design points;  represent a predicted point).
represent a predicted point).
 represent design points;
represent design points;  represent a predicted point).
represent a predicted point).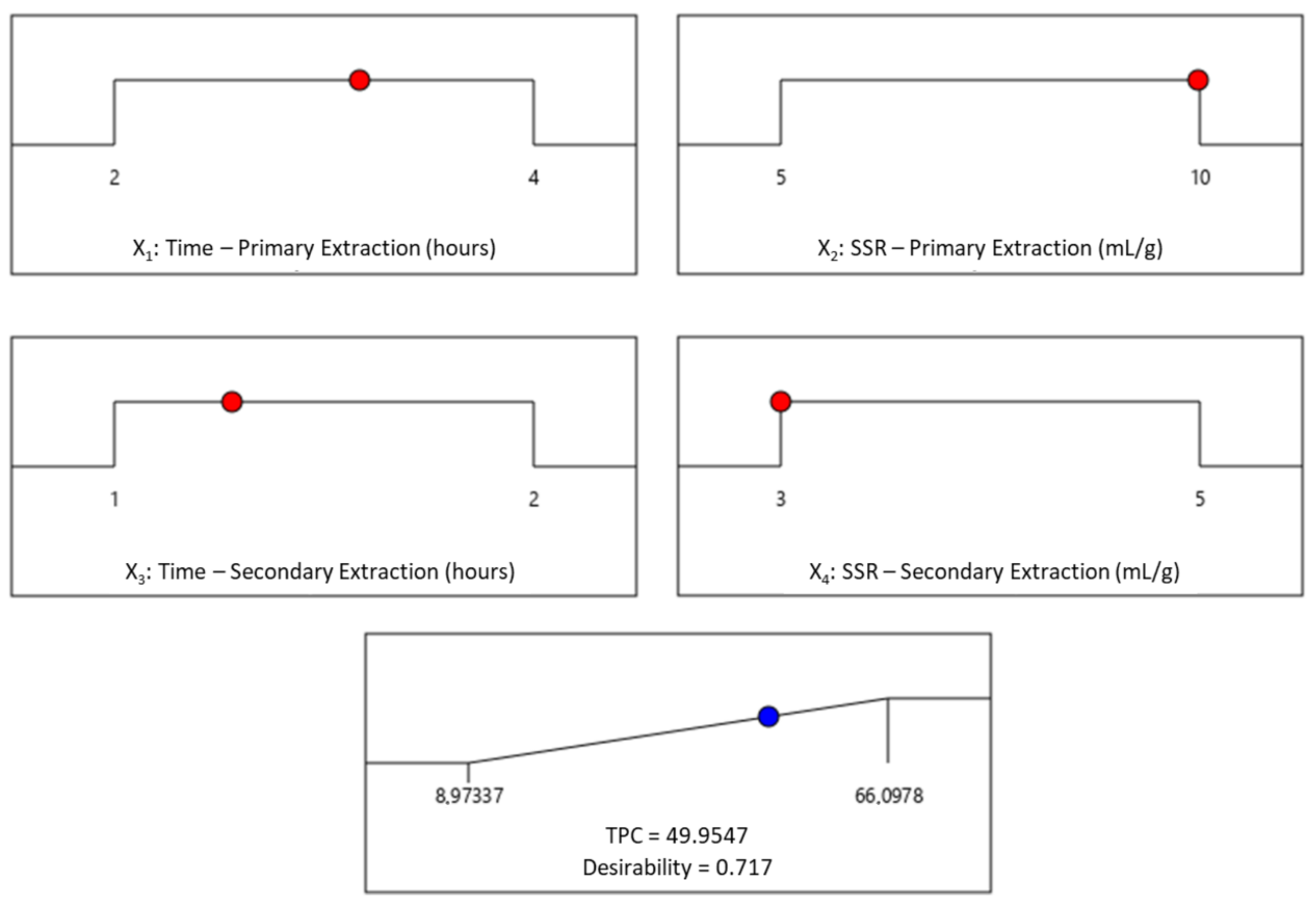
| Run No. | Process Variables (Coded Variables) | Response | |||||
|---|---|---|---|---|---|---|---|
| X1: Time P1E (hours) a | X2: P1E-SSR (mL/g) a | X3: Time P2E (hours) a | X4: P2E-SSR (mL/g) a | Y: TPC (mg GAE/g dw OMP) | |||
| OV | PV | RE | |||||
| 1 | 3.0 (0) | 7.5 (0) | 0.5 (−2) | 4.0 (0) | 31.3 | 29.0 | 2.3 |
| 2 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 36.9 | 36.6 | 0.4 |
| 3 | 3.0 (0) | 12.5 (2) | 1.5 (0) | 4.0 (0) | 37.0 | 34.1 | 2.9 |
| 4 | 2.0 (−1) | 5.0 (−1) | 1.0 (−1) | 5.0 (1) | 43.1 | 45.0 | −2.0 |
| 5 | 4.0 (1) | 5.0 (−1) | 1.0 (−1) | 5.0 (1) | 39.1 | 39.4 | −0.3 |
| 6 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 39.9 | 36.6 | 3.3 |
| 7 | 2.0 (−1) | 5.0 (−1) | 1.0 (−1) | 3.0 (−1) | 40.4 | 40.5 | −0.1 |
| 8 | 2.0 (−1) | 10.0 (1) | 2.0 (1) | 5.0 (1) | 41.8 | 4.0 | −0.3 |
| 9 | 4.0 (1) | 10.0 (1) | 1.0 (−1) | 3.0 (−1) | 45.4 | 46.2 | −0.8 |
| 10 | 4.0 (1) | 5.0 (−1) | 1.0 (−1) | 3.0 (−1) | 44.7 | 43.0 | 1.7 |
| 11 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 6.0 (2) | 66.1 | 62.4 | 3.7 |
| 12 | 1.0 (−2) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 25.3 | 23.5 | 1.7 |
| 13 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 37.4 | 36.6 | 0.8 |
| 14 | 4.0 (1) | 10.0 (1) | 2.0 (1) | 3.0 (−1) | 43.8 | 40.4 | 3.4 |
| 15 | 2.0 (−1) | 10.0 (1) | 1.0 (−1) | 3.0 (−1) | 43.7 | 44.3 | −0.6 |
| 16 | 4.0 (1) | 5.0 (−1) | 2.0 (1) | 5.0 (1) | 34.3 | 32.3 | 2.0 |
| 17 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 35.1 | 36.6 | −1.5 |
| 18 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 2.0 (−2) | 58.1 | 60.6 | −2.5 |
| 19 | 2.0 (−1) | 5.0 (−1) | 2.0 (1) | 5.0 (1) | 34.3 | 36.1 | −1.9 |
| 20 | 4.0 (1) | 10.0 (1) | 2.0 (1) | 5.0 (1) | 35.0 | 37.6 | −2.6 |
| 21 | 2.0 (−1) | 5.0 (−1) | 2.0 (1) | 3.0 (−1) | 9.7 | 5.9 | 3.9 |
| 22 | 2.0 (−1) | 10.0 (1) | 2.0 (1) | 3.0 (−1) | 34.2 | 36.7 | −2.4 |
| 23 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 34.2 | 36.6 | −2.3 |
| 24 | 4.0 (1) | 5.0 (−1) | 2.0 (1) | 3.0 (−1) | 9.0 | 10.3 | −1.3 |
| 25 | 3.0 (0) | 7.5 (0) | 2.5 (2) | 4.0 (0) | 13.3 | 14.3 | −1.0 |
| 26 | 4.0 (1) | 10.0 (1) | 1.0 (−1) | 5.0 (1) | 15.2 | 17.7 | −2.4 |
| 27 | 5.0 (2) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 21.2 | 21.7 | −0.5 |
| 28 | 2.0 (−1) | 10.0 (1) | 1.0 (−1) | 5.0 (1) | 22.5 | 23.9 | −1.4 |
| 29 | 3.0 (0) | 2.5 (−2) | 1.5 (0) | 4.0 (0) | 23.4 | 25.0 | −1.7 |
| 30 | 3.0 (0) | 7.5 (0) | 1.5 (0) | 4.0 (0) | 35.9 | 36.6 | −0.7 |
| Y: TPC (mg GAE/g dw OMP) | Model | Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Remarks |
| Sequential model sum of squares | Mean | 3544.7 | 1 | 35,447.7 | - | - | - | |
| Linear | 454.5 | 4 | 113.6 | 0.65 | 0.6300 | - | ||
| 2FI | 2081.8 | 6 | 347.0 | 2.90 | 0.0400 | - | ||
| Quadratic | 2151.2 | 4 | 537.8 | 64.51 | <0.0001 | Suggested | ||
| Cubic | 83.3 | 8 | 10.4 | 1.75 | 0.2400 | Aliased | ||
| Residual | 41.8 | 7 | 6.0 | - | - | - | ||
| Total | 40,260.2 | 30 | 1342.0 | - | - | - | ||
| Model | Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Remarks | |
| Lack-of-fit Tests | Linear | 4338.0 | 20 | 216.9 | 54.27 | 0.0002 | - | |
| 2FI | 2256.3 | 14 | 161.1 | 40.33 | 0.0003 | - | ||
| Quadratic | 105.1 | 10 | 10.5 | 2.63 | 0.1488 | Suggested | ||
| Cubic | 21.8 | 2 | 10.9 | 2.73 | 0.1583 | Aliased | ||
| Pure Error | 20.0 | 5 | 4.0 | - | - | - | ||
| Model | Source | Std. dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | Remarks | |
| Summary Statistics | Linear | 13.2 | 0.094 | −0.050 | −0.410 | 6785.6 | - | |
| 2FI | 11.0 | 0.527 | 0.278 | 0.205 | 3827.5 | - | ||
| Quadratic | 2.9 | 0.074 | 0.950 | 0.868 | 634.0 | Suggested | ||
| Cubic | 2.4 | 0.991 | 0.964 | 0.342 | 3165.4 | Aliased | ||
| Fit Summary | Source | Sequential p-Value | Lack of fit p-Value | Adjusted R2 | Predicted R2 | Remarks | ||
| Linear | 0.6310 | 0.0002 | −0.054 | −0.410 | - | |||
| 2FI | 0.0354 | 0.0003 | 0.278 | 0.201 | - | |||
| Quadratic | <0.0001 | 0.1488 | 0.950 | 0.868 | Suggested | |||
| Cubic | 0.2387 | 0.1583 | 0.964 | 0.342 | Aliased | |||
| Y: TPC (mg GAE/g dw OMP) | Source | Coefficient Estimate | Standard Error | Sum of Squares | DF | Mean Square | F-Value | p-Value | Remarks |
| Model | - | - | 4687.5 | 14 | 334.8 | 40.16 | <0.0001 | S | |
| Intercept | 36.6 | 1.2 | - | 1 | - | - | - | - | |
| X1 | −0.5 | 0.6 | 5.1 | 1 | 5.1 | 0.62 | 0.4459 | - | |
| X2 | 2.3 | 0.6 | 122.7 | 1 | 122.7 | 14.71 | 0.0016 | - | |
| X3 | −3.7 | 0.6 | 322.3 | 1 | 322.3 | 38.66 | <0.0001 | - | |
| X4 | 0.4 | 0.6 | 4.4 | 1 | 4.4 | 0.53 | 0.4781 | - | |
| X1X2 | −0.2 | 0.7 | 0.4 | 1 | 0.4 | 0.05 | 0.8313 | - | |
| X1X3 | 0.5 | 0.7 | 3.4 | 1 | 3.4 | 0.41 | 0.5313 | - | |
| X1X4 | −2.1 | 0.7 | 67.0 | 1 | 67.0 | 8.03 | 0.0126 | - | |
| X2X3 | 6.8 | 0.7 | 729.8 | 1 | 729.8 | 87.54 | <0.0001 | - | |
| X2X4 | −6.2 | 0.7 | 620.7 | 1 | 620.7 | 74.45 | <0.0001 | - | |
| X3X4 | 6.4 | 0.7 | 660.5 | 1 | 660.5 | 79.22 | <0.0001 | - | |
| X12 | −3.5 | 0.6 | 332.7 | 1 | 332.7 | 39.90 | <0.0001 | - | |
| X22 | −1.6 | 0.6 | 83.8 | 1 | 83.8 | 10.05 | 0.0063 | - | |
| X32 | −3.7 | 0.6 | 381.3 | 1 | 381.3 | 45.73 | <0.0001 | - | |
| X42 | 6.2 | 0.6 | 1066.5 | 1 | 1066.5 | 127.93 | <0.0001 | - | |
| Residual | - | - | 125.06 | 15 | 8.34 | - | - | - | |
| Lack of Fit | - | - | 105.07 | 10 | 10.51 | 2.63 | 0.1488 | NS | |
| Pure Error | - | - | 19.98 | 5 | 4.00 | - | - | - | |
| Cor Error | - | - | 4812.52 | 29 | - | - | - | - | |
| Std. Dev. | Mean | CV (%) | R2 | Adjusted R2 | Predicted R2 | Adeq. precision | PRESS | - | |
| 2.9 | 34.4 | 8.4 | 0.974 | 0.950 | 0.868 | 27.7 | 634.0 | - |
| Post Analysis | |
|---|---|
| Predicted (mg GAE/g of dw OMP) | 50.0 |
| 95% PI low (mg GAE/g of dw OMP) | 45.2 |
| 95% PI high (mg GAE/g of dw OMP) | 54.7 |
| Experimental (mg GAE/g of dw OMP) a | 50.5 ± 1.5 |
| Residual Error | 0.5 |
| %Error | 1.0 |
| Extract Fraction | Hydrolyzed Extracted Samples | Non-Hydrolyzed Extracted Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g dw OMP) | TPC Proportion (%) | TAA (%) | TAA Proportion (%) | TPC (mg GAE/g dw OMP) | TPC Proportion (%) | TAA (%) | TAA Proportion (%) | |
| TPA | 50.5 ± 1.5 | 100 | 79.1 ± 7.9 | 100 | 21.8 ± 0.7 | 100 | 53.5 ± 3.9 | 100 |
| BPA | 13.6 ± 1.1 | 26.9 ± 2.2 | 29.1 ± 3.9 | 36.8 ± 4.9 | 2.0 ± 0.1 | 9.2 ± 0.5 | 18.8 ± 4.6 | 35.1 ± 7.3 |
| FPA | 36.9 ± 0.4 | 73.1 ± 0.8 | 49.9 ± 4.7 | 63.1 ± 5.9 | 19.8 ± 0.6 | 91.2 ± 2.8 | 34.7 ± 3.8 | 64.9 ± 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, F.; Tavares, L.; Santos, L. Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace. Molecules 2022, 27, 8620. https://doi.org/10.3390/molecules27238620
Paulo F, Tavares L, Santos L. Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace. Molecules. 2022; 27(23):8620. https://doi.org/10.3390/molecules27238620
Chicago/Turabian StylePaulo, Filipa, Loleny Tavares, and Lúcia Santos. 2022. "Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace" Molecules 27, no. 23: 8620. https://doi.org/10.3390/molecules27238620
APA StylePaulo, F., Tavares, L., & Santos, L. (2022). Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace. Molecules, 27(23), 8620. https://doi.org/10.3390/molecules27238620






