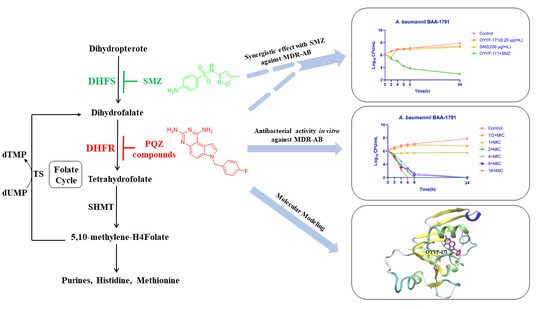
The Anti-Multidrug-Resistant Acinetobacter baumannii Study on 1,3-diamino-7H-pyrrolo[3,2-f]quinazoline Compounds
Abstract
1. Introduction
2. Results
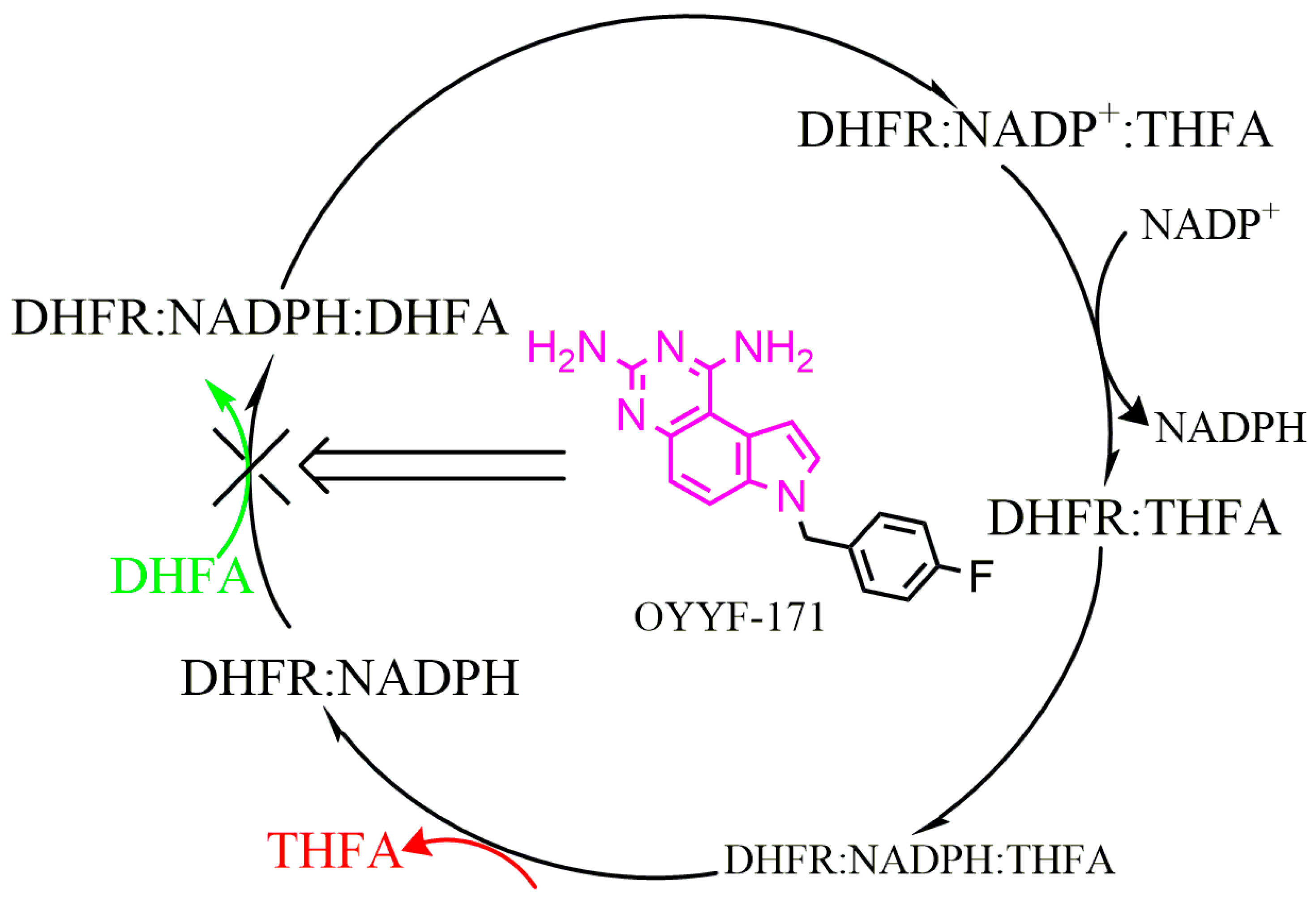
2.1. Molecular Modeling
2.2. In Vitro Antibacterial Activity against A. baumannii Isolates
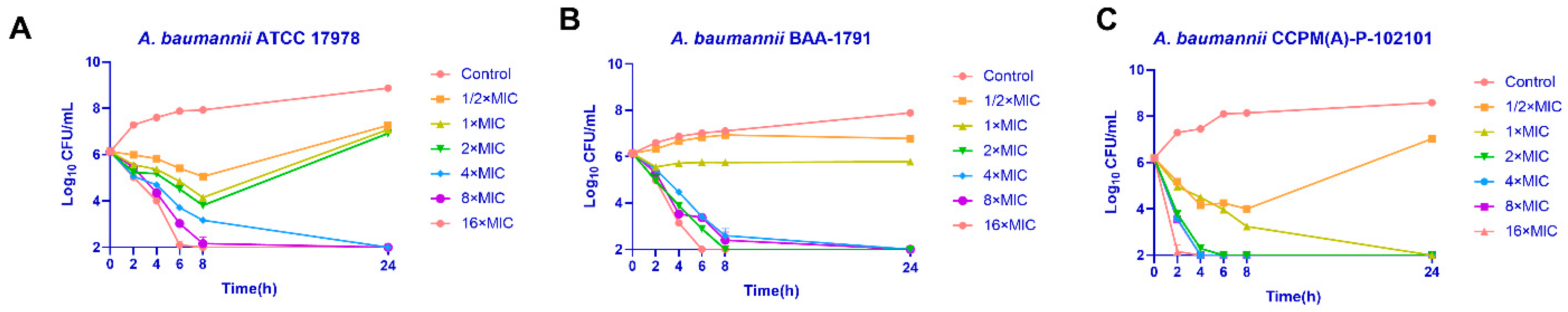
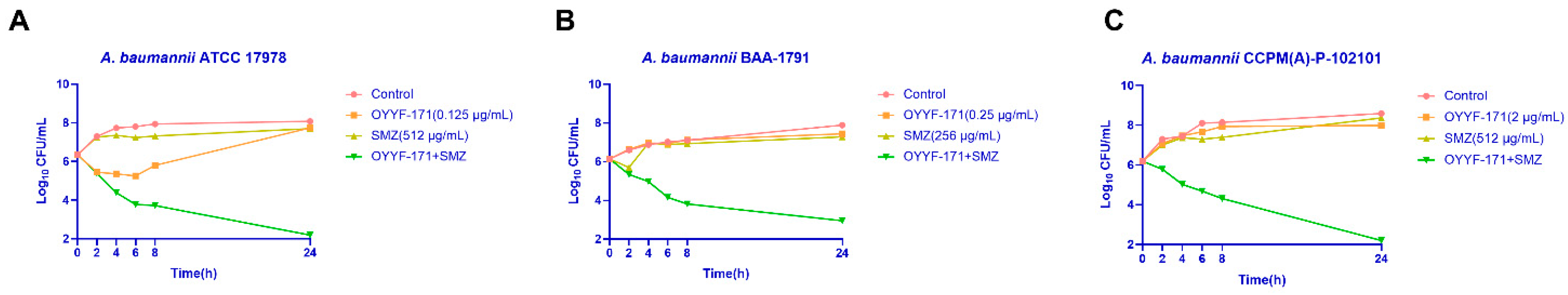
2.3. Time-Killing Assay
2.4. Synergistic Effect with Sulfamethoxazole (SMZ)
3. Discussion
4. Materials and Methods
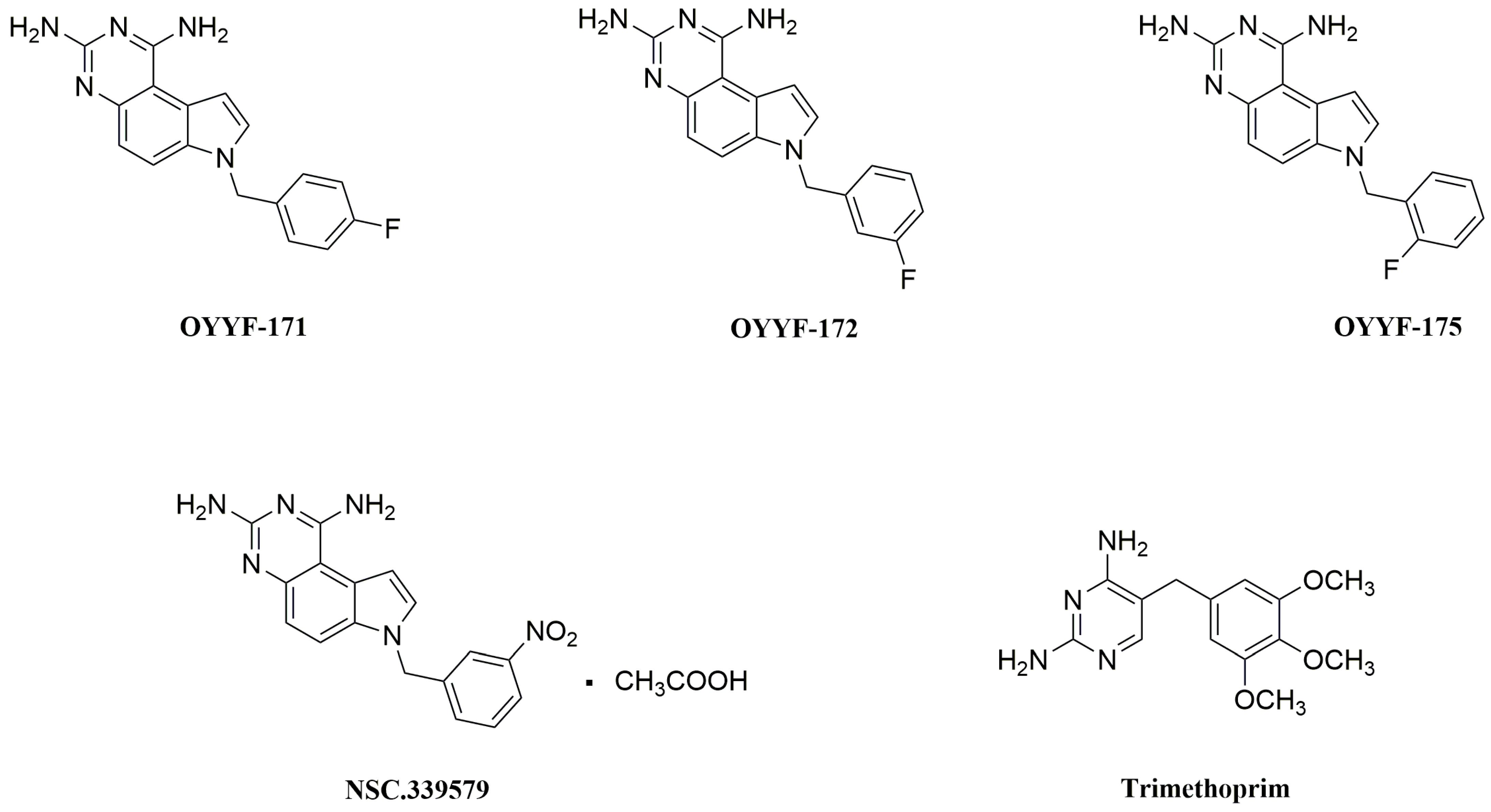
4.1. Preparation of Compounds OYYF-171, -172, and -175
4.2. Molecular Simulation
4.3. Bacterial Strains and Culture Condition
4.4. Antimicrobial Agents and Susceptibility Test
4.5. Time-Killing Assay
4.6. Synergy Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef] [PubMed]
- Perez, S.; Innes, G.K.; Walters, M.S.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions—New Jersey, February–July 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- Maynard, C.; Cummins, I.; Green, J.; Weinkove, D. A bacterial route for folic acid supplementation. BMC Biol. 2018, 16, 67. [Google Scholar] [CrossRef]
- Galassi, R.; Oumarou, C.S.; Burini, A.; Dolmella, A.; Micozzi, D.; Vincenzetti, S.; Pucciarelli, S. A study on the inhibition of dihydrofolate reductase (DHFR) from Escherichia coli by gold(i) phosphane compounds. X-ray crystal structures of (4,5-dichloro-1H-imidazolate-1-yl)-triphenylphosphane-gold(i) and (4,5-dicyano-1H-imidazolate-1-yl)-triphenylphosphane-gold(i). Dalton Trans. 2015, 44, 3043–3056. [Google Scholar] [CrossRef]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate reductase inhibitors for use as antimicrobial agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef]
- Hong, W.; Wang, Y.; Chang, Z.; Yang, Y.; Pu, J.; Sun, T.; Kaur, S.; Sacchettini, J.C.; Jung, H.; Lin Wong, W.; et al. The identification of novel Mycobacterium tuberculosis DHFR inhibitors and the investigation of their binding preferences by using molecular modelling. Sci. Rep. 2015, 5, 15328. [Google Scholar] [CrossRef]
- Li, Y.; Ouyang, Y.; Wu, H.; Wang, P.; Huang, Y.; Li, X.; Chen, H.; Sun, Y.; Hu, X.; Wang, X.; et al. The discovery of 1, 3-diamino-7H-pyrrol[3, 2-f]quinazoline compounds as potent antimicrobial antifolates. Eur. J. Med. Chem. 2022, 228, 113979. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, R.B.; van den Bedem, H.; Fraser, J.S.; Wright, P.E. Integrated description of protein dynamics from room-temperature X-ray crystallography and NMR. Proc. Natl. Acad. Sci. USA 2014, 111, E445–E454. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Sobtop, Version 1.0(dev3.1). Available online: http://sobereva.com/soft/Sobtop (accessed on 1 November 2022).
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M100-Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Yang, X.Y.; Li, C.R.; Lou, R.H.; Wang, Y.M.; Zhang, W.X.; Chen, H.Z.; Huang, Q.S.; Han, Y.X.; Jiang, J.D.; You, X.F. In vitro activity of recombinant lysostaphin against Staphylococcus aureus isolates from hospitals in Beijing, China. J. Med. Microbiol. 2007, 56, 71–76. [Google Scholar] [CrossRef]
- Lu, X.; Yang, X.; Li, X.; Lu, Y.; Ren, Z.; Zhao, L.; Hu, X.; Jiang, J.; You, X. In vitro activity of sodium new houttuyfonate alone and in combination with oxacillin or netilmicin against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e68053. [Google Scholar] [CrossRef] [PubMed]

| Molecules | ΔGMM-GBSA | TΔS | ΔGbinding |
|---|---|---|---|
| OYYF-171 | −30.31 ± 0.34 | −11.91 ± 1.66 | −18.40 |
| OYYF-172 | −35.91 ± 0.85 | −13.55 ± 0.55 | −22.36 |
| OYYF-175 | −34.47 ± 0.74 | −17.11 ± 0.04 | −17.36 |
| TMP | −35.56 ± 0.35 | −10.22 ± 0.77 | −25.35 |
| Strain (Phenotype) | MIC (μg/mL) | ||||
|---|---|---|---|---|---|
| OYYF-171 | OYYF-172 | OYYF-175 | TMP | ||
| A. baumannii | ATCC 17978 | 0.5 | 1 | 1 | 8 |
| ATCC BAA-1605 (MDR) | 2 | 2 | 2 | 64 | |
| ATCC BAA-1789 (MDR) | 4 | 4 | 8 | 128 | |
| ATCC BAA-1791 (MDR) | 1 | 1 | 2 | 64 | |
| ATCC BAA-1793 (MDR) | 8 | 8 | 16 | 128 | |
| ATCC BAA-1794 (MDR) | 8 | 4 | 8 | 64 | |
| ATCC BAA-1795 (MDR) | 16 | 16 | 32 | >256 | |
| ATCC BAA-1796 (MDR) | 8 | 8 | 16 | 64 | |
| Antibiotic | MIC (μg/mL) | |||
|---|---|---|---|---|
| MIC50 | MIC90 | MIC Range | ||
| β-lactam | AMP | >32 | >32 | >32 |
| CAZ | >32 | >32 | 2–>32 | |
| AZT | 64 | >64 | 4–>64 | |
| MEM | >8 | >8 | 0.06–>8 | |
| AMP/SUL | >32 | >32 | 2–>32 | |
| Aminoglycoside | GEM | >16 | >16 | 0.5–>16 |
| Quinolone | CIP | >4 | >4 | 0.125–>4 |
| LEV | 8 | >8 | 0.06–>8 | |
| Folate metabolism inhibitor | TMP | 128 | >128 | 8–>128 |
| PQZ compound | OYYF-171 | 8 | 8 | 0.25–32 |
| OYYF-172 | 8 | 8 | 0.25–32 | |
| OYYF-175 | 8 | 16 | 0.25–>32 | |
| Combination of Drugs | Strain | MIC (µg/mL) | FICI | FICI Median | Checkerboard Effect | |||
|---|---|---|---|---|---|---|---|---|
| Alone | In Combination | |||||||
| OYYF-171 | SMZ | OYYF-171 | SMZ | |||||
| OYYF-171/SMZ | ATCC 17978 | 0.5 | 2048 | 0.125 | 128 | 0.313 | 0.185 | Synergism |
| ATCC BAA-1791 | 1 | 1024 | 0.25 | 128 | 0.375 | Synergism | ||
| ATCC BAA-1793 | 8 | 1024 | 0.125 | 4 | 0.020 | Synergism | ||
| ATCC BAA-1794 | 8 | 32 | 0.125 | 4 | 0.141 | Synergism | ||
| ATCC BAA-1789 | 8 | 2048 | 1 | 128 | 0.188 | Synergism | ||
| ATCC BAA-1605 | 4 | 2048 | 0.5 | 32 | 0.141 | Synergism | ||
| CCPM(A)-P-102101 | 8 | 2048 | 2 | 256 | 0.375 | Synergism | ||
| CCPM(A)-P-102102 | 0.25 | 16 | 0.03 | 1 | 0.183 | Synergism | ||
| CCPM(A)-P-102103 | 8 | 2048 | 1 | 128 | 0.188 | Synergism | ||
| CCPM(A)-P-102105 | 0.5 | 1024 | 0.03 | 64 | 0.123 | Synergism | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Chen, H.; Zhang, J.; Hu, X.; Xie, C.; Cao, W.; Zhao, Z.; Xiao, Z.; Ren, Y.; Dong, L.; et al. The Anti-Multidrug-Resistant Acinetobacter baumannii Study on 1,3-diamino-7H-pyrrolo[3,2-f]quinazoline Compounds. Molecules 2022, 27, 8609. https://doi.org/10.3390/molecules27238609
Wu H, Chen H, Zhang J, Hu X, Xie C, Cao W, Zhao Z, Xiao Z, Ren Y, Dong L, et al. The Anti-Multidrug-Resistant Acinetobacter baumannii Study on 1,3-diamino-7H-pyrrolo[3,2-f]quinazoline Compounds. Molecules. 2022; 27(23):8609. https://doi.org/10.3390/molecules27238609
Chicago/Turabian StyleWu, Han, Hongtong Chen, Jungan Zhang, Xinxin Hu, Chunyang Xie, Weiting Cao, Ziqi Zhao, Zengshuo Xiao, Yixin Ren, Luyao Dong, and et al. 2022. "The Anti-Multidrug-Resistant Acinetobacter baumannii Study on 1,3-diamino-7H-pyrrolo[3,2-f]quinazoline Compounds" Molecules 27, no. 23: 8609. https://doi.org/10.3390/molecules27238609
APA StyleWu, H., Chen, H., Zhang, J., Hu, X., Xie, C., Cao, W., Zhao, Z., Xiao, Z., Ren, Y., Dong, L., Sun, P., You, X., Yang, X., Hong, W., & Wang, H. (2022). The Anti-Multidrug-Resistant Acinetobacter baumannii Study on 1,3-diamino-7H-pyrrolo[3,2-f]quinazoline Compounds. Molecules, 27(23), 8609. https://doi.org/10.3390/molecules27238609