The Use of Polydialkylsiloxanes/Triflic Acid as Derivatization Agents in the Analysis of Sulfur-Containing Aromatics by “Soft”-Ionization Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Optimization of Derivatization Procedure
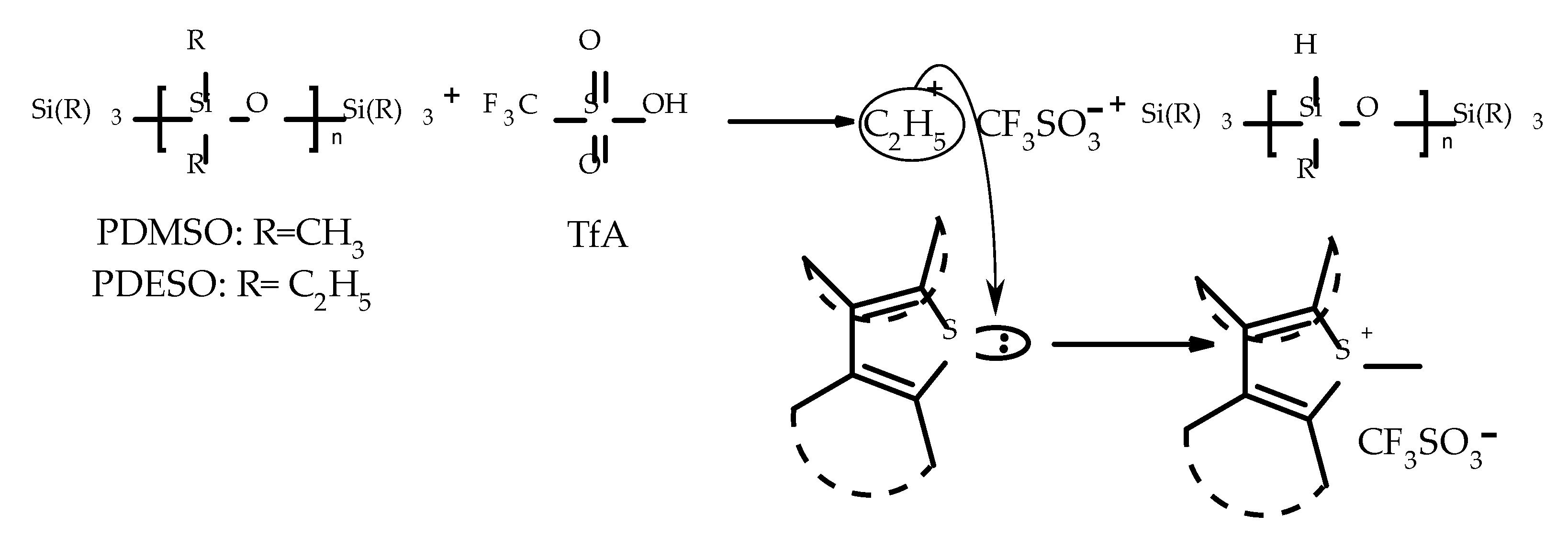
2.2. Possible Mechanism of the Described Derivatization Reaction
2.3. Features of MALDI and ESI Mass Spectra of S-Alkyldibenzothiophenium Cations Extracted from Salts
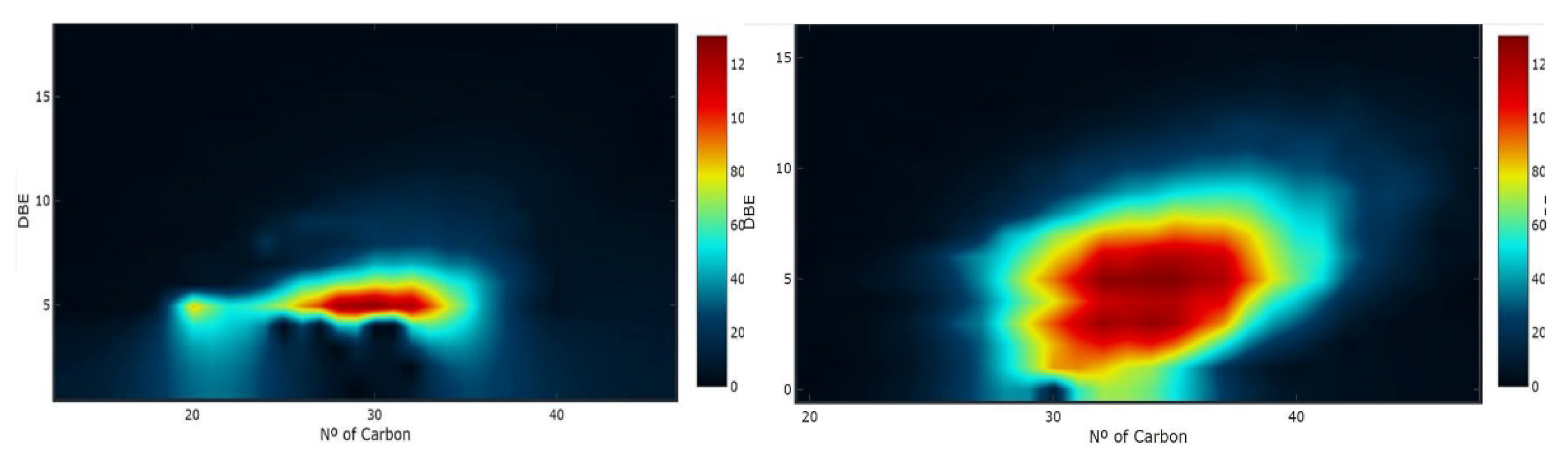
2.4. Example of Application of Preliminary Derivatization by Poly(dialkyl)siloxanes to Analysis of Petroleum Sulfur-Containing Aromatics by High-Resolution ESI Mass Spectrometry
3. Materials and Methods
3.1. Chemicals
3.2. Derivatization of Dibenzothiophenes and Their Analogues by Silicon Fluids
3.3. Derivatization of Dibenzothiophenes by Silicon Fluids and Characterization of the Derivatives
3.3.1. S-Methyldibenzothiophenium Triflate
3.3.2. S-Ethyldibenzothiophenium Triflate
3.4. Sample Preparation for Analysis by MALDI Mass Spectrometry
3.5. Sample Preparation for Analysis by ESI Mass Spectrometry
3.6. Sample Preparation for Analysis by GC/MS
3.7. Instrumentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rajendran, A.; Cui, T.; Fan, H.; Yang, Z.; Feng, J.; Li, W. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Abro, R.; Abdeltawab, A.A.; Al-Deyab, S.S.; Yu, G.; Qazi, A.B.; Gao, S.; Chen, X. A Review of extractive desulfurization of fuel oils using ionic liquids. RSC Adv. 2014, 4, 35302–35317. [Google Scholar] [CrossRef]
- Drobyshev, A.I.; Glebova, S.M.; Tikhonov, V.A. X-Ray fluorescence determination of sulfur and jther elements in customs control of oil and liquid oil Products. J. Anal. Chem. 2006, 61, 777–780. [Google Scholar] [CrossRef]
- Santelli, R.E.; Oliveira, E.P.; de Carvalho, M.d.F.B.; Bezerra, M.A.; Freire, A.S. Total sulfur determination in gasoline, kerosene and diesel fuel using inductively coupled plasma optical emission spectrometry after direct sample introduction as detergent emulsions. Spectrochimica Acta Part B At. Spectrosc. 2008, 63, 800–804. [Google Scholar] [CrossRef]
- Pollo, B.J.; Alexandrino, G.L.; Augusto, F.; Hantao, L.W. The Impact of Comprehensive Two-Dimensional Gas Chromatography on Oil Gas Analysis: Recent Advances and Applications in Petroleum Industry. TrAC Trends Anal. Chem. 2018, 105, 202–217. [Google Scholar] [CrossRef]
- López García, C.; Becchi, M.; Grenier-Loustalot, M.F.; Païsse, O.; Szymanski, R. Analysis of Aromatic Sulfur Compounds in Gas Oils Using GC with Sulfur Chemiluminescence Detection and High-Resolution MS. Anal. Chem. 2002, 74, 3849–3857. [Google Scholar] [CrossRef]
- Chin, S.-T.; Wu, Z.-Y.; Morrison, P.D.; Marriott, P.J. Observations on Comprehensive Two Dimensional Gas Chromatography Coupled with Flame Photometric Detection for Sulfur- and Phosphorus-Containing Compounds. Anal. Methods 2010, 2, 243. [Google Scholar] [CrossRef]
- Borisov, R.S.; Kulikova, L.N.; Zaikin, V.G. Mass Spectrometry in petroleum chemistry (petroleomics) (review). Pet. Chem. 2019, 59, 1055–1076. [Google Scholar] [CrossRef]
- Zhan, D.; Fenn, J.B. Electrospray Mass Spectrometry of Fossil Fuels. Int. J. Mass Spectrom. 2000, 194, 197–208. [Google Scholar] [CrossRef]
- Hsu, C.S.; Hendrickson, C.L.; Rodgers, R.P.; McKenna, A.M.; Marshall, A.G. Petroleomics: Advanced Molecular Probe for Petroleum Heavy Ends. J. Mass Spectrom. 2011, 46, 337–343. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Thomas, M.J.; Jones, H.E.; Barrow, M.P. Petroleomics: Tools, challenges, and developments. Annu. Rev. Anal. Chem. 2020, 13, 405–430. [Google Scholar] [CrossRef]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the mechanism of electrospray ionization. Anal. Chem. 2012, 85, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Huang, L.C.L.; Wang, Y.-S.; Peng, W.-P.; Chang, H.C.; Hsu, N.Y.; Yang, W.B.; Chen, C.H. Matrix-Assisted Laser Desorption/Ionization (MALDI) Mechanism Revisited. Anal. Chim. Acta 2007, 582, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zaikin, V.G.; Borisov, R.S. Options of the main derivatization approaches for analytical ESI and MALDI mass spectrometry. Crit. Rev. Anal. Chem. 2021, 52, 1287–1342. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.-L.; Liu, P.; Wang, Q.-Y.; Cai, W.-J.; Yuan, B.-F.; Feng, Y.-Q. Derivatization for liquid chromatography-mass spectrometry. TrAC Trends Anal. Chem. 2014, 59, 121–132. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Kozlov, A.V.; Borisov, R.S.; Shchapin, I.Y. Regio-isomeric effects in tandem mass spectra of sulfonium cations generated from thiacyclane based sulfonium salts under soft ionization conditions. Eur. J. Mass Spectrom. 2018, 24, 108–115. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Borisov, R.S.; Zaikin, V.G. Permanent-charge generation derivatization for the analysis of thiols by “soft” ionization mass spectrometry methods. J. Anal. Chem. 2019, 74, 1396–1404. (Original paper: Mass-spektrometriya (In Russian) 2019, 16, 40–48) [Google Scholar] [CrossRef]
- Starkova, J.E.; Polovkov, N.Y.; Kanat’eva, A.Y.; Borisov, R.S.; Zaikin, V.G. Effects of isomerism in the mass spectra of stimulated dissociation of sulfonium cations desorbed from S-alkylated thiacyclanes under MALDI conditions. A convenient way to derivatize sulfides under the influence of alkyl formats. Mass-spektrometriya 2021, 18, 101–108. (In Russian) [Google Scholar]
- Müller, H.; Andersson, J.T.; Schrader, W. Characterization of high-molecular-weight sulfur-containing aromatics in vacuum residues using Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2005, 77, 2536–2543. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Wu, J.; Zhang, Y.; Liu, X.; Shi, Q.; Zhao, S.; Xu, C.; Hsu, C.S. Selective methylation of sulfides in petroleum for electrospray ionization mass spectrometry analysis. Energy Fuels 2019, 33, 1797–1802. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, J.; Zhang, Y.; Liang, Y.; Shi, Q. Characterization of sulfur-containing compounds in petroleum using AgSbF6 as a methylation reagent. Energy Fuels 2020, 34, 10842–10848. [Google Scholar] [CrossRef]
- Eaborn, C.; Lickiss, P.D.; Ramadan, N.A. Cleavage of silicon–carbon bonds in tris(trimethylsilyl)methylsilicon compounds by trifluoroacetic acid. Rearrangements and anchimeric assistance. J. Chem. Soc. Perkin Trans. 1984, 2, 267–270. [Google Scholar] [CrossRef]
- Valk, J.-M.; Boersma, J.; van Koten, G. Selective intramolecular cleavage of the carbon-silicon bond by palladium salts. J. Organomet. Chem. 1994, 483, 213–216. [Google Scholar] [CrossRef][Green Version]
- Aggarwal, V.K.; Thompson, A.; Jones, R.V.H. Synthesis of sulfonium salts by sulfide alkylation; an alternative approach. Tetrahedron Lett. 1994, 35, 8659–8660. [Google Scholar] [CrossRef]
- Rosa, T.R.; Folli, G.S.; Pacheco, W.L.S.; Castro, M.P.; Romão, W.; Filgueiras, P.R. DropMS: Petroleomics data treatment based in Web server for high-resolution mass spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 1483–1490. [Google Scholar] [CrossRef]
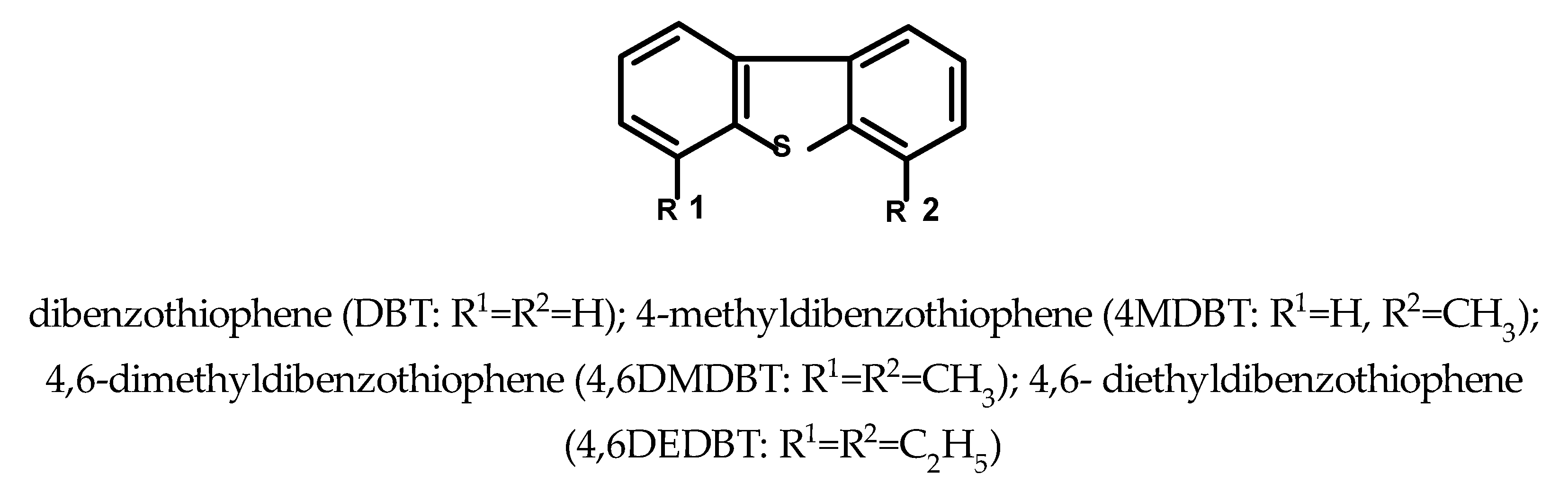
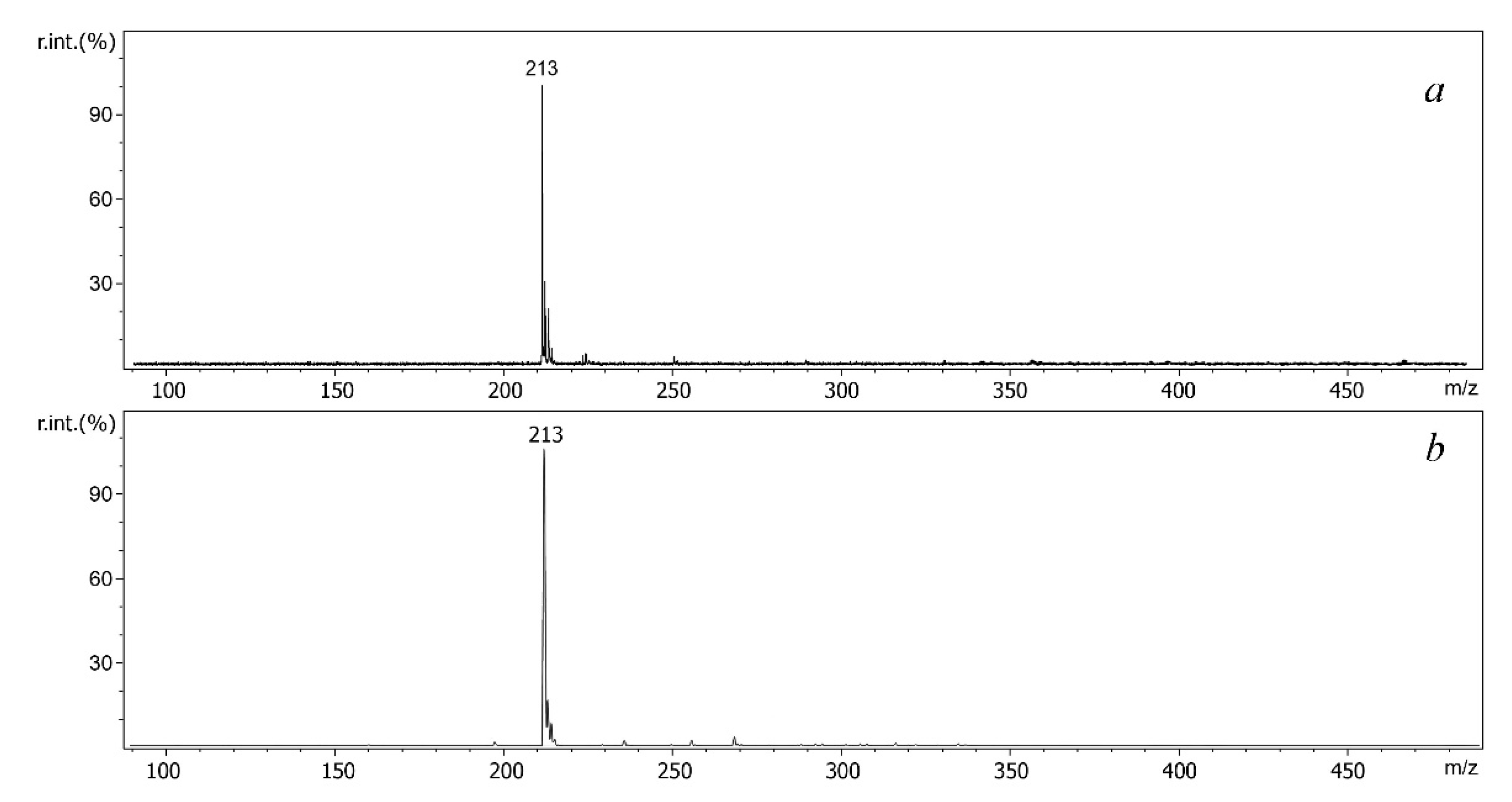
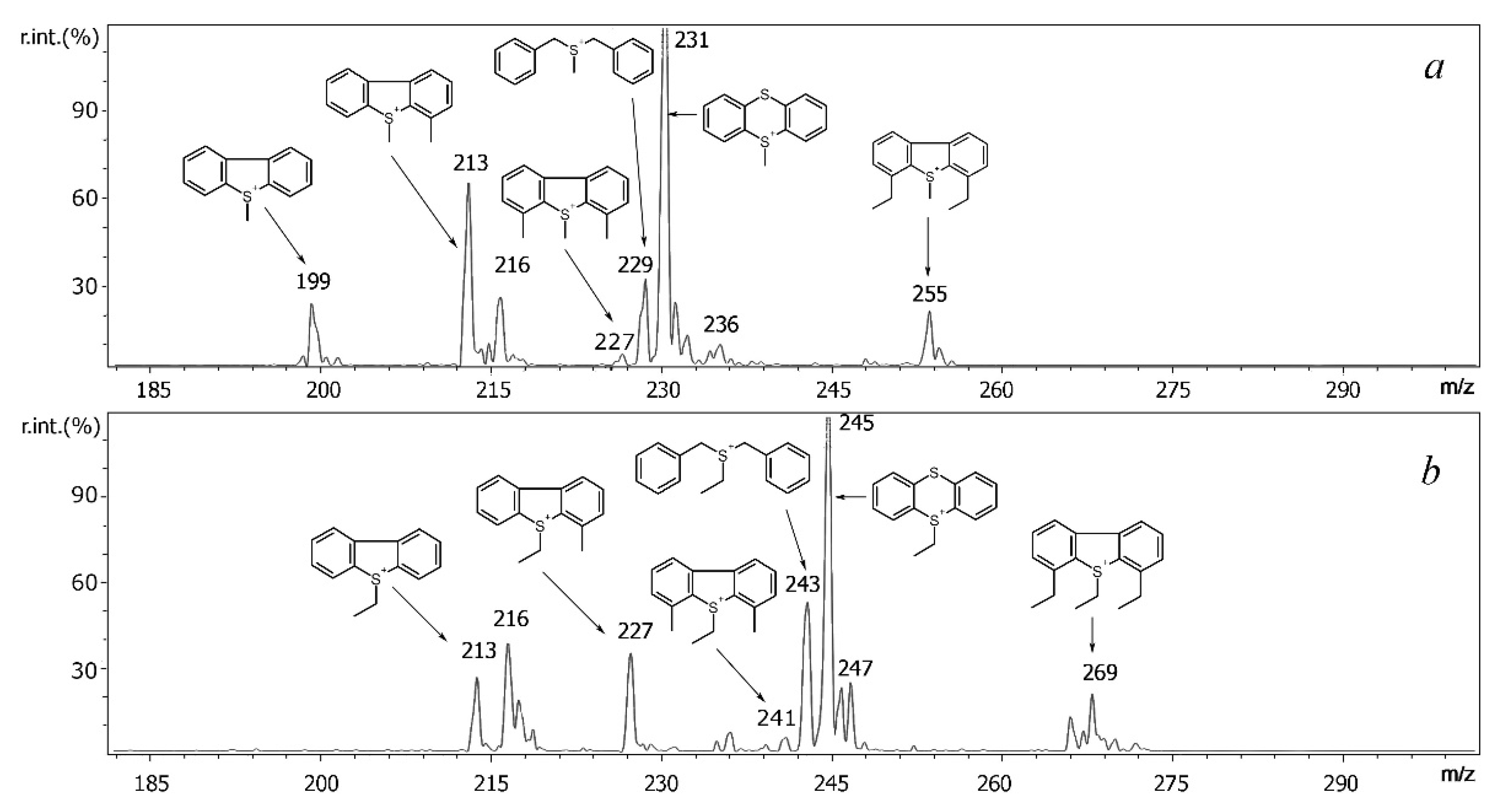
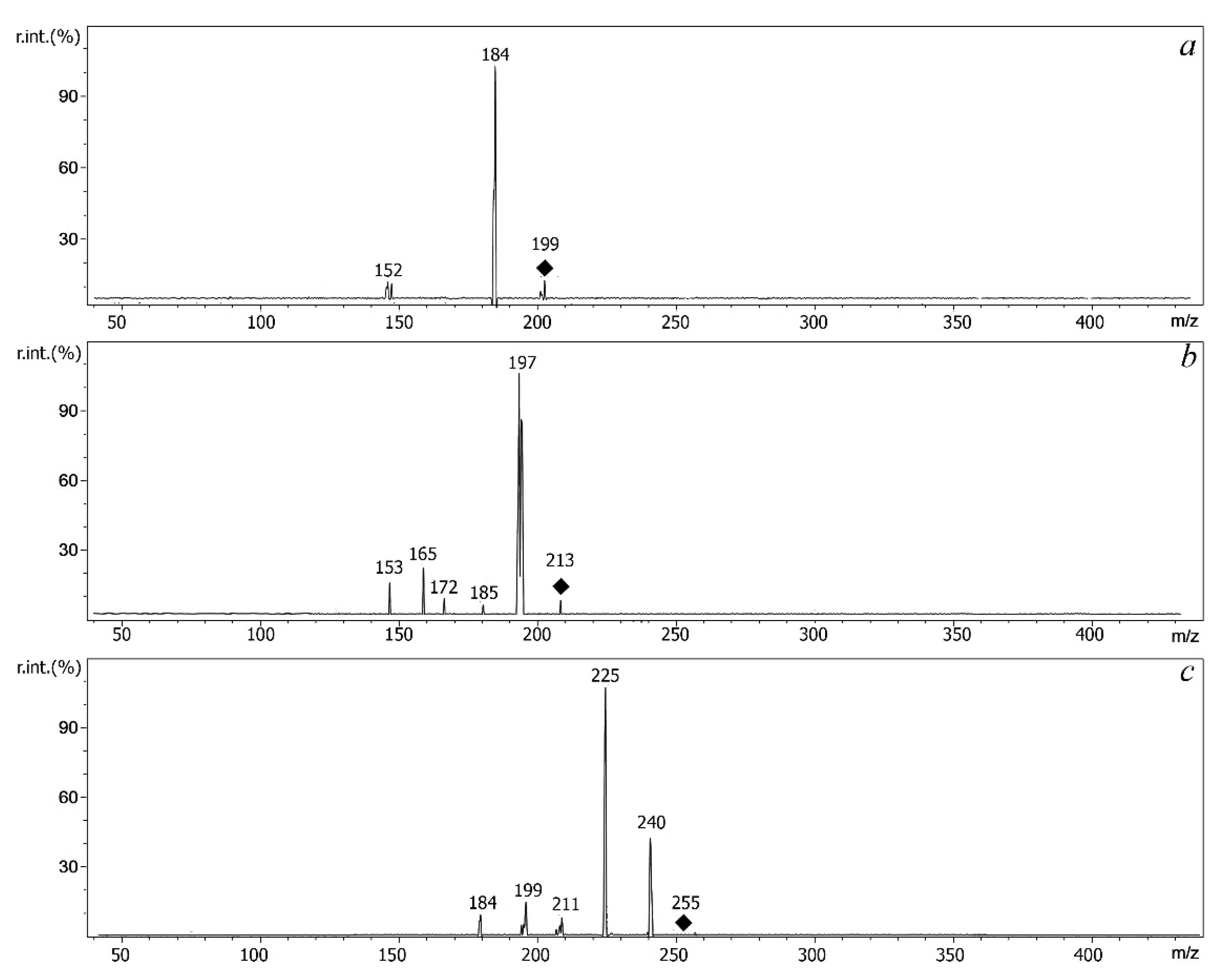
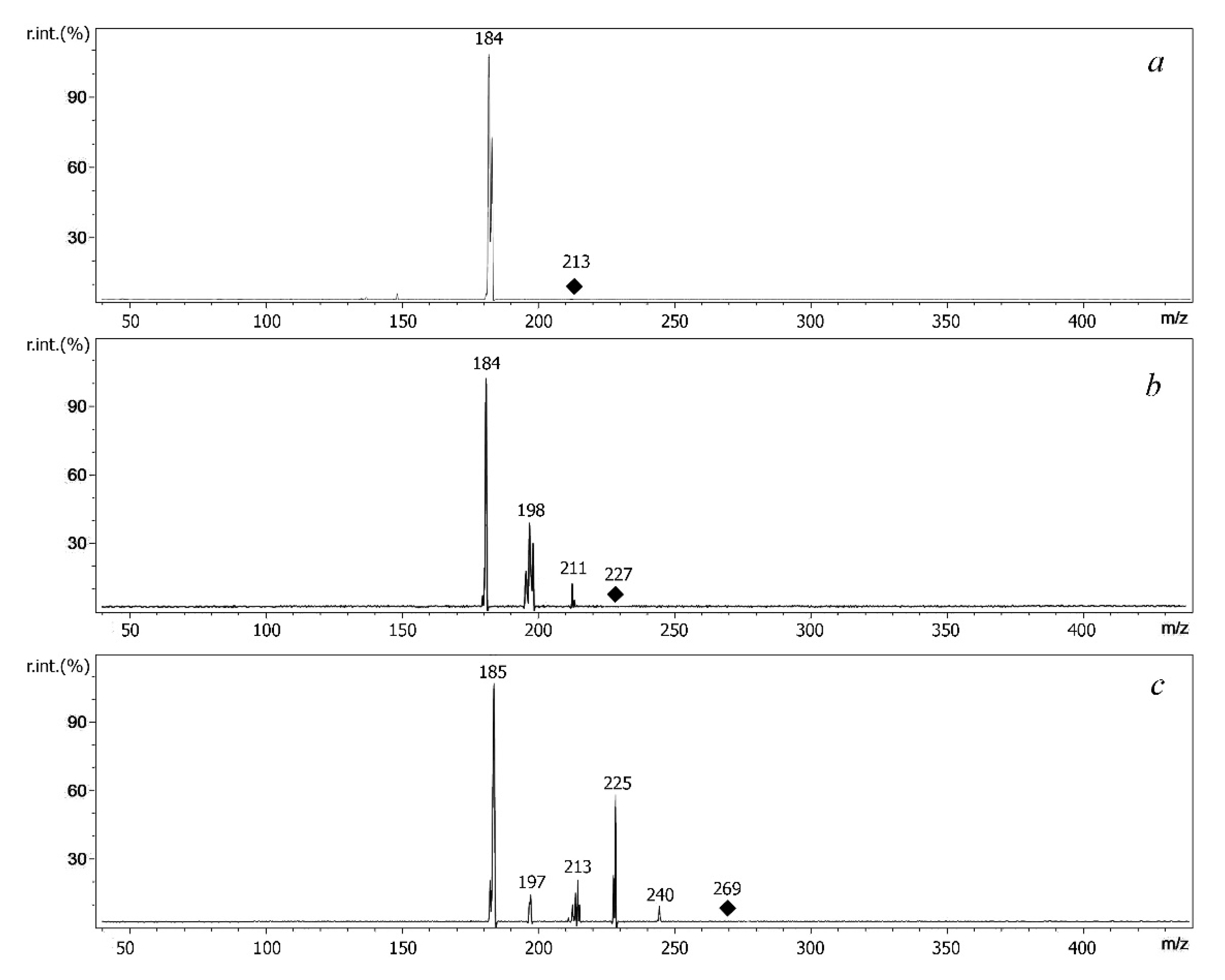
| Compound | Molecular Weight | Methylated Derivative, m/z | Ethylated Derivative, m/z |
|---|---|---|---|
| DBT | 184 | 199 | 213 |
| 4MeDBT | 198 | 213 | 227 |
| 4,6DMDBT | 212 | 227 | 241 |
| 4,6DEDBT | 240 | 255 | 269 |
| Dibenzyl sulfide | 214 | 229 | 243 |
| Thianthrene | 216 | 231 | 245 |
| Compound | Derivative | Precursor Ion, m/z | Product Ions |
|---|---|---|---|
| DBT | Me | 199 | [M-CH3]+ (m/z 184) |
| Et | 213 | [M-C2H4]+ (m/z 185), [M-C2H5]+ (m/z 184) | |
| 4MDBT | Me | 213 | [M-CH3]+ (m/z 198) [M-CH3-H]+· (m/z 197) |
| Et | 227 | [M-C2H4]+ (m/z 199), [M-C2H5]+ (m/z 198), [M- C2H4-CH3]+ (m/z 184) | |
| 4,6DEDBT | Me | 255 | [M-CH3]+ (m/z 240), [M- CH3-CH3]+ (m/z 225) |
| Et | 269 | [M-C2H5]+ (m/z 240), [M- C2H5-CH3]+ (m/z 225) [M-3C2H4]+ (m/z 185) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starkova, Z.; Ilyushenkova, V.; Polovkov, N.; Voskressenskaya, D.; Pikovskoi, I.; Tebenikhin, M.; Vtorushina, E.; Kanateva, A.; Borisov, R.; Zaikin, V. The Use of Polydialkylsiloxanes/Triflic Acid as Derivatization Agents in the Analysis of Sulfur-Containing Aromatics by “Soft”-Ionization Mass Spectrometry. Molecules 2022, 27, 8600. https://doi.org/10.3390/molecules27238600
Starkova Z, Ilyushenkova V, Polovkov N, Voskressenskaya D, Pikovskoi I, Tebenikhin M, Vtorushina E, Kanateva A, Borisov R, Zaikin V. The Use of Polydialkylsiloxanes/Triflic Acid as Derivatization Agents in the Analysis of Sulfur-Containing Aromatics by “Soft”-Ionization Mass Spectrometry. Molecules. 2022; 27(23):8600. https://doi.org/10.3390/molecules27238600
Chicago/Turabian StyleStarkova, Zhanna, Valentina Ilyushenkova, Nikolay Polovkov, Daria Voskressenskaya, Ilya Pikovskoi, Mikhail Tebenikhin, Ella Vtorushina, Anastasiia Kanateva, Roman Borisov, and Vladimir Zaikin. 2022. "The Use of Polydialkylsiloxanes/Triflic Acid as Derivatization Agents in the Analysis of Sulfur-Containing Aromatics by “Soft”-Ionization Mass Spectrometry" Molecules 27, no. 23: 8600. https://doi.org/10.3390/molecules27238600
APA StyleStarkova, Z., Ilyushenkova, V., Polovkov, N., Voskressenskaya, D., Pikovskoi, I., Tebenikhin, M., Vtorushina, E., Kanateva, A., Borisov, R., & Zaikin, V. (2022). The Use of Polydialkylsiloxanes/Triflic Acid as Derivatization Agents in the Analysis of Sulfur-Containing Aromatics by “Soft”-Ionization Mass Spectrometry. Molecules, 27(23), 8600. https://doi.org/10.3390/molecules27238600








