An Efficient Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Method for the Analysis of Methyl Farnesoate Released in Growth Medium by Daphnia pulex
Abstract
1. Introduction
2. Results and Discussion
2.1. SPME Optimization
2.2. SPME-GC-MS Method Validation
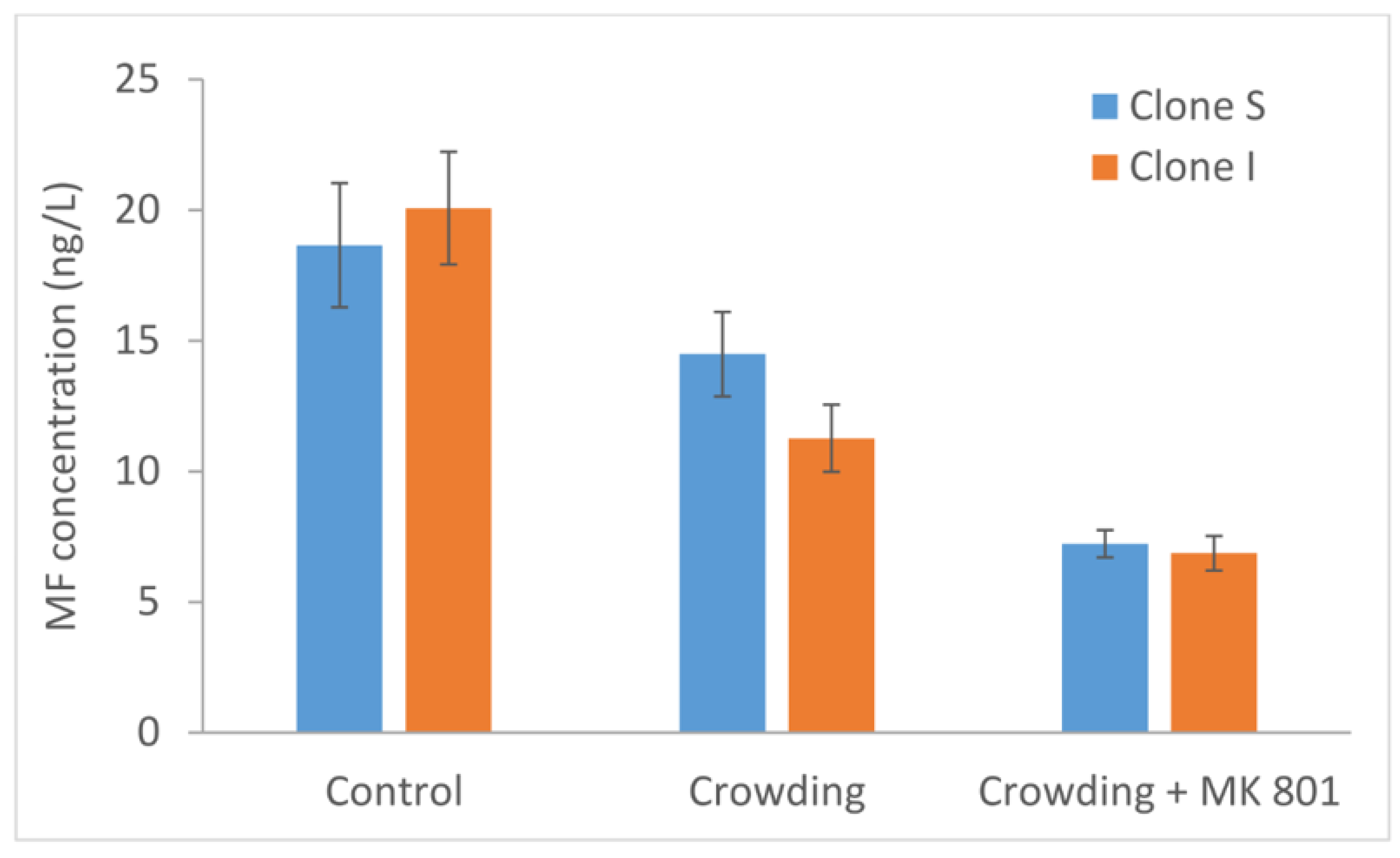
2.3. Release of Methyl Farnesoate under Stress Conditions
3. Materials and Methods
3.1. Chemicals and Materials
3.2. GC-MS Analysis
3.3. Method Optimization
3.4. Method Validation
3.5. Daphnia pulex Culturing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartfelder, K.; Emlen, D.J. Endocrine Control of Insect Polyphenism; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123847492. [Google Scholar]
- Truman, J.W.; Hiruma, K.; Allee, J.P.; MacWhinnie, S.G.B.; Champlin, D.T.; Riddiford, L.M. Juvenile Hormone Is Required to Couple Imaginal Disc Formation with Nutrition in Insects. Science 2006, 312, 1385–1388. [Google Scholar] [CrossRef]
- Cornette, R.; Gotoh, H.; Koshikawa, S.; Miura, T. Juvenile Hormone Titers and Caste Differentiation in the Damp-Wood Termite Hodotermopsis Sjostedti (Isoptera, Termopsidae). J. Insect Physiol. 2008, 54, 922–930. [Google Scholar] [CrossRef]
- Riddiford, L.M. Juvenile Hormone Action: A 2007 Perspective. J. Insect Physiol. 2008, 54, 895–901. [Google Scholar] [CrossRef]
- Laufer, H.; Ahl, J.S.B.; Sagi, A. The Role of Juvenile Hormones in Crustacean Reproduction. Integr. Comp. Biol. 1993, 33, 365–374. [Google Scholar] [CrossRef]
- Olmstead, A.W.; LeBlanc, G.A. The Environmental-Endocrine Basis of Gynandromorphism (Intersex) in a Crustacean. Int. J. Biol. Sci. 2007, 3, 77–84. [Google Scholar] [CrossRef]
- Imai, M.; Naraki, Y.; Tochinai, S.; Miura, T. Elaborate Regulations of the Predator-Induced Polyphenism in the Water Flea Daphnia Pulex: Kairomone-Sensitive Periods and Life-History Tradeoffs. J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 788–795. [Google Scholar] [CrossRef]
- LeBlanc, G.A.; Wang, Y.H.; Holmes, C.N.; Kwon, G.; Medlock, E.K. A Transgenerational Endocrine Signaling Pathway in Crustacea. PLoS ONE 2013, 8, e61715. [Google Scholar] [CrossRef]
- Miyakawa, H.; Toyota, K.; Hirakawa, I.; Ogino, Y.; Miyagawa, S.; Oda, S.; Tatarazako, N.; Miura, T.; Colbourne, J.K.; Iguchi, T. A Mutation in the Receptor Methoprene-Tolerant Alters Juvenile Hormone Response in Insects and Crustaceans. Nat. Commun. 2013, 4, 1856. [Google Scholar] [CrossRef]
- Toyota, K.; Miyakawa, H.; Hiruta, C.; Furuta, K.; Ogino, Y.; Shinoda, T.; Tatarazako, N.; Miyagawa, S.; Shaw, J.R.; Iguchi, T. Methyl Farnesoate Synthesis Is Necessary for the Environmental Sex Determination in the Water Flea Daphnia Pulex. J. Insect Physiol. 2015, 80, 22–30. [Google Scholar] [CrossRef]
- Toyota, K.; Miyakawa, H.; Yamaguchi, K.; Shigenobu, S.; Ogino, Y.; Tatarazako, N.; Miyagawa, S.; Iguchi, T. NMDA Receptor Activation Upstream of Methyl Farnesoate Signaling for Short Day-Induced Male Offspring Production in the Water Flea, Daphnia Pulex. BMC Genom. 2015, 16, 186. [Google Scholar] [CrossRef]
- Olmstead, A.W.; Leblanc, G.A. Juvenoid Hormone Methyl Farnesoate Is a Sex Determinant in the Crustacean Daphnia Magna. J. Exp. Zool. 2002, 293, 736–739. [Google Scholar] [CrossRef]
- Rider, C.V.; Gorr, T.A.; Olmstead, A.W.; Wasilak, B.A.; LeBlanc, G.A. Stress Signaling: Coregulation of Hemoglobin and Male Sex Determination through a Terpenoid Signaling Pathway in a Crustacean. J. Exp. Biol. 2005, 208, 15–23. [Google Scholar] [CrossRef]
- Camp, A.A.; Yun, J.; Chambers, S.A.; Haeba, M.H.; LeBlanc, G.A. Involvement of Glutamate and Serotonin Transmitter Systems in Male Sex Determination in Daphnia Pulex. J. Insect Physiol. 2020, 121, 104015. [Google Scholar] [CrossRef]
- Suppa, A.; Gorbi, G.; Marková, S.; Buschini, A.; Rossi, V. Transgenerational Effects of Methyl Farnesoate on Daphnia Pulex Clones: Male and Ephippia Production and Expression of Genes Involved in Sex Determination. Freshw. Biol. 2021, 66, 374–390. [Google Scholar] [CrossRef]
- Borst, D.W.; Tsukimura, B. Quantification of Methyl Farnesoate Levels in Hemolymph by High-Performance Liquid Chromatography. J. Chromatogr. A 1991, 545, 71–78. [Google Scholar] [CrossRef]
- Rotllant, G.; Pascual, N.; Sardà, F.; Takac, P.; Laufer, H. Identification of Methyl Farnesoate in the Hemolymph of the Mediterranean Deep-Sea Species Norway Lobster, Nephrops Norvegicus. J. Crustacean Biol. 2001, 21, 328–333. [Google Scholar] [CrossRef][Green Version]
- Lovett, D.L.; Verzi, M.P.; Clifford, P.D.; Borst, D.W. Hemolymph Levels of Methyl Farnesoate Increase in Response to Osmotic Stress in the Green Crab, Carcinus Maenas. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 299–306. [Google Scholar] [CrossRef]
- Nagaraju, G.P.C.; Reddy, P.R.; Reddy, P.S. In Vitro Methyl Farnesoate Secretion by Mandibular Organs Isolated from Different Molt and Reproductive Stages of the Crab Oziotelphusa Senex Senex. Fish. Sci. 2006, 72, 410–414. [Google Scholar] [CrossRef]
- Ramirez, C.E.; Nouzova, M.; Michalkova, V.; Fernandez-Lima, F.; Noriega, F.G. Common Structural Features Facilitate the Simultaneous Identification and Quantification of the Five Most Common Juvenile Hormones by Liquid Chromatography-Tandem Mass Spectrometry. Insect Biochem. Mol. Biol. 2020, 116, 103287. [Google Scholar] [CrossRef]
- Navare, A.T.; Mayoral, J.G.; Nouzova, M.; Noriega, F.G.; Fernández, F.M. Rapid Direct Analysis in Real Time (DART) Mass Spectrometric Detection of Juvenile Hormone III and Its Terpene Precursors. Anal. Bioanal. Chem. 2010, 398, 3005–3013. [Google Scholar] [CrossRef]
- Wainwright, G.; Prescott, M.C.; Rees, H.H.; Webster, S.G. Mass Spectrometric Determination of Methyl Farnesoate Profiles and Correlation with Ovarian Development in the Edible Crab, Cancer Pagurus. J. Mass Spectrom. 1996, 31, 1338–1344. [Google Scholar] [CrossRef]
- Smith, P.A.; Clare, A.S.; Rees, H.H.; Prescott, M.C.; Wainwright, G.; Thorndyke, M.C. Identification of Methyl Farnesoate in the Cypris Larva of the Barnacle, Balanus Amphitrite, and Its Role as a Juvenile Hormone. Insect Biochem. Mol. Biol. 2000, 30, 885–890. [Google Scholar] [CrossRef]
- Laufer, H.; Demir, N.; Pan, X.; Stuart, J.D.; Ahl, J.S.B. Methyl Farnesoate Controls Adult Male Morphogenesis in the Crayfish, Procambarus Clarkii. J. Insect Physiol. 2005, 51, 379–384. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, D.; Li, Y.; Qiu, X.; Cui, X.; Tang, J. Hemolymph Levels of Methyl Farnesoate during Ovarian Development of the Swimming Crab Portunus Trituberculatus, and Its Relation to Transcript Levels of HMG-CoA Reductase and Farnesoic Acid O-Methyltransferase. Biological. Bull. 2015, 228, 118–124. [Google Scholar] [CrossRef]
- Jones, D.; Jones, G.; Teal, P.; Hammac, C.; Messmer, L.; Osborne, K.; Belgacem, Y.H.; Martin, J.R. Suppressed Production of Methyl Farnesoid Hormones Yields Developmental Defects and Lethality in Drosophila Larvae. Gen. Comp. Endocrinol. 2010, 165, 244–254. [Google Scholar] [CrossRef]
- Teal, P.E.A.; Proveaux, A.T.; Heath, R.R. Analysis and Quantitation of Insect Juvenile Hormones Using Chemical Ionization Ion-Trap Mass Spectrometry. Anal. Biochem. 2000, 277, 206–213. [Google Scholar] [CrossRef][Green Version]
- Teal, P.E.A.; Jones, D.; Jones, G.; Torto, B.; Nyasembe, V.; Borgemeister, C.; Alborn, H.T.; Kaplan, F.; Boucias, D.; Lietze, V.U. Identification of Methyl Farnesoate from the Hemolymph of Insects. J. Nat. Prod. 2014, 77, 402–405. [Google Scholar] [CrossRef]
- Montes, R.; Rodil, R.; Neuparth, T.; Santos, M.M.; Cela, R.; Quintana, J.B. A Simple and Sensitive Approach to Quantify Methyl Farnesoate in Whole Arthropods by Matrix-Solid Phase Dispersion and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2017, 1508, 158–162. [Google Scholar] [CrossRef]
- Kai, P.Z.; Yin, Y.; Zhang, Z.R.; Huang, J.; Tobe, S.S.; Chen, S.S. A Rapid Quantitative Assay for Juvenile Hormones and Intermediates in the Biosynthetic Pathway Using Gas Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1538, 67–74. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Riboni, N.; Fornari, F.; Bianchi, F.; Careri, M. Recent Advances in in Vivo Spme Sampling. Separations 2020, 7, 6. [Google Scholar] [CrossRef]
- Huang, S.; Chen, G.; Ye, N.; Kou, X.; Zhu, F.; Shen, J.; Ouyang, G. Solid-Phase Microextraction: An Appealing Alternative for the Determination of Endogenous Substances—A Review. Anal. Chim. Acta 2019, 1077, 67–86. [Google Scholar] [CrossRef]
- Groothuis, F.A.; Heringa, M.B.; Nicol, B.; Hermens, J.L.M.; Blaauboer, B.J.; Kramer, N.I. Dose Metric Considerations in in Vitro Assays to Improve Quantitative in Vitro-in Vivo Dose Extrapolations. Toxicology 2015, 332, 30–40. [Google Scholar] [CrossRef]
- Savelieva, E.I.; Gavrilova, O.P.; Gagkaeva, T.Y. Using Solid-Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry for the Study of the Volatile Products of Biosynthesis Released by Plants and Microorganisms. J. Anal. Chem. 2014, 69, 609–615. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Zhou, L.D.; Chen, H.; Wang, C.Z.; Xia, Z.N.; Yuan, C.S. Solid-Phase Microextraction Technology for in Vitro and in Vivo Metabolite Analysis. Trends Anal. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef]
- Choi, K.S.; Lee, J.M.; Park, J.H.; Cho, J.R.; Song, J.H.; Kim, D.S.; Boo, K.S. Sex Pheromone Composition of the Cotton Caterpillar, Palpita Indica (Lepidoptera: Pyralidae), in Korea. J. Asia Pac. Entomol. 2009, 12, 269–275. [Google Scholar] [CrossRef]
- Lengyel, F.; Westerlund, S.A.; Kaib, M. Juvenile Hormone III Influences Task-Specific Cuticular Hydrocarbon Profile Changes in the Ant Myrmicaria Eumenoides. J. Chem. Ecol. 2007, 33, 167–181. [Google Scholar] [CrossRef]
- Chen, J. Freeze-Thaw Sample Preparation Method Improves Detection of Volatile Compounds in Insects Using Headspace Solid-Phase Microextraction. Anal. Chem. 2017, 89, 8366–8371. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Mirabelli, M.F. Solid Phase Microextraction-Mass Spectrometry: Metanoia. Trends Anal. Chem. 2019, 112, 201–211. [Google Scholar] [CrossRef]
- Augusto, F.; Carasek, E.; Silva, R.G.C.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New Sorbents for Extraction and Microextraction Techniques. J. Chromatogr. A 2010, 1217, 2533–2542. [Google Scholar] [CrossRef]
- Riboni, N.; Fornari, F.; Bianchi, F.; Careri, M. A Simple and Efficient Solid-Phase Microextraction—Gas Chromatography—Mass Spectrometry Method for the Determination of Fragrance Materials at Ultra-Trace Levels in Water Samples Using Multi-Walled Carbon Nanotubes as Innovative Coating. Talanta 2021, 224, 121891. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q.; Liu, X.; Wei, S.; Zheng, J.; Lin, W.; Ye, Y.; Zhu, F.; Ouyang, G. Determination of the Mass Transfer Coefficients in Direct Immersion Solid-phase Microextraction. J. Sep. Sci. 2020, 43, 1847–1853. [Google Scholar] [CrossRef]
- Zhang, L.; Gionfriddo, E.; Acquaro, V.; Pawliszyn, J. Direct Immersion Solid-Phase Microextraction Analysis of Multi-Class Contaminants in Edible Seaweeds by Gas Chromatography-Mass Spectrometry. Anal. Chim. Acta 2018, 1031, 83–97. [Google Scholar] [CrossRef]
- Nagaraju, G.P.C. Is Methyl Farnesoate a Crustacean Hormone? Aquaculture 2007, 272, 39–54. [Google Scholar] [CrossRef]
- Booksmythe, I.; Gerber, N.; Ebert, D.; Kokko, H. Daphnia Females Adjust Sex Allocation in Response to Current Sex Ratio and Density. Ecol. Lett. 2018, 21, 629–637. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Mattarozzi, M.; Musci, M. Experimental Design for the Optimization of the Extraction Conditions of Polycyclic Aromatic Hydrocarbons in Milk with a Novel Diethoxydiphenylsilane Solid-Phase Microextraction Fiber. J. Chromatogr. A 2008, 1196, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Zug, Switzerland, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- Suppa, A.; Caleffi, S.; Gorbi, G.; Marková, S.; Kotlík, P.; Rossi, V. Environmental Conditions as Proximate Cues of Predation Risk Inducing Defensive Response in Daphnia Pulex. Biologia 2021, 76, 623–632. [Google Scholar] [CrossRef]
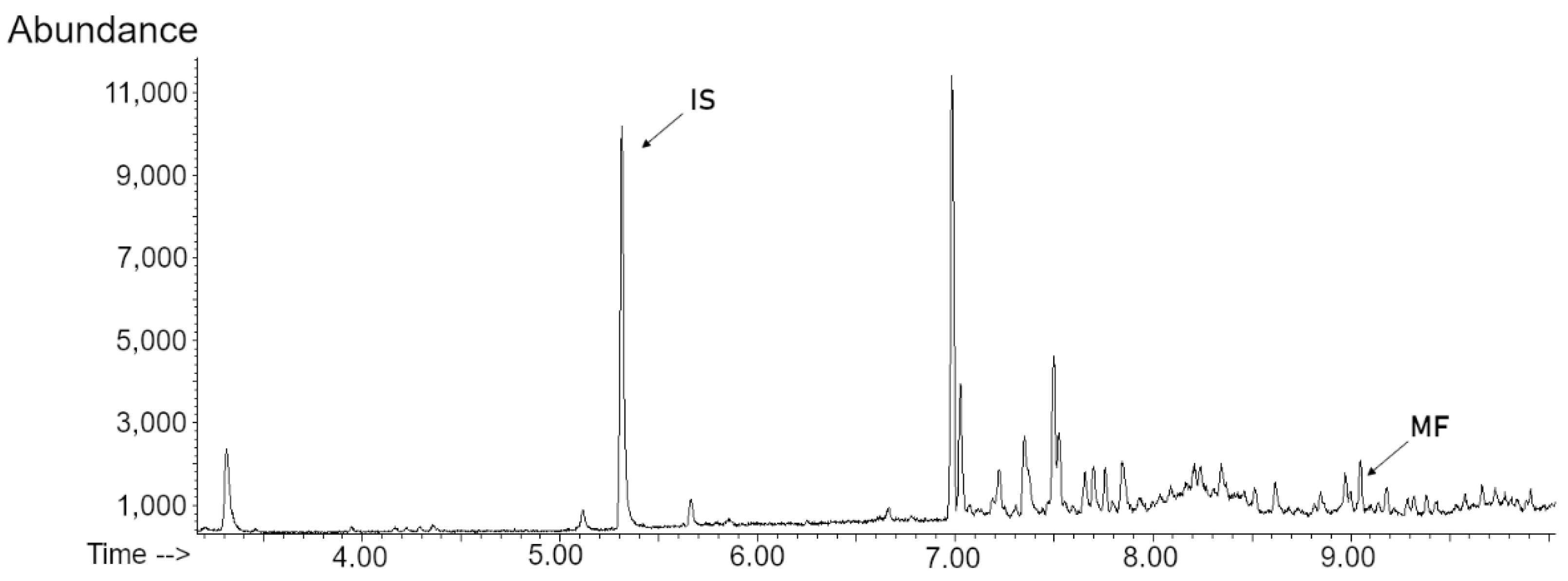
| Concentration Level (ng/L) | |||
|---|---|---|---|
| LOQ | 30 | 80 | |
| Repeatability RSD% | 13 | 9.5 | 7.5 |
| Intermediate Precision RSD% | 14 | 11 | 7.8 |
| Trueness RSD% (±st.dev.) | 105 ± 13 | 98.1 ± 9.0 | 90.5 ± 5.9 |
| Article | LOD (µg/L) | Extraction Method | Analytical Technique | Organism | Matrix |
|---|---|---|---|---|---|
| This study | 0.0013 | SPME | GC-(EI)MS | Daphnia pulex | Growth medium |
| [16] | 0.25 | LLE | HPLC-UV | Homarus americanus, Libinia emarginata, Carcinus maenas | Hemolymph |
| [22] | 50 | LLE | GC-(EI)MS | Cancer pagurus | Hemolymph |
| [23] | 100 | LLE | GC-(EI)MS | Balanus amphitrite | Dry-blotted cyprids |
| [24] | 0.3 | LLE + preparative HPLC | GC-(EI)MS | Procambarus clarkii | Hemolymph |
| [21] | 160 | - | DART-MS | - | - |
| [30] | 1 | LLE | GC-MS/MS | Diploptera punctata | Hemolymph and corpora allata |
| [29] | 0.5 | MSPD | GC-(CI)MS | Gammarus locusta, Artemia franciscana, Apis mellifera | Whole arthropod |
| [20] | 0.007 | LLE | UHPLC-MS/MS | Aedes aegypti, Sarcophaga bullata, Oncopeltus fasciatus, Manduca sexta, Bombyx mori, Drosophila melanogaster, Megalopta genalis, Anopheles albimanus, Dipetalogaster maxima | Hemolymph, corpora allata–corpora cardiaca and brain and head capsule |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riboni, N.; Suppa, A.; Buschini, A.; Bianchi, F.; Rossi, V.; Gorbi, G.; Careri, M. An Efficient Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Method for the Analysis of Methyl Farnesoate Released in Growth Medium by Daphnia pulex. Molecules 2022, 27, 8591. https://doi.org/10.3390/molecules27238591
Riboni N, Suppa A, Buschini A, Bianchi F, Rossi V, Gorbi G, Careri M. An Efficient Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Method for the Analysis of Methyl Farnesoate Released in Growth Medium by Daphnia pulex. Molecules. 2022; 27(23):8591. https://doi.org/10.3390/molecules27238591
Chicago/Turabian StyleRiboni, Nicolò, Antonio Suppa, Annamaria Buschini, Federica Bianchi, Valeria Rossi, Gessica Gorbi, and Maria Careri. 2022. "An Efficient Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Method for the Analysis of Methyl Farnesoate Released in Growth Medium by Daphnia pulex" Molecules 27, no. 23: 8591. https://doi.org/10.3390/molecules27238591
APA StyleRiboni, N., Suppa, A., Buschini, A., Bianchi, F., Rossi, V., Gorbi, G., & Careri, M. (2022). An Efficient Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Method for the Analysis of Methyl Farnesoate Released in Growth Medium by Daphnia pulex. Molecules, 27(23), 8591. https://doi.org/10.3390/molecules27238591







