Inhibitory Effects of Varespladib, CP471474, and Their Potential Synergistic Activity on Bothrops asper and Crotalus durissus cumanensis Venoms
Abstract
1. Introduction
2. Results
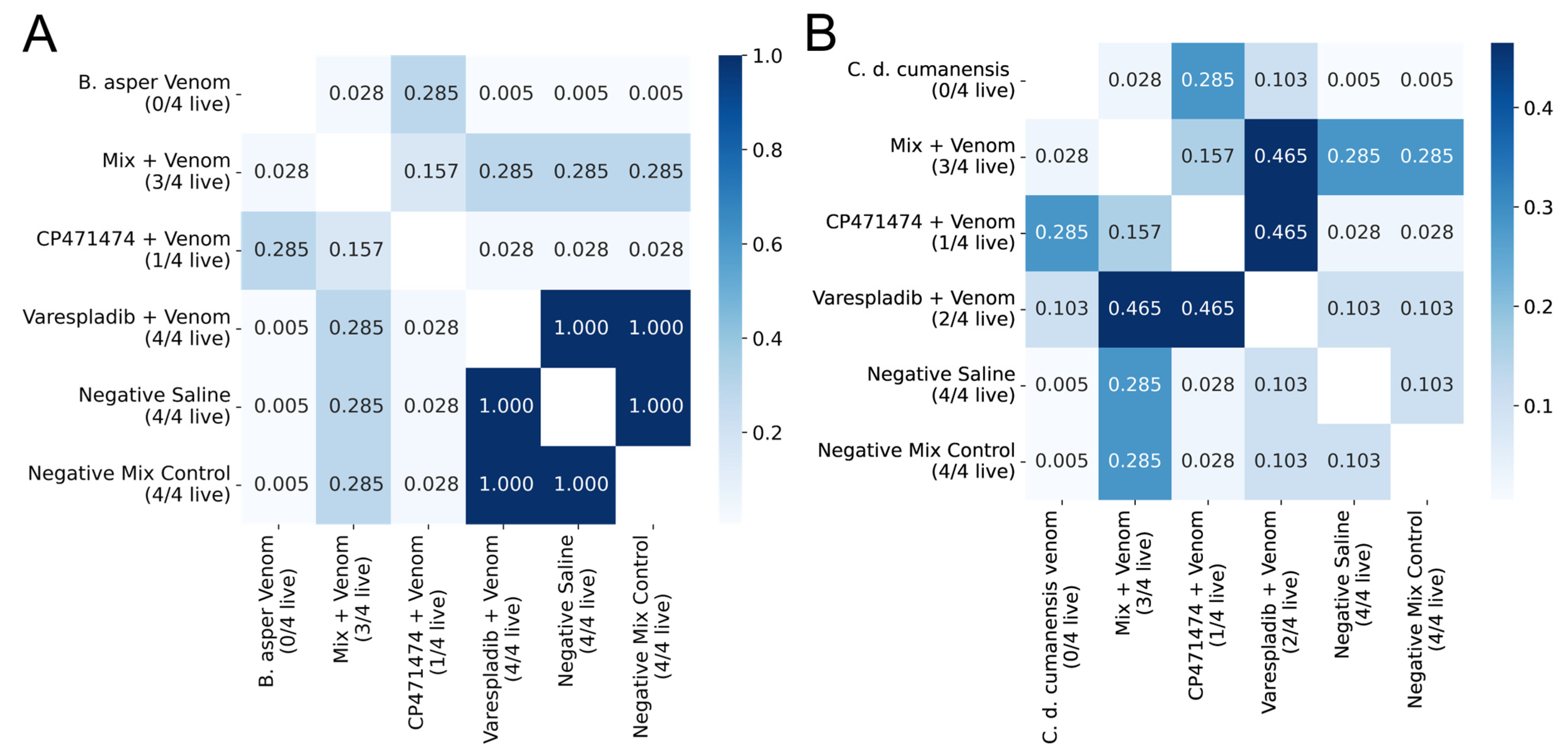
2.1. Inhibition of Lethal Activity in Preincubation Assays
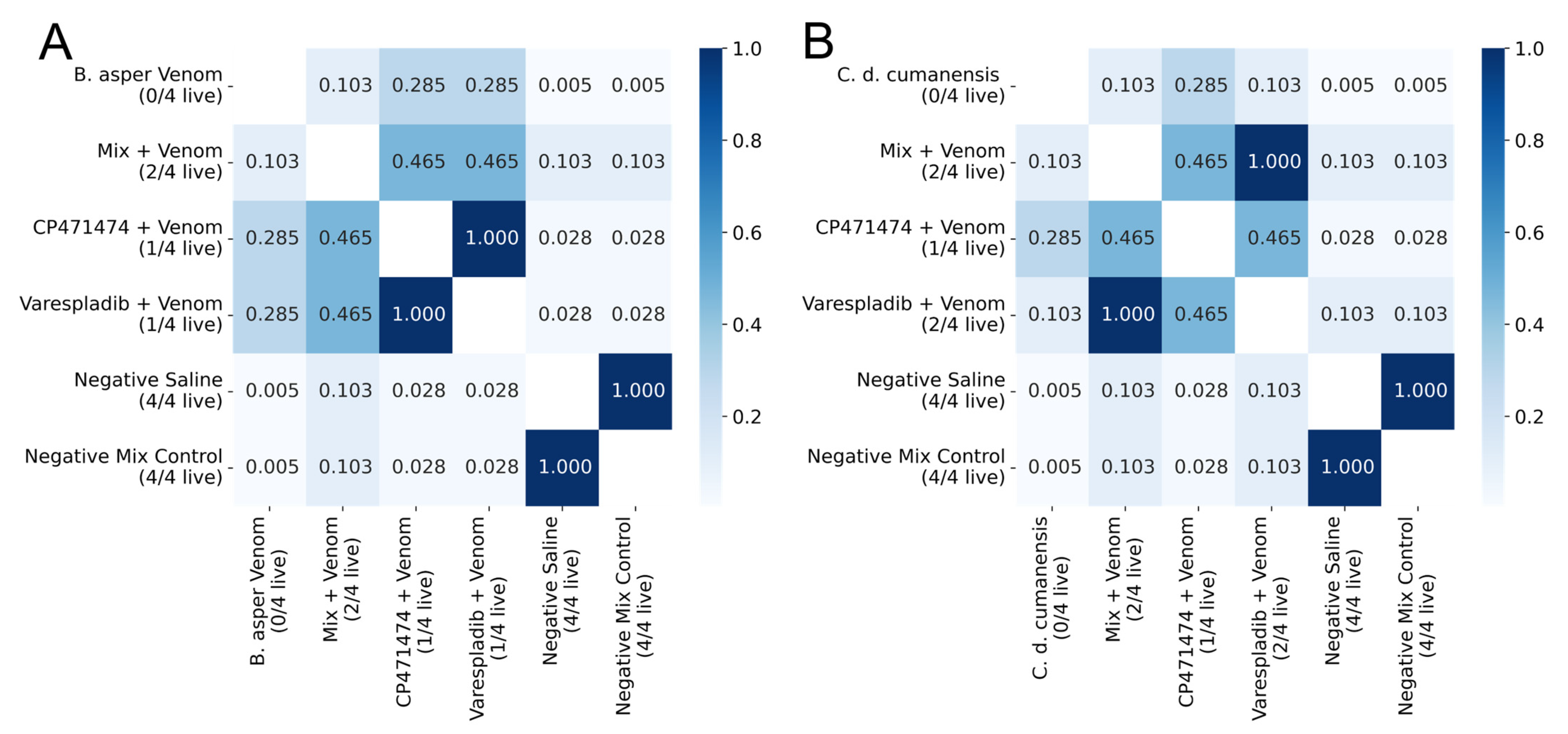
2.2. Inhibition of Lethal Activity in Independent Injection Assays
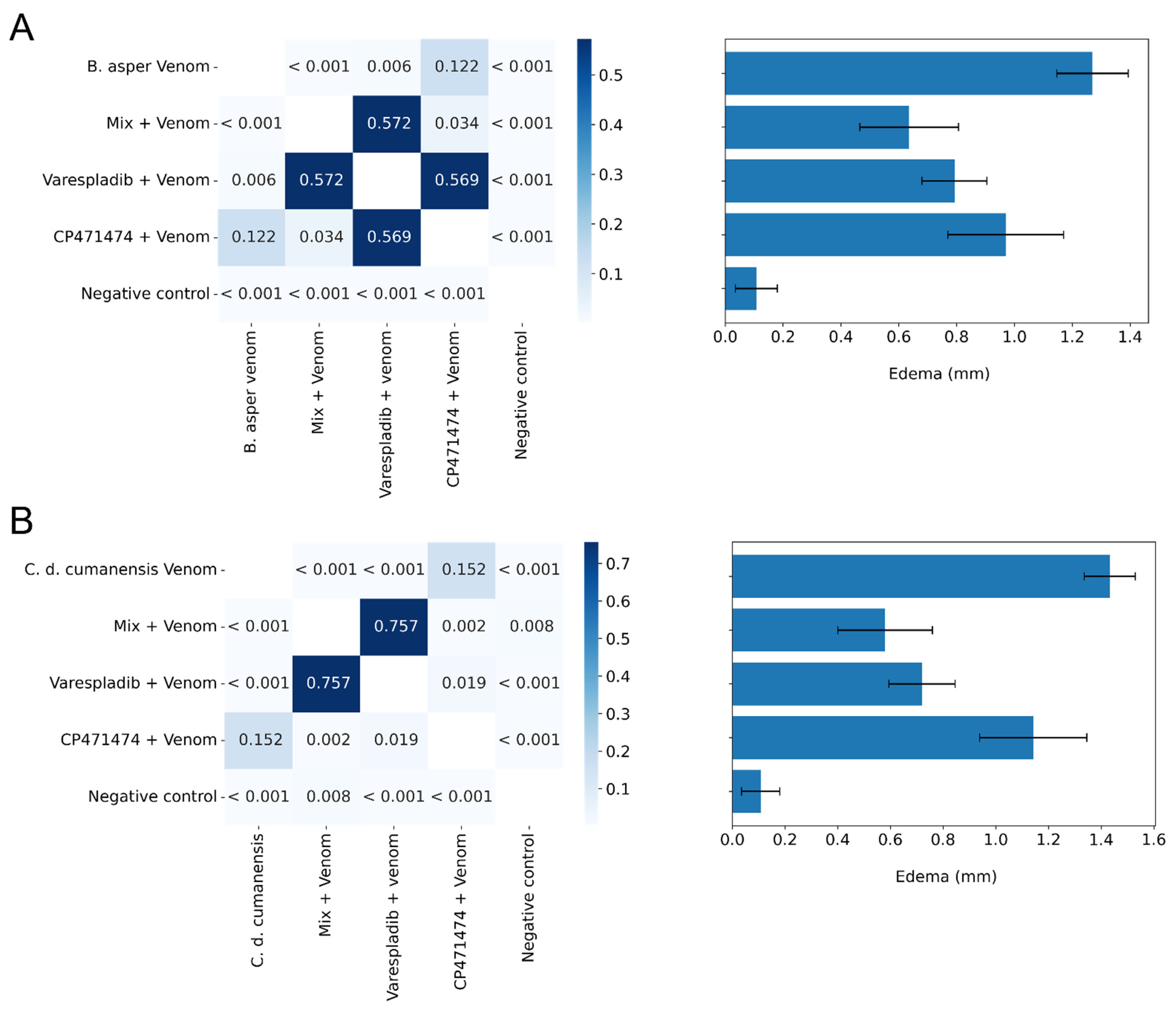
2.3. Inhibition of Edema-Forming Activity in Independent Injection Assays
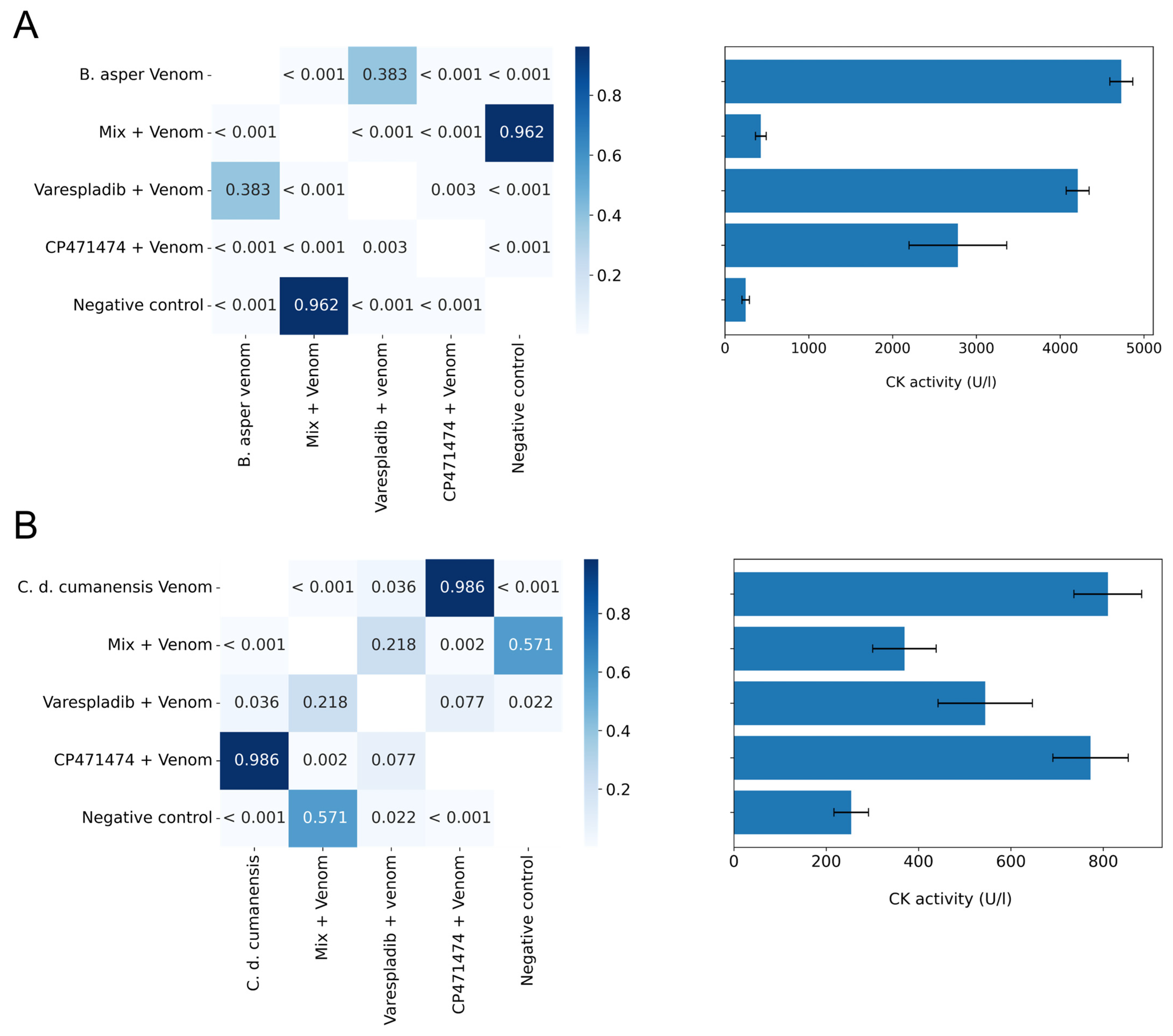
2.4. Inhibition of Myotoxic Activity in Preincubation Assays
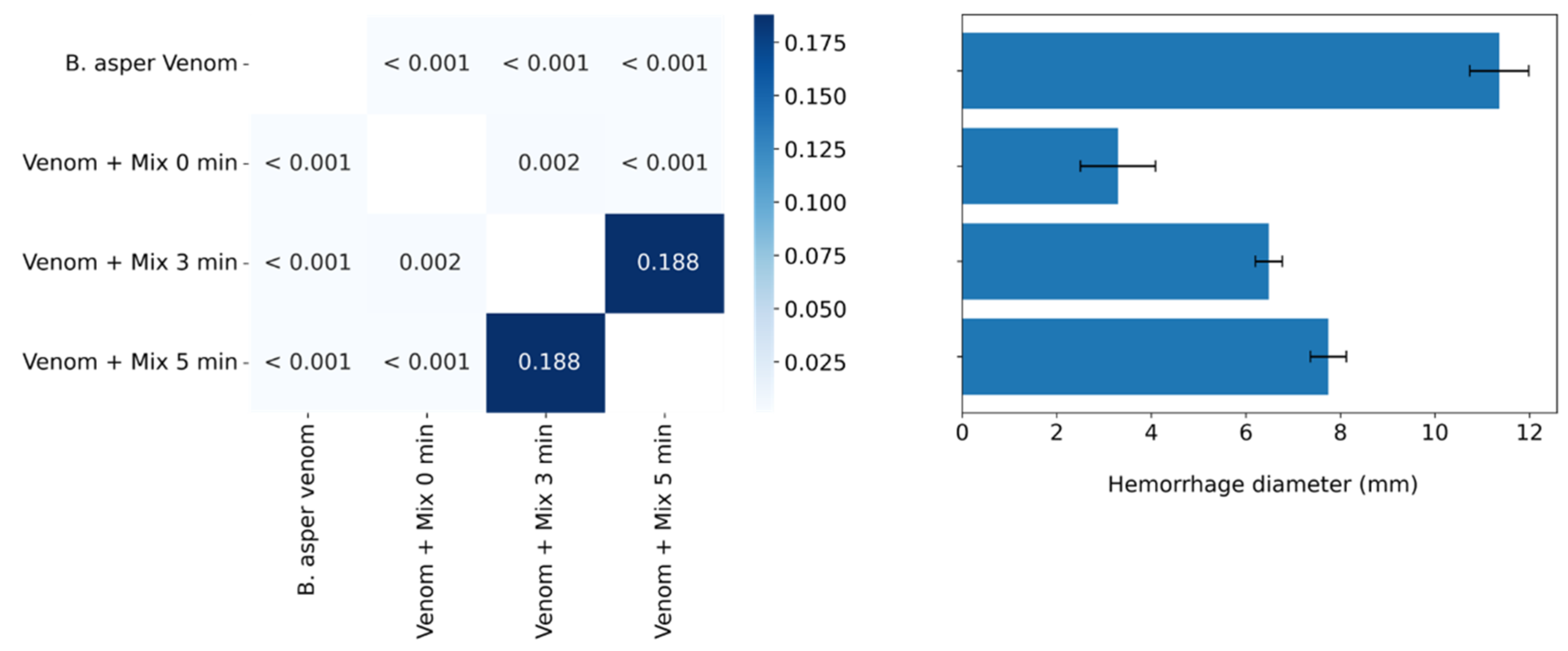
2.5. Inhibition of the Hemorrhagic Activity of B. asper venom in Preincubation and Independent Injection Assays
2.6. Inhibition of the Hemorrhagic Activity of B. asper venom with Independent Injection at Different Time Intervals
2.7. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Venoms
4.2. Chemicals and Reagents
4.3. Animals
4.4. Inhibition of the Lethal Activity
4.5. Inhibition of Edema-Forming Activity
4.6. Inhibition of Myotoxicity
4.7. Inhibition of Hemorrhagic Activity
4.8. Molecular Docking Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Lalloo, D.G.; Janaka de Silva, H. Chronic Health Effects and Cost of Snakebite. Toxicon X 2021, 9–10, 100074. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A.; Gutiérrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar] [PubMed]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the Therapeutics of Anti-Snake Venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef]
- The Lancet Snake-Bite Envenoming: A Priority Neglected Tropical Disease. Lancet 2017, 390, 2. [CrossRef]
- Boletin Epidemiológico Semana 52 del 2021. Available online: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2022_Bolet%C3%ADn_epidemiologico_semana_27.pdf (accessed on 24 September 2022).
- Otero-Patiño, R. Snake Bites in Colombia. In Clinical Toxinology; Gopalakrishnakone, P., Faiz, S.M.A., Gnanathasan, C.A., Habib, A.G., Fernando, R., Yang, C.-C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–42. ISBN 978-94-007-6288-6. [Google Scholar]
- León-Núñez, L.J.; Camero-Ramos, G.; Gutiérrez, J.M. Epidemiology of Snakebites in Colombia (2008–2016). Rev. Salud Pública 2020, 22, e202. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake Venomics of the Lancehead Pitviper Bothrops asper: Geographic, Individual, and Ontogenetic Variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Salazar-Valenzuela, D.; Pla, D.; Lomonte, B.; Guerrero-Vargas, J.A.; Ayerbe, S.; Gibbs, H.L.; Calvete, J.J. Venom Variation in Bothrops asper Lineages from North-Western South America. J. Proteom. 2020, 229, 103945. [Google Scholar] [CrossRef]
- Quintana-Castillo, J.; Vargas, L.; Segura, C.; Estrada-Gómez, S.; Bueno-Sánchez, J.; Alarcón, J. Characterization of the Venom of C. d. Cumanesis of Colombia: Proteomic Analysis and Antivenomic Study. Toxins 2018, 10, 85. [Google Scholar] [CrossRef]
- Hawgood, B.J.; Smith, J.W. The mode of action at the mouse neuromuscular junction of the phospholipase a-crotapotin complex isolated from venom of the south american rattlesnake. Br. J. Pharmacol. 1977, 61, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and Biological Characterization of a PLA2 from Crotoxin Complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Gómez, I.D.; Patiño, A.C. Relationship between the Structure and the Enzymatic Activity of Crotoxin Complex and Its Phospholipase A2 Subunit: An in Silico Approach. J. Mol. Graph. Model. 2012, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Lomonte, B.; Leon, G.; Rucavado, A.; Chaves, F.; Angulo, Y. Trends in Snakebite Envenomation Therapy: Scien tific, Technological and Public Health Considerations. CPD 2007, 13, 2935–2950. [Google Scholar] [CrossRef]
- Silva, A.; Isbister, G.K. Current Research into Snake Antivenoms, Their Mechanisms of Action and Applications. Biochem. Soc. Trans. 2020, 48, 537–546. [Google Scholar] [CrossRef]
- Hamza, M.; Knudsen, C.; Gnanathasan, C.A.; Monteiro, W.; Lewin, M.R.; Laustsen, A.H.; Habib, A.G. Clinical Management of Snakebite Envenoming: Future Perspectives. Toxicon X 2021, 11, 100079. [Google Scholar] [CrossRef]
- Varespladib. Am. J. Cardiovasc. Drugs 2011, 11, 137–143. [CrossRef]
- Nicholls, S.J.; Kastelein, J.J.P.; Schwartz, G.G.; Bash, D.; Rosenson, R.S.; Cavender, M.A.; Brennan, D.M.; Koenig, W.; Jukema, J.W.; Nambi, V.; et al. Varespladib and Cardiovascular Events in Patients with an Acute Coronary Syndrome: The VISTA-16 Randomized Clinical Trial. JAMA 2014, 311, 252. [Google Scholar] [CrossRef]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Lewin, M.; Gutiérrez, J.; Samuel, S.; Herrera, M.; Bryan-Quirós, W.; Lomonte, B.; Bickler, P.; Bulfone, T.; Williams, D. Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus scutellatus) Venom. Toxins 2018, 10, 380. [Google Scholar] [CrossRef]
- Lewin, M.; Gilliam, L.; Gilliam, J.; Samuel, S.; Bulfone, T.; Bickler, P.; Gutiérrez, J. Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus fulvius (Eastern Coral Snake) Venom. Toxins 2018, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Quirós, W.; Fernández, J.; Gutiérrez, J.M.; Lewin, M.R.; Lomonte, B. Neutralizing Properties of LY315920 toward Snake Venom Group I and II Myotoxic Phospholipases A2. Toxicon 2019, 157, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.M.; Borges, R.J.; Lomonte, B.; Lewin, M.R.; Fontes, M.R.M. The Synthetic Varespladib Molecule Is a Multi-Functional Inhibitor for PLA2 and PLA2-like Ophidic Toxins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129913. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Lewin, M.R.; Zdenek, C.N.; Carter, R.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors against Anticoagulant Activities of African Spitting Cobra (Naja Species) Venoms. Front. Immunol. 2021, 12, 752442. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Youngman, N.J.; Liu, J.; Lewin, M.R.; Carter, R.W.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors against Anticoagulant Activities of Black Snake (Pseudechis Spp.) Venoms. Toxicol. Lett. 2022, 366, 26–32. [Google Scholar] [CrossRef]
- Youngman, N.J.; Lewin, M.R.; Carter, R.; Naude, A.; Fry, B.G. Efficacy and Limitations of Chemically Diverse Small-Molecule Enzyme-Inhibitors against the Synergistic Coagulotoxic Activities of Bitis Viper Venoms. Molecules 2022, 27, 1733. [Google Scholar] [CrossRef]
- Xie, C.; Albulescu, L.-O.; Still, K.B.M.; Slagboom, J.; Zhao, Y.; Jiang, Z.; Somsen, G.W.; Vonk, F.J.; Casewell, N.R.; Kool, J. Varespladib Inhibits the Phospholipase A2 and Coagulopathic Activities of Venom Components from Hemotoxic Snakes. Biomedicines 2020, 8, 165. [Google Scholar] [CrossRef]
- Selman, M.; Cisneros-Lira, J.; Gaxiola, M.; Ramírez, R.; Kudlacz, E.M.; Mitchell, P.G.; Pardo, A. Matrix Metalloproteinases Inhibition Attenuates Tobacco Smoke-Induced Emphysema in Guinea Pigs. Chest 2003, 123, 1633–1641. [Google Scholar] [CrossRef]
- Preciado, L.M.; Pereañez, J.A.; Comer, J. Potential of Matrix Metalloproteinase Inhibitors for the Treatment of Local Tissue Damage Induced by a Type P-I Snake Venom Metalloproteinase. Toxins 2019, 12, 8. [Google Scholar] [CrossRef]
- Albulescu, L.-O.; Xie, C.; Ainsworth, S.; Alsolaiss, J.; Crittenden, E.; Dawson, C.A.; Softley, R.; Bartlett, K.E.; Harrison, R.A.; Kool, J.; et al. A Therapeutic Combination of Two Small Molecule Toxin Inhibitors Provides Broad Preclinical Efficacy against Viper Snakebite. Nat. Commun. 2020, 11, 6094. [Google Scholar] [CrossRef]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev. Proteom. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, Venomics, Antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Karatt-Vellatt, A.; Masters, E.W.; Arias, A.S.; Pus, U.; Knudsen, C.; Oscoz, S.; Slavny, P.; Griffiths, D.T.; Luther, A.M.; et al. In Vivo Neutralization of Dendrotoxin-Mediated Neurotoxicity of Black Mamba Venom by Oligoclonal Human IgG Antibodies. Nat. Commun. 2018, 9, 3928. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Patiño, A.C.; Núñez, V.; Osorio, E. The Biflavonoid Morelloflavone Inhibits the Enzymatic and Biological Activities of a Snake Venom Phospholipase A2. Chem.-Biol. Interact. 2014, 220, 94–101. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic Toxins from Snake Venom: Structural Characterization and Mechanism of Catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Maciel, F.V.; Ramos Pinto, Ê.K.; Valério Souza, N.M.; Gonçalves de Abreu, T.A.; Ortolani, P.L.; Fortes-Dias, C.L.; Garrido Cavalcante, W.L. Varespladib (LY315920) Prevents Neuromuscular Blockage and Myotoxicity Induced by Crotoxin on Mouse Neuromuscular Preparations. Toxicon 2021, 202, 40–45. [Google Scholar] [CrossRef]
- Gutierres, P.G.; Pereira, D.R.; Vieira, N.L.; Arantes, L.F.; Silva, N.J.; Torres-Bonilla, K.A.; Hyslop, S.; Morais-Zani, K.; Nogueira, R.M.B.; Rowan, E.G.; et al. Action of Varespladib (LY-315920), a Phospholipase A2 Inhibitor, on the Enzymatic, Coagulant and Haemorrhagic Activities of Lachesis muta rhombeata (South-American Bushmaster) Venom. Front. Pharmacol. 2022, 12, 812295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the Inhibitory Potential of Varespladib for Snakebite Envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef]
- Scott, D.L.; White, S.P.; Otwinowski, Z.; Yuan, W.; Gelb, M.H.; Sigler, P.B. Interfacial Catalysis: The Mechanism of Phospholipase A2. Science 1990, 250, 1541–1546. [Google Scholar] [CrossRef]
- Berg, O.G.; Gelb, M.H.; Tsai, M.-D.; Jain, M.K. Interfacial Enzymology: The Secreted Phospholipase A2-Paradigm. Chem. Rev. 2001, 101, 2613–2654. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Berg, O. Coupling of the I-Face and the Active Site of Phospholipase A2 for Interfacial Activation. Curr. Opin. Chem. Biol. 2006, 10, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Mamede, C.C.N.; de Sousa Simamoto, B.B.; da Cunha Pereira, D.F.; de Oliveira Costa, J.; Ribeiro, M.S.M.; de Oliveira, F. Edema, Hyperalgesia and Myonecrosis Induced by Brazilian Bothropic Venoms: Overview of the Last Decade. Toxicon 2020, 187, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental Pathology of Local Tissue Damage Induced by Bothrops asper Snake Venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á.; Vargas, M.; Villalta, M.; Sánchez, A.; Sanz, L.; Lomonte, B.; et al. Preclinical Evaluation of the Efficacy of Antivenoms for Snakebite Envenoming: State-of-the-Art and Challenges Ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef]
- Lomonte, B.; Tarkowski, A.; Hanson, L.A. Host Response to Bothrops asper Snake Venom. Analysis of Edema Formation, Inflammatory Cells, and Cytokine Release in a Mouse Model. Inflammation 1993, 17, 93–105. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; Cerdas, L. Isolation and Partial Characterization of a Myotoxin from the Venom of the Snake Bothrops nummifer. Toxicon 1986, 24, 885–894. [Google Scholar] [CrossRef]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R. Studies on the Quantitative Method for Determination of Hemorrhagic Activity of Habu Snake Venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–52. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. Gauss View, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09, Revision, B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Ownby, C.L.; Odell, G.V. Pathogenesis of Myonecrosis Induced by Crude Venom and a Myotoxin of Bothrops asper. Exp. Mol. Pathol. 1984, 40, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Lomonte, B.; Ovadia, M.; Gutiérrez, J.M. Local Tissue Damage Induced by BaP1, a Metalloproteinase Isolated from Bothrops asper (Terciopelo) Snake Venom. Exp. Mol. Pathol. 1995, 63, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.C.; Hyslop, S.; Fontes, M.R.M.; Prado-Franceschi, J.; Zambelli, V.O.; Magro, A.J.; Brigatte, P.; Gutierrez, V.P.; Cury, Y. Crotoxin: Novel Activities for a Classic β-Neurotoxin. Toxicon 2010, 55, 1045–1060. [Google Scholar] [CrossRef] [PubMed]

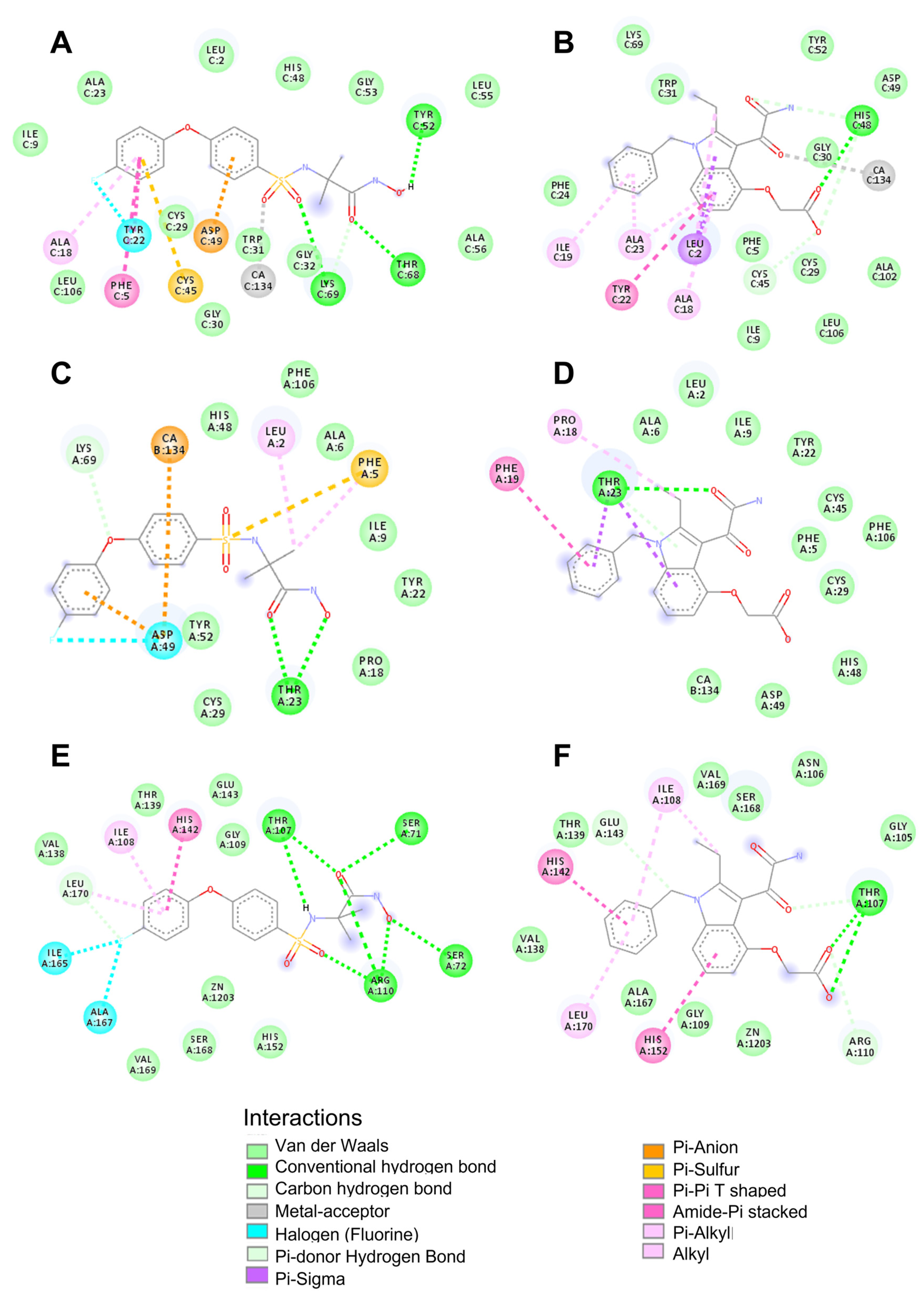
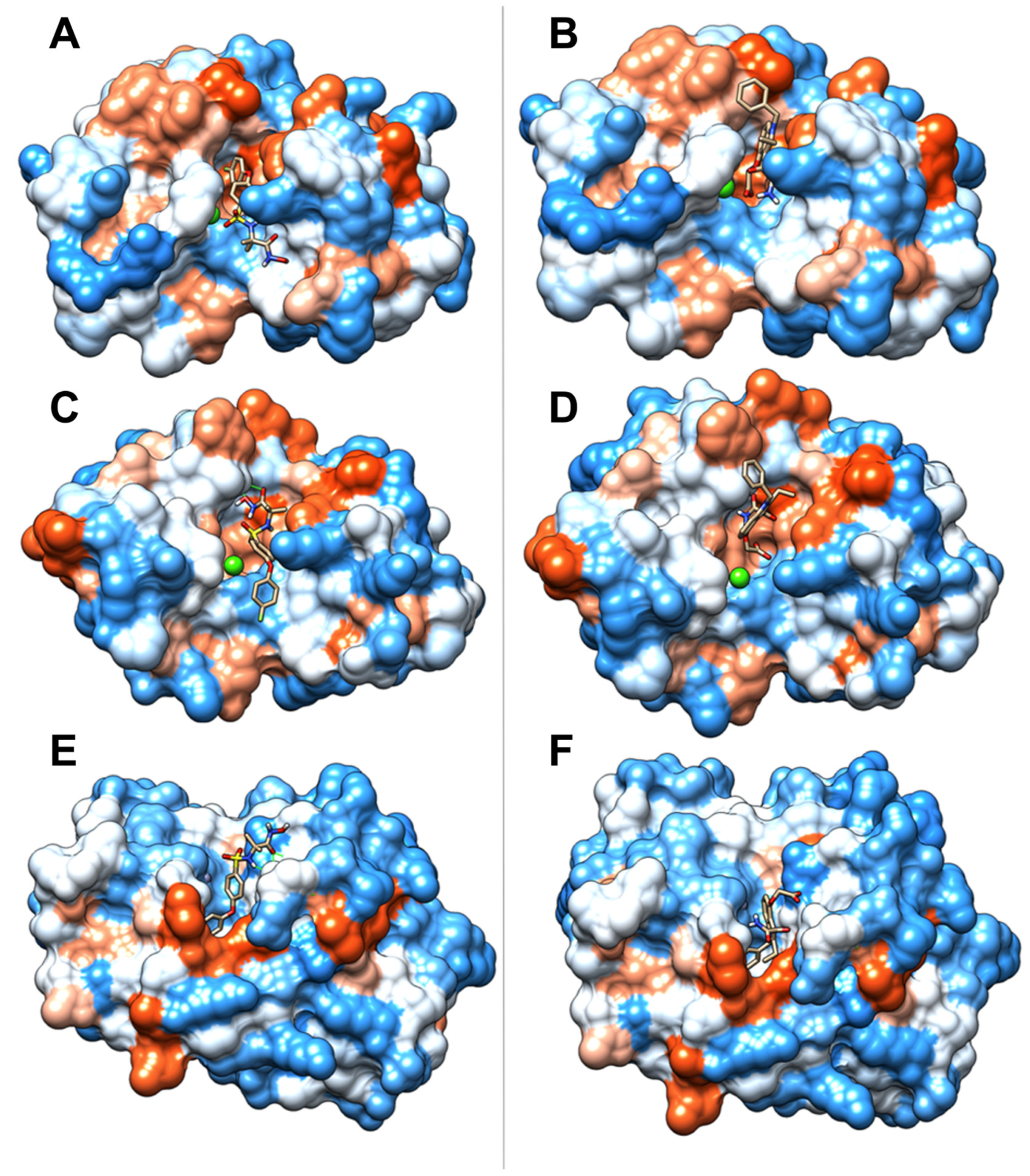
| Compound | Protein | Affinity (kcal/mol) | Main Weak Interactions | |
|---|---|---|---|---|
| Hidrogen Bonds | Van der Waals | |||
| Varespladib | PLA2 myotoxin I (PDB code 5TFV_A) | −6.5 | Thr23 | Ca2+, His48,Trp31 |
| PLA2 from the crotoxin complex (PDB code 2QOG) | −8.3 | His48 | Asp49, Ca2+ | |
| Metalloproteinase BaP1 (PDB code 2W15), | −7.6 | Thr107 | His142, His152 | |
| CP471474 | PLA2 myotoxin I (PDB code 5TFV_A) | −7.1 | Thr23 | Asp49, Phe5 |
| PLA2 from the crotoxin complex (PDB code 2QOG) | −7.5 | Lys49, Thr68, Tyr52 | Asp49, Cys45, Ca2+ | |
| Metalloproteinase BaP1 (PDB code 2W15) | −8.2 | Ser71, Ser72, Thr107, Thr110 | His142, Zn2+ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz, S.; Henao Castañeda, I.C.; Granados, J.; Patiño, A.C.; Preciado, L.M.; Pereañez, J.A. Inhibitory Effects of Varespladib, CP471474, and Their Potential Synergistic Activity on Bothrops asper and Crotalus durissus cumanensis Venoms. Molecules 2022, 27, 8588. https://doi.org/10.3390/molecules27238588
Quiroz S, Henao Castañeda IC, Granados J, Patiño AC, Preciado LM, Pereañez JA. Inhibitory Effects of Varespladib, CP471474, and Their Potential Synergistic Activity on Bothrops asper and Crotalus durissus cumanensis Venoms. Molecules. 2022; 27(23):8588. https://doi.org/10.3390/molecules27238588
Chicago/Turabian StyleQuiroz, Sara, Isabel C. Henao Castañeda, Johan Granados, Arley Camilo Patiño, Lina María Preciado, and Jaime Andrés Pereañez. 2022. "Inhibitory Effects of Varespladib, CP471474, and Their Potential Synergistic Activity on Bothrops asper and Crotalus durissus cumanensis Venoms" Molecules 27, no. 23: 8588. https://doi.org/10.3390/molecules27238588
APA StyleQuiroz, S., Henao Castañeda, I. C., Granados, J., Patiño, A. C., Preciado, L. M., & Pereañez, J. A. (2022). Inhibitory Effects of Varespladib, CP471474, and Their Potential Synergistic Activity on Bothrops asper and Crotalus durissus cumanensis Venoms. Molecules, 27(23), 8588. https://doi.org/10.3390/molecules27238588






