Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres as Efficient Heterogeneous Catalysts for the Selective Oxidation and Desulfurization of Sulfides
Abstract
1. Introduction
2. Results and Discussion
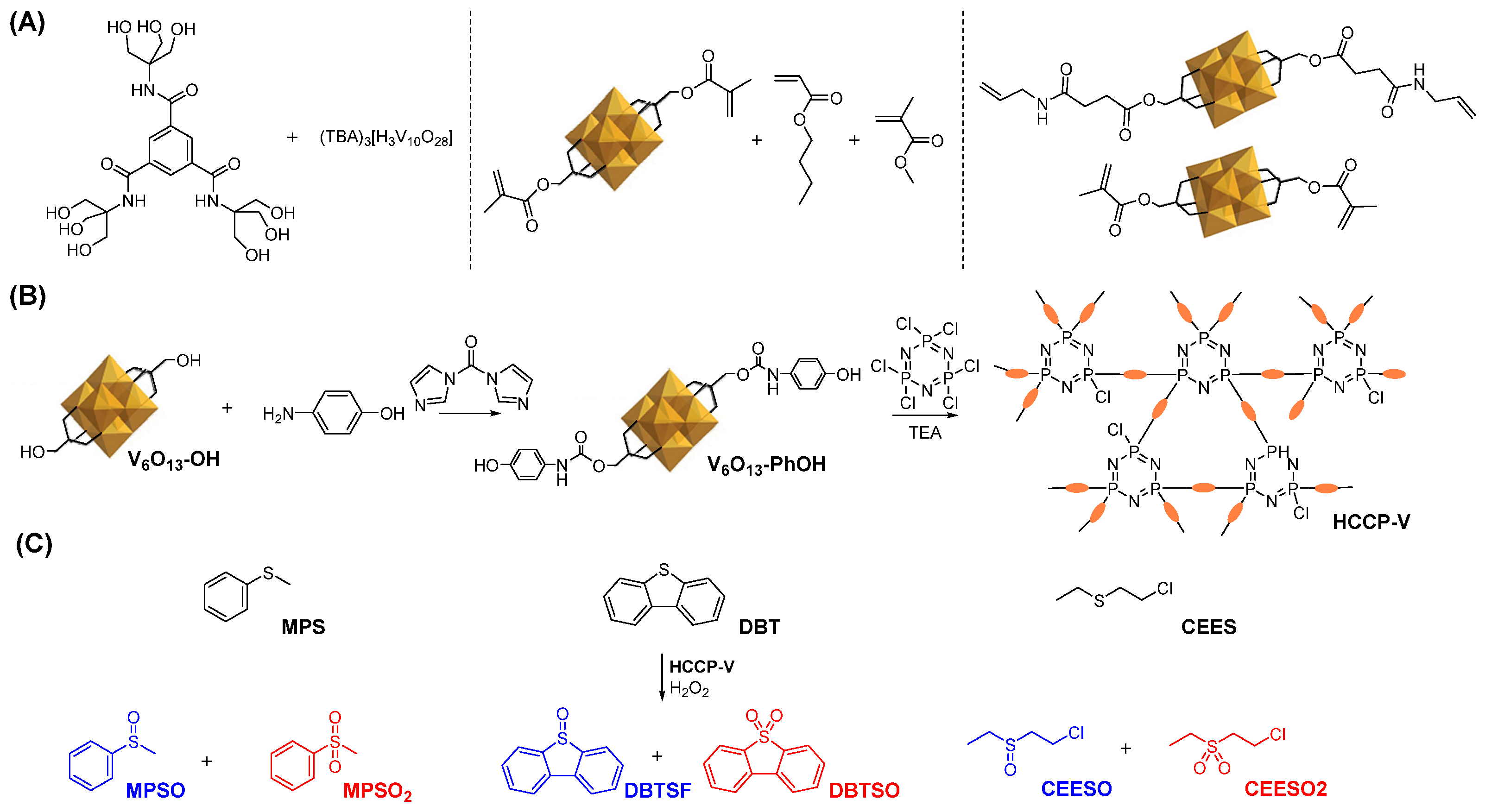
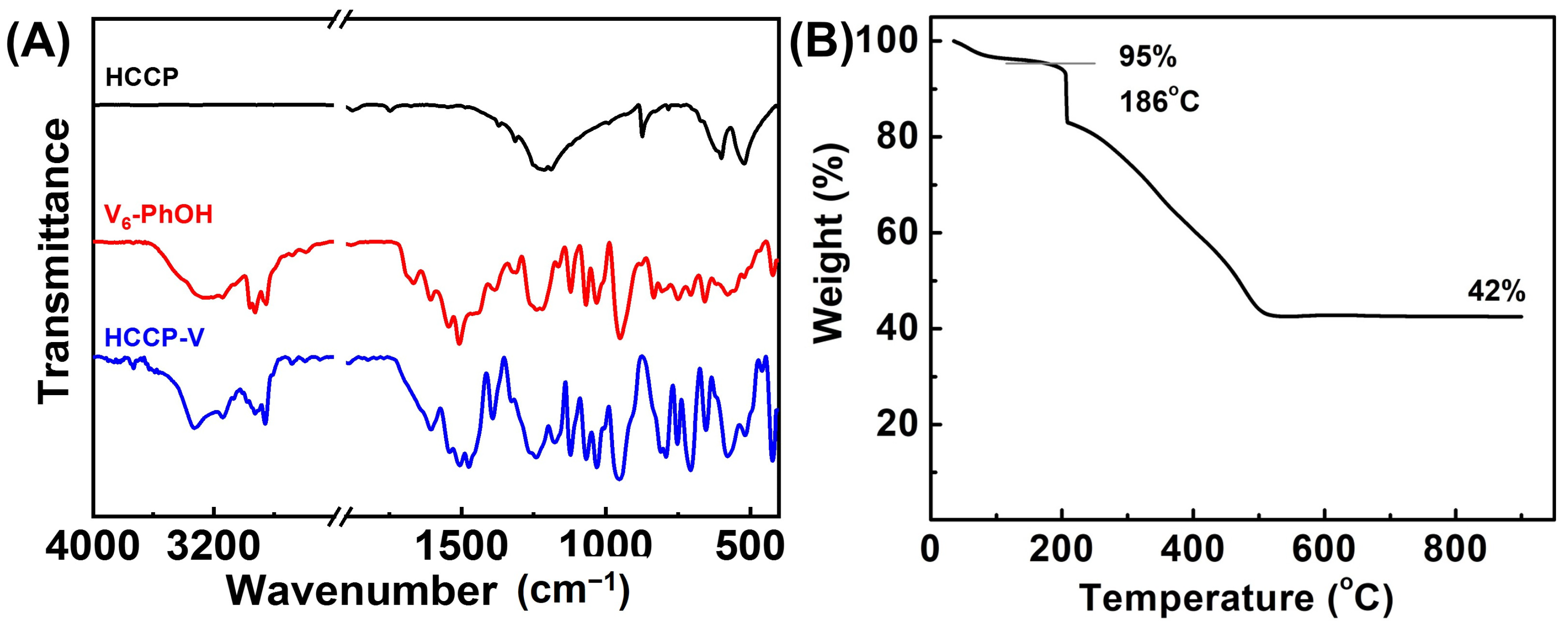
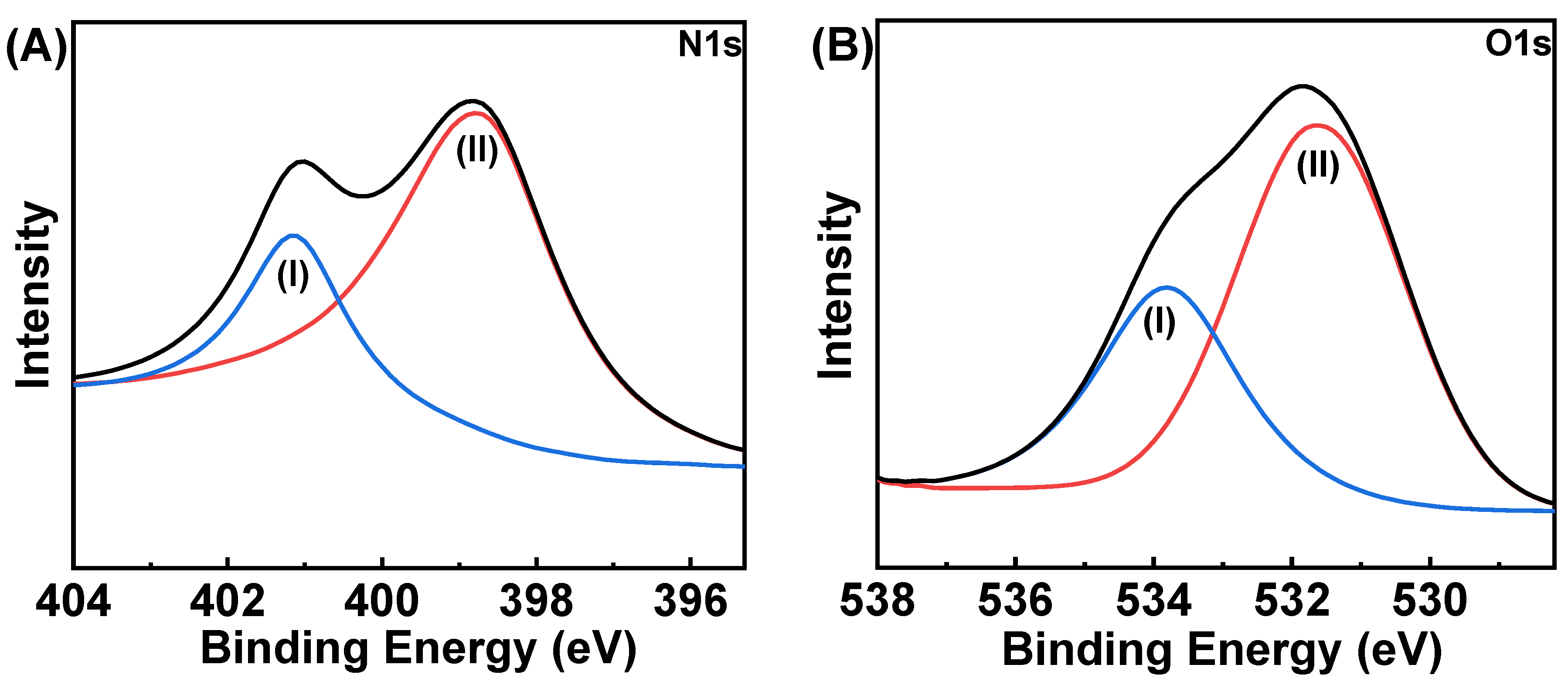
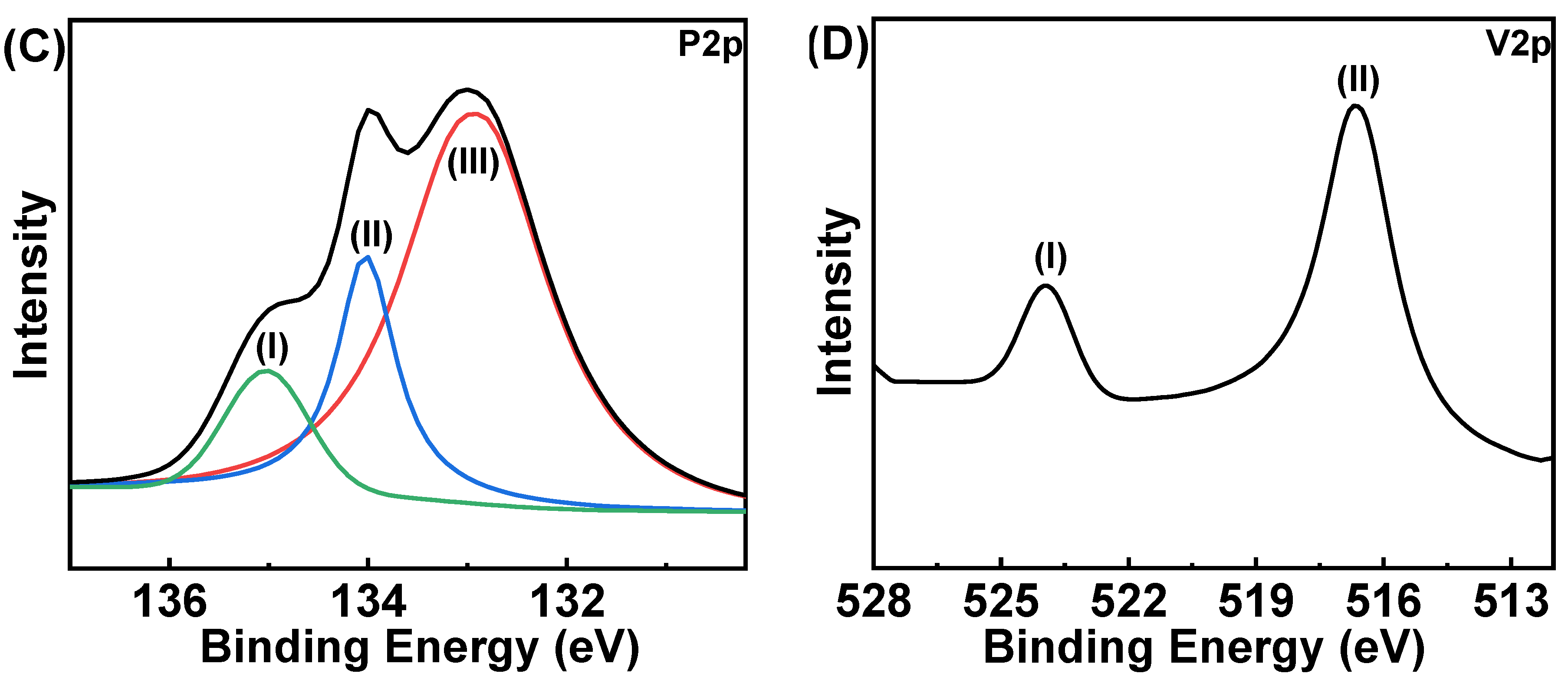
2.1. Structural and Morphological Characterization of HCCP-V
2.2. Catalytic Oxidation of MPS by HCCP-V
2.3. Catalytic Oxidation of DBT by HCCP-V
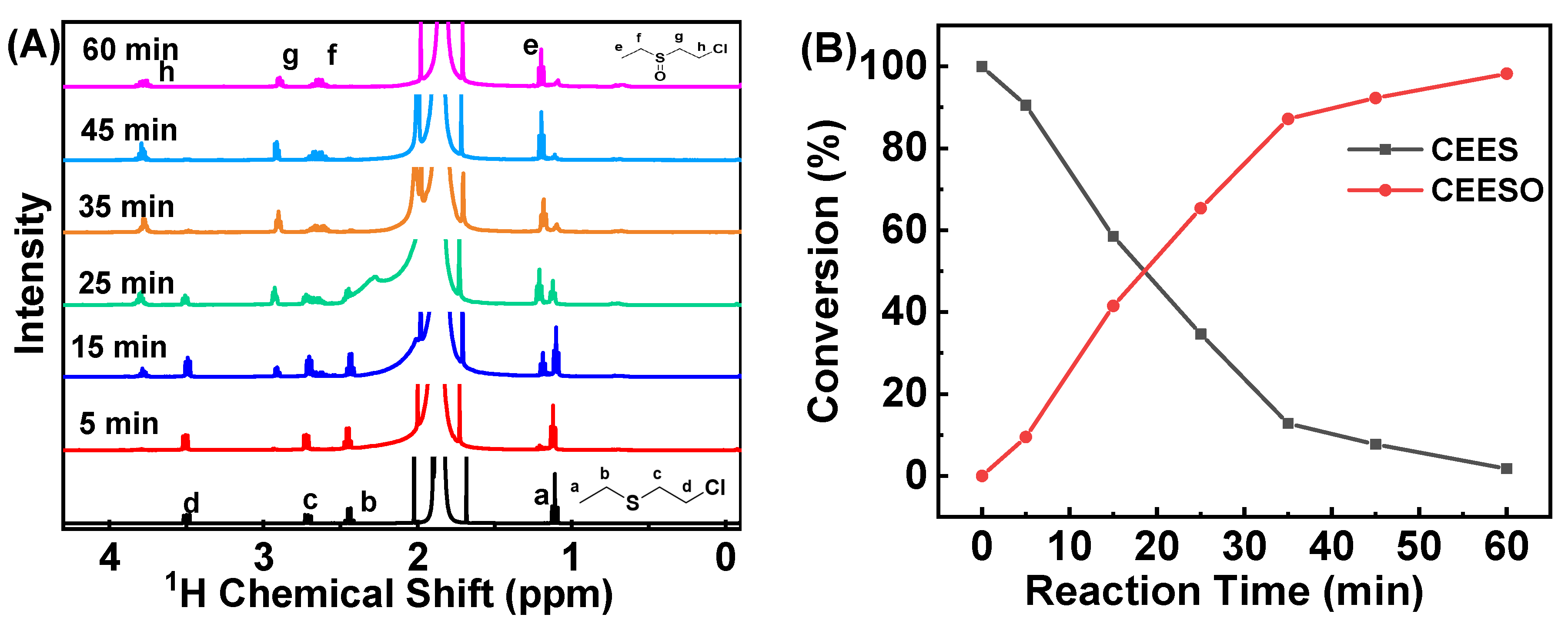
2.4. Catalytic Oxidation of CEES by HCCP-V
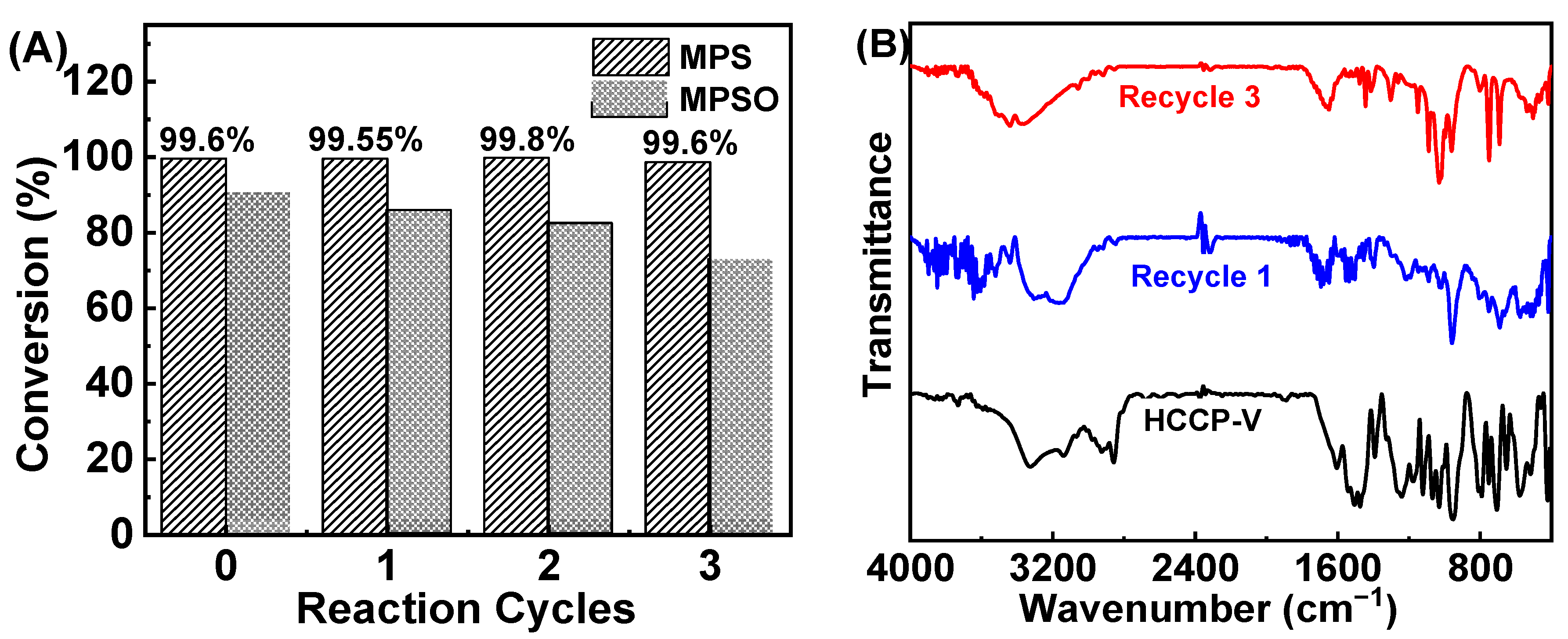
2.5. Recyclability of HCCP-V
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures of Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres
3.2.1. Synthesis of [N(C4H9)4]2[V6O13{(OCH2)3CCH2OH}2] (V6O13-OH)
3.2.2. Synthesis of [N(C4H9)4]2[V6O13{(OCH2)3CCH2OCONHC6H4OH}2] (V6O13-PhOH)
3.2.3. Precipitation Polymerization to Prepare Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres (HCCP-V)
3.3. Characterization
3.4. The Catalytic Oxidation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ho, C.L.; Wong, W.Y. Metal-containing polymers: Facile tuning of photophysical traits and emerging applications in organic electronics and photonics. Coord. Chem. Rev. 2011, 255, 2469–2502. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.Y.; Ren, L.X.; Tang, C.B. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef]
- Friebe, C.; Hager, M.D.; Winter, A.; Schubert, U.S. Metal-containing Polymers via Electropolymerization. Adv. Mater. 2012, 24, 332–345. [Google Scholar] [CrossRef]
- Hailes, R.L.N.; Oliver, A.M.; Gwyther, J.; Whittell, G.R.; Manners, I. Polyferrocenylsilanes: Synthesis, properties, and applications. Chem. Soc. Rev. 2016, 45, 5358–5407. [Google Scholar] [CrossRef]
- Hardy, C.G.; Zhang, J.Y.; Yan, Y.; Ren, L.X.; Tang, C.B. Metallopolymers with transition metals in the side-chain by living and controlled polymerization techniques. Prog. Polym. Sci. 2014, 39, 1742–1796. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.W.; Yao, J.H.; Xu, P.; Miao, Z.L.; Li, J.L.; Lv, Z.D.; Zhang, Q.Y.; Yan, Y. Metallopolymers from organically modified polyoxometalates (MOMPs): A review. J. Organomet. Chem. 2019, 884, 1–16. [Google Scholar] [CrossRef]
- Thorimbert, S.; Hasenknopf, B.; Lacote, E. Cross-Linking Organic and Polyoxometalate Chemistries. Isr. J. Chem. 2011, 51, 275–280. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, L.X. Polyoxometalate-Incorporated Supramolecular Self-Assemblies: Structures and Functional Properties. Isr. J. Chem. 2011, 51, 181–190. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Li, H.L.; Wu, L.X. Ionic Complexes of Metal Oxide Clusters for Versatile Self Assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Long, D.L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef]
- Li, C.Z.; Zhao, X.C.; Wang, A.Q.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Zhang, J.P.; Miao, Z.L.; Yan, J.; Zhang, X.; Li, X.Z.; Zhang, Q.Y.; Yan, Y. Synthesis of Negative-Charged Metal-Containing Cyclomatrix Polyphosphazene Microspheres Based on Polyoxometalates and Application in Charge-Selective Dye Adsorption. Macromol. Rapid Commun. 2019, 40, e1800730. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar]
- Streb, C. Structure and Bonding in Molecular Vanadium Oxides: From Templates via Host-Guest Chemistry to Applications. In Polyoxometalate-Based Assemblies and Functional Materials; Song, Y.F., Ed.; Springer, Berlin, Germany, 2018; Volume 176, pp. 31–47.
- Zhou, T.; Xiao, H.R.; Xie, L.L.; Han, Q.; Qiu, X.J.; Xiao, Y.M.; Yang, X.L.; Zhu, L.M.; Cao, X.Y. Research on the electrochemical performance of polyoxovanadate material K4Na2V10O28 as a novel aqueous zinc-ion batteries cathode. Electrochim. Acta 2022, 424, 140621. [Google Scholar] [CrossRef]
- Vannathan, A.A.; Chandewar, P.R.; Shee, D.; Mal, S.S. Polyoxovanadate-Activated Carbon-Based Hybrid Materials for High-Performance Electrochemical Capacitors. J. Electrochem. Soc. 2022, 169, 050538. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrao, G.; Goncalves, J.; Sanchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, X.M.; Du, Z.Y.; Xu, Y. Organo-functionalized polyoxovanadates: Crystal architecture and property aspects. Dalton Trans. 2021, 50, 7871–7886. [Google Scholar] [CrossRef]
- Anjass, M.; Lowe, G.A.; Streb, C. Molecular Vanadium Oxides for Energy Conversion and Energy Storage: Current Trends and Emerging Opportunities. Angew. Chem. Int. Ed. 2021, 60, 7522–7532. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.F.; Zhang, Y.N.; Lin, F.; Lu, H.Y.; Jiang, Z.X.; Li, C. Oxidation of dibenzothiophene catalyzed by C8H17N(CH3)(3) (3)H3V10O28 using molecular oxygen as oxidant. Chem. Commun. 2012, 48, 11647–11649. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Hu, J.F.; Chi, Y.N.; Lin, Z.G.; Zou, B.; Yang, S.; Hill, C.L.; Hu, C.W. A Polyoxoniobate-Polyoxovanadate Double-Anion Catalyst for Simultaneous Oxidative and Hydrolytic Decontamination of Chemical Warfare Agent Simulants. Angew. Chem. Int. Ed. 2017, 56, 4473–4477. [Google Scholar] [CrossRef] [PubMed]
- Nohra, B.; El Moll, H.; Albelo, L.M.R.; Mialane, P.; Marrot, J.; Mellot-Draznieks, C.; O’Keeffe, M.; Biboum, R.N.; Lemaire, J.; Keita, B.; et al. Polyoxometalate-Based Metal Organic Frameworks (POMOFs): Structural Trends, Energetics, and High Electrocatalytic Efficiency for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 13363–13374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Li, Y.H.; Lin, J.F.; Li, H.; Wang, X.L. In Situ Ligand-Transformation-Involved Synthesis of Inorganic-Organic Hybrid Polyoxovanadates as Efficient Heterogeneous Catalysts for the Selective Oxidation of Sulfides. Inorg. Chem. 2020, 59, 17583–17590. [Google Scholar] [CrossRef]
- Lu, B.B.; Yang, J.; Liu, Y.Y.; Ma, J.F. A Polyoxovanadate Resorcin 4 arene-Based Porous Metal Organic Framework as an Efficient Multifunctional Catalyst for the Cycloaddition of CO2 with Epoxides and the Selective Oxidation of Sulfides. Inorg. Chem. 2017, 56, 11710–11720. [Google Scholar] [CrossRef]
- Li, J.K.; Huang, X.Q.; Yang, S.; Xu, Y.Q.; Hu, C.W. Controllable Synthesis, Characterization, and Catalytic Properties of Three Inorganic-Organic Hybrid Copper Vanadates in the Highly Selective Oxidation of Sulfides and Alcohols. Cryst. Growth Des. 2015, 15, 1907–1914. [Google Scholar] [CrossRef]
- Monakhov, K.Y.; Bensch, W.; Kogerler, P. Semimetal-functionalised polyoxovanadates. Chem. Soc. Rev. 2015, 44, 8443–8483. [Google Scholar] [CrossRef]
- Miao, W.K.; Yan, Y.K.; Wang, X.L.; Xiao, Y.; Ren, L.J.; Zheng, P.; Wang, C.H.; Ren, L.X.; Wang, W. Incorporation of Polyoxometalates into Polymers to Create Linear Poly(polyoxometalate)s with Catalytic Function. Acs Macro Lett. 2014, 3, 211–215. [Google Scholar] [CrossRef]
- Sullivan, K.P.; Neiwert, W.A.; Zeng, H.D.; Mehta, A.K.; Yin, Q.S.; Hillesheim, D.A.; Vivek, S.; Yin, P.C.; Collins-Wildman, D.L.; Weeks, E.R.; et al. Polyoxometalate-based gelating networks for entrapment and catalytic decontamination. Chem. Commun. 2017, 53, 11480–11483. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Wemyss, A.M.; Brown, O.B.; Huang, Q.Y.; Wan, C.Y. Structure and electrochemical properties of hierarchically porous carbon nanomaterials derived from hybrid ZIF-8/ZIF-67 bi-MOF coated cyclomatrix poly(organophosphazene) nanospheres. New J. Chem. 2020, 44, 4353–4362. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.Q.; Zhu, W.; Yin, P.C. Surface Engineering Promoted Insulin-Sensitizing Activities of Sub-Nanoscale Vanadate Clusters through Regulated Pharmacokinetics and Bioavailability. Small 2022, 18, 2203957. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.T.; Xu, Z.W.; Chen, Y.D.; Zhang, M.X.; Yin, J.F.; Li, M.; Chen, K.; Yin, P.C. Sub-nanoscaled Metal Oxide Cluster-Integrated Polymer Network for Quasi-Homogeneous Catalysis. Acs Appl. Mater. Interfaces 2020, 12, 38655–38661. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, Z.C.; Wu, P.F. A New Scheme to Prepare Polyoxovanadate-Polymer Hybrid Materials. J. Clust. Sci. 2021, 32, 1739–1745. [Google Scholar] [CrossRef]
- Ahmad, M.; Nawaz, T.; Hussain, I.; Chen, X.; Imran, M.; Hussain, R.; Assiri, M.A.; Ali, S.; Wu, Z.P. Phosphazene Cyclomatrix Network-Based Polymer: Chemistry, Synthesis, and Applications. Acs Omega 2022, 7, 28694–28707. [Google Scholar] [CrossRef]
- Fu, J.W.; Huang, X.B.; Zhu, L.; Tang, X.Z. One-pot synthesis of porous cyclomatrix-type polyphosphazene nanotubes with closed ends via an in situ template approach. Scr. Mater. 2008, 58, 1047–1049. [Google Scholar] [CrossRef]
- Maaskant, E.; Gojzewski, H.; Hempenius, M.A.; Vancso, G.J.; Benes, N.E. Thin cyclomatrix polyphosphazene films: Interfacial polymerization of hexachlorocyclotriphosphazene with aromatic biphenols. Polym. Chem. 2018, 9, 3169–3180. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.W.; Li, L.; Zhang, H.B.; Chen, Z.; Yang, Y.C.; Jiang, Z.H.; Mu, J.X. Preparation and Properties of Novel Crosslinked Polyphosphazene-Aromatic Ethers Organic-Inorganic Hybrid Microspheres. Polymers 2022, 14, 2411. [Google Scholar] [CrossRef]
- Orum, S.M. Novel cyclomatrix polyphosphazene nanospheres: Preparation, characterization and dual anticancer drug release application. Polym. Bull. 2022, 79, 2851–2869. [Google Scholar] [CrossRef]
- Han, J.W.; Hill, C.L. A coordination network that catalyzes O-2-based oxidations. J. Am. Chem. Soc. 2007, 129, 15094–15095. [Google Scholar] [CrossRef] [PubMed]
- Akopyan, A.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A. Deep Oxidative Desulfurization of Fuels in the Presence of Brönsted Acidic Polyoxometalate-Based Ionic Liquids. Molecules 2020, 25, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, W.S.; Xun, S.H.; Li, H.M.; Gu, Q.Q.; Zhao, Z.; Wang, Q. Deep oxidative desulfurization of dibenzothiophene with POM-based hybrid materials in ionic liquids. Chem. Eng. J. 2013, 220, 328–336. [Google Scholar] [CrossRef]
- Hao, L.W.; Sun, L.L.; Su, T.; Hao, D.M.; Liao, W.P.; Deng, C.L.; Ren, W.Z.; Zhang, Y.M.; Lu, H.Y. Polyoxometalate-based ionic liquid catalyst with unprecedented activity and selectivity for oxidative desulfurization of diesel in Omin BF4. Chem. Eng. J. 2019, 358, 419–426. [Google Scholar] [CrossRef]
- Hou, Y.J.; An, H.Y.; Zhang, Y.M.; Hu, T.; Yang, W.; Chang, S.Z. Rapid Destruction of Two Types of Chemical Warfare Agent Simulants by Hybrid Polyoxomolybdates Modified by Carboxylic Acid Ligands. Acs Catal. 2018, 8, 6062–6069. [Google Scholar] [CrossRef]
- Tian, H.R.; Zhang, Z.; Liu, S.M.; Dang, T.Y.; Li, X.H.; Lu, Y.; Liu, S.X. A novel polyoxovanadate-based Co-MOF: Highly efficient and selective oxidation of a mustard gas simulant by two-site synergetic catalysis. J. Mater. Chem. A 2020, 8, 12398–12405. [Google Scholar] [CrossRef]
- Achim Miiller, J.M. Hartmut Bogge, Anja Stammler, Alexandru Botar, Cis-/trans-isomerism of bis-(trisalkoxy)-hexavanadates: Cis-Na-2[V(IV)6O7(OH)6{(OCH2)3CCH2OH}2]•8H–O, cis-(CN3H6)3[(V(IV)V(V)5O13){(OCH2)3CCH2OH}2]•4,5H2O and trans-(CN3H6)2[(V(V)6O13){(OCH2)3CCH2OH}2] •H2O. Z. Anorg. Allg. Chem. 1995, 621, 1818–1831. [Google Scholar] [CrossRef]
- Yalcin, G.; Kayan, A. Synthesis and characterization of Zr, Ti, Al-phthalate and pyridine-2-carboxylate compounds and their use in ring opening polymerization. Appl. Catal. A Gen. 2012, 433–434, 223–228. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, S.; Lu, X.; Lu, Q. Water-triggered self-assembly polycondensation for the one-pot synthesis of cyclomatrix polyphosphazene nanoparticles from amino acid ester. Chem. Commun. 2015, 51, 8373–8376. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Z.; Dang, T.; Liu, S.; Lu, Y.; Liu, S. Hollow lindqvist-like-shaped {V6} cluster-based metal-organic framework for the highly efficient detoxification of mustard gas simulant. Inorg. Chem. 2021, 60, 840–845. [Google Scholar] [CrossRef]
- Hou, Y.; An, H.; Chang, S.; Zhang, J. Versatile catalysts constructed from hybrid polyoxomolybdates for simultaneously detoxifying sulfur mustard and organophosphate simulants. Catal. Sci. Technol. 2019, 9, 2445–2455. [Google Scholar] [CrossRef]
- An, H.; Hou, Y.; Wang, L.; Zhang, Y.; Yang, W.; Chang, S. Evans-showell-type polyoxometalates constructing high-dimensional inorganic-organic hybrid compounds with copper-organic coordination complexes: Synthesis and oxidation catalysis. Inorg. Chem. 2017, 56, 11619–11632. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, C.; Guo, D.; Wang, C.; Han, Y.; He, G.; Zhang, J.; Huang, X.; Hu, C. Inorganic-organic hybrid polyoxovanadates based on [V4O12]4- or [VO3]22- clusters: Controllable synthesis, crystal structures and catalytic properties in selective oxidation of sulfides. Dalton Trans. 2020, 49, 14148–14157. [Google Scholar] [CrossRef]
- An, H.; Hou, Y.; Chang, S.; Zhang, J.; Zhu, Q. Highly efficient oxidation of various thioethers catalyzed by organic ligand-modified polyoxomolybdates. Inorg. Chem. Front. 2020, 7, 169–176. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Qiu, J.; Wang, N.; Zhang, Q.; Lei, Q.; Hu, Y.L.; Zhang, Y. Catalytic oxidative desulfurization of model fuel using [HPMo][HTAC]2/SiO2 as a amphiphilic catalyst. Adv. Mater. Res. 2011, 396–398, 827–832. [Google Scholar]
- Ding, Y.; Wang, J.; Liao, M.; Li, J.; Zhang, L.; Guo, J.; Wu, H. Deep oxidative desulfurization of dibenzothiophene by novel POM-based IL immobilized on well-ordered KIT-6. Chem. Eng. J. 2021, 418, 129470. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, L.; Gao, R.; Hu, G.; Zhao, J. Deep desulfurization of fuels using supported ionic liquid-polyoxometalate hybrid as catalyst: A comparison of different types of ionic liquids. J. Hazard Mater. 2021, 401, 123267. [Google Scholar] [CrossRef]
- Cedeño, L.; Gomez, H.; Fraustro, A.; Guerra, H.; Cuevas, R. Oxidative desulfurization of synthetic diesel using supported catalysts. Catal. Today 2008, 133, 244–254. [Google Scholar] [CrossRef]


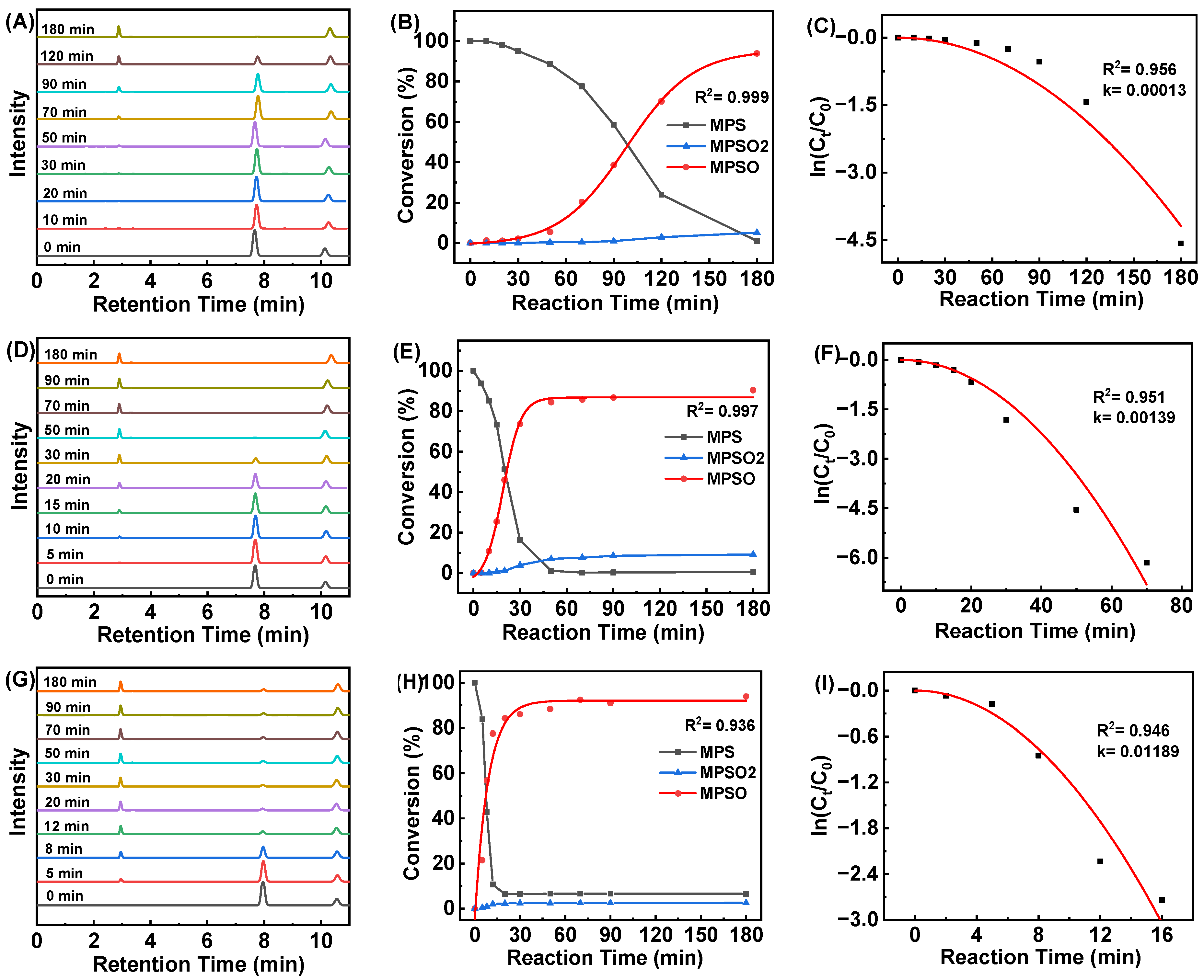
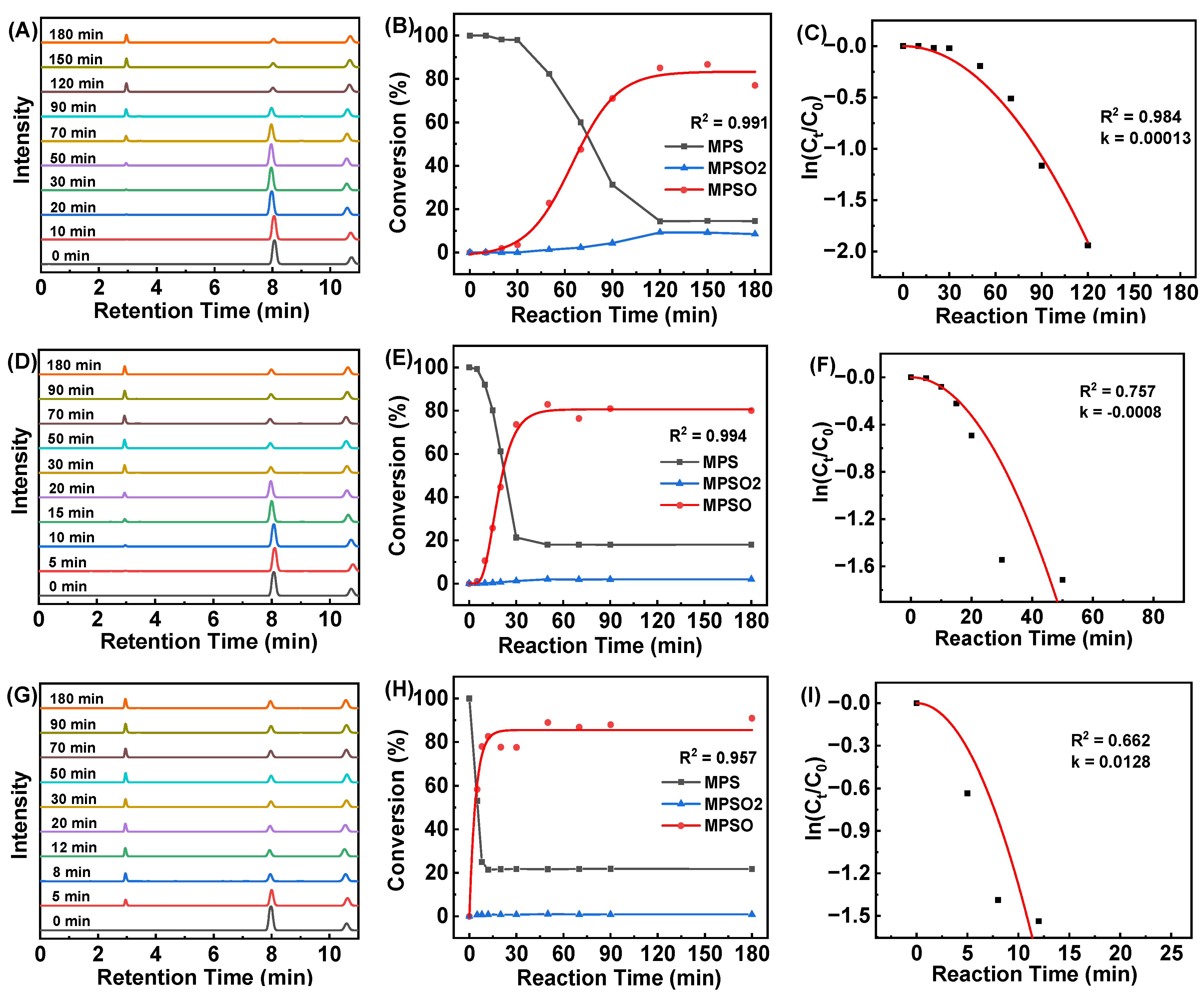
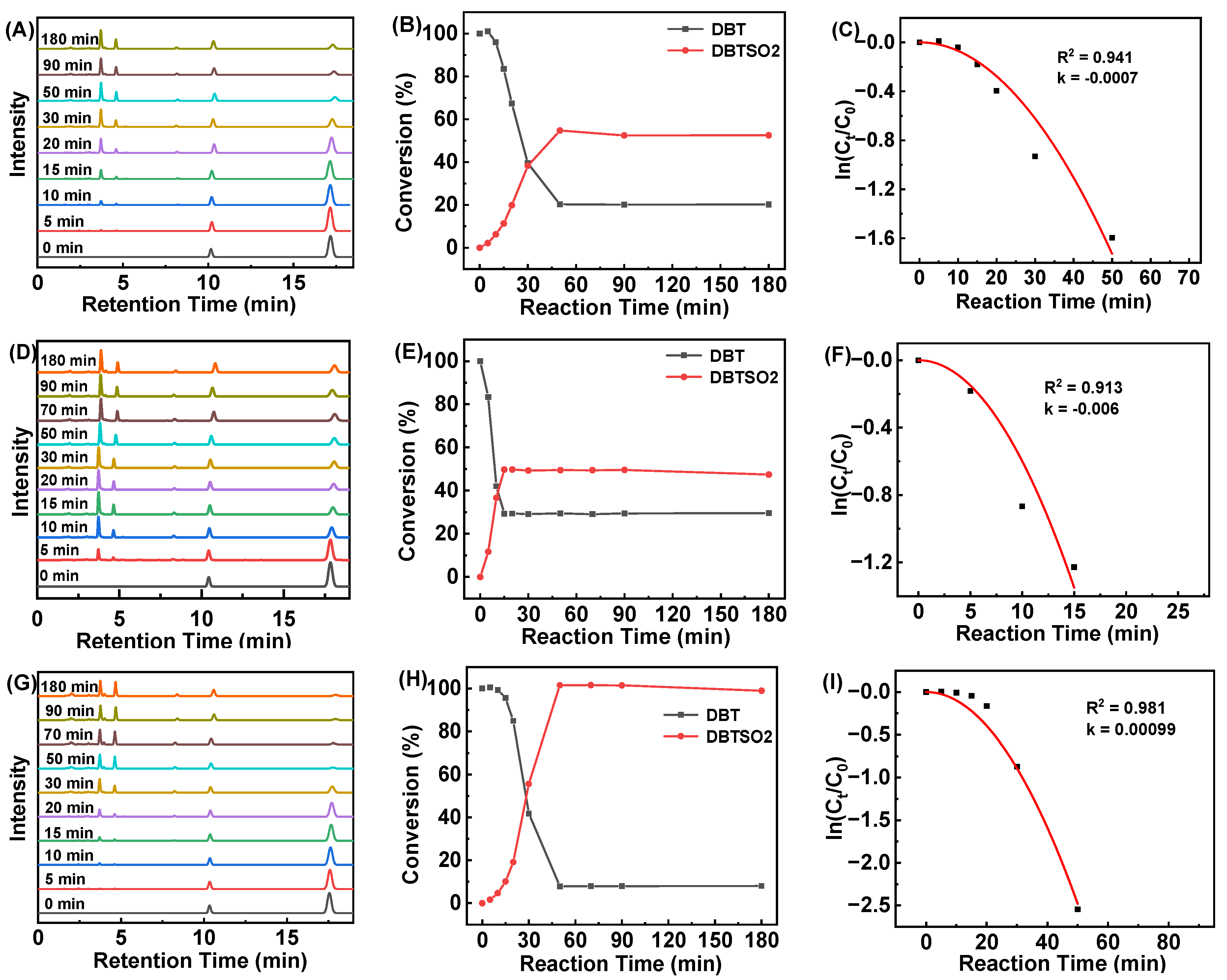
| Catalyst | T/°C | [MPS]:[H2O2]:[Catalyst] | t/min | k/min−1 | TOF/min−1 | Conversion/% |
|---|---|---|---|---|---|---|
| HCCP-BPS | 40 | 1:1.2:1/400 | 180 | - | - | - |
| HCCP-V | 25 | 1:1.2:1/400 | 180 | 0.00013 | 2.20 | 99.0 |
| HCCP-V | 40 | 1:1.2:1/400 | 50 | 0.00139 | 7.98 | 99.6 |
| HCCP-V | 55 | 1:1.2:1/400 | 20 | 0.01189 | 18.71 | 93.4 |
| HCCP-V | 25 | 1:1:1/400 | 120 | 0.00013 | 2.86 | 85.6 |
| HCCP-V | 40 | 1:1:1/400 | 50 | 0.0008 | 6.58 | 82.1 |
| HCCP-V | 55 | 1:1:1/400 | 12 | 0.0128 | 25.21 | 75.5 |
| Catalyst | T/°C | [DBT]:[H2O2]:[Catalyst] | t/min | k/min−1 | TOF/min−1 | Conversion/% |
|---|---|---|---|---|---|---|
| HCCP-V | 70 | 1:5:1/100 | 50 | 0.0007 | 1.60 | 79.8 |
| HCCP-V | 70 | 1:8:1/100 | 50 | 0.00099 | 1.84 | 92.2 |
| HCCP-V | 80 | 1:5:1/100 | 15 | 0.006 | 4.70 | 70.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Huang, D.; Yan, J.; Miao, Z.; Yu, L.; Cai, N.; Fang, Q.; Zhang, Q.; Yan, Y. Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres as Efficient Heterogeneous Catalysts for the Selective Oxidation and Desulfurization of Sulfides. Molecules 2022, 27, 8560. https://doi.org/10.3390/molecules27238560
Hu Y, Huang D, Yan J, Miao Z, Yu L, Cai N, Fang Q, Zhang Q, Yan Y. Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres as Efficient Heterogeneous Catalysts for the Selective Oxidation and Desulfurization of Sulfides. Molecules. 2022; 27(23):8560. https://doi.org/10.3390/molecules27238560
Chicago/Turabian StyleHu, Yinghui, Diping Huang, Jing Yan, Zhiliang Miao, Lize Yu, Ningjing Cai, Quanhai Fang, Qiuyu Zhang, and Yi Yan. 2022. "Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres as Efficient Heterogeneous Catalysts for the Selective Oxidation and Desulfurization of Sulfides" Molecules 27, no. 23: 8560. https://doi.org/10.3390/molecules27238560
APA StyleHu, Y., Huang, D., Yan, J., Miao, Z., Yu, L., Cai, N., Fang, Q., Zhang, Q., & Yan, Y. (2022). Polyoxovanadate-Based Cyclomatrix Polyphosphazene Microspheres as Efficient Heterogeneous Catalysts for the Selective Oxidation and Desulfurization of Sulfides. Molecules, 27(23), 8560. https://doi.org/10.3390/molecules27238560





