Drugs for COVID-19: An Update
Abstract
1. Introduction
3. Antiviral Drugs
3.1. Remdesivir (Veklury)
3.2. Favipiravir
3.3. Lopinavir/Ritonavir
3.4. Molnupiravir (Lagevrio)
3.5. Paxlovid (Nirmatrelvir/Ritonavir)
3.6. Simeprevir
4. Antimalarial Drugs
Chloroquine and Hydroxychloroquine
5. Antibiotics
Azithromycin
6. Interleukine Inhibitors
6.1. Anakinra
6.2. Tocilizumab (RoActemra)
7. Janus Kinase (JAKs) Inhibitors
Baricitinib
8. Corticosteroids
Dexamethasone
9. Anticoagulants
Low Molecular Weight Heparin (LMWH)
10. Non-Steroid Anti-Inflammatory Drugs (NSAIDs)
11. Recent Studies for New Drugs
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 November 2022).
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Pellegrino, M.; Mariconda, A.; Longo, P.; Sinicropi, M.S.; Aquaro, S. COVID-19 at a glance: An up-to-date overview on variants, drug design and therapies. Viruses 2022, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Malani, P.N.; Omer, S.B. Confronting the Delta variant of SARS-CoV-2, summer 2021. JAMA 2021, 326, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Hong, V.; Tartof, S.Y. Association of SARS-CoV-2 BA.4/BA.5 Omicron lineages with immune escape and clinical outcome. medRxiv 2022. [Google Scholar] [CrossRef]
- Sheward, D.J.; Kim, C.; Fischbach, J.; Sato, K.; Muschiol, S.; Ehling, R.A.; Björkström, N.K.; Hedestam, G.B.K.; Reddy, S.T.; Albert, J.; et al. Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralising antibodies. Lancet Infect. Dis. 2022, 22, 1538–1540. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Maio, A.C.; Basile, G.; Giuzio, F.; Bonomo, M.G.; Aquaro, S.; Walsh, T.J.; Sinicropi, M.S.; et al. Are Nutraceuticals Effective in COVID-19 and Post-COVID Prevention and Treatment? Foods 2022, 11, 2884. [Google Scholar] [CrossRef]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, D.D.; Han, I.; Kumar, Y.; Choi, E.H. Current Potential Therapeutic Approaches against SARS-CoV-2: A Review. Biomedicines 2021, 9, 1620. [Google Scholar] [CrossRef]
- Gil Martinez, V.; Avedillo Salas, A.; Santander Ballestin, S. Antiviral therapeutic approaches for SARS-CoV-2 infection: A systematic review. Pharmaceuticals 2021, 14, 736. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Baron, R.J.; Ejnes, Y.D. Physicians spreading misinformation on social media—Do right and wrong answers still exist in medicine? N. Engl. J. Med. 2022, 387, 1–3. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; Devnarain, N. Time to stop using ineffective COVID-19 drugs. N. Engl. J. Med. 2022, 387, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Banerjee, S.; Ghosh, K.; Gayen, S.; Jha, T. Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors. Bioorg. Med. Chem. 2021, 29, 115860. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Gheware, A.; Ray, A.; Rana, D.; Bajpai, P.; Nambirajan, A.; Arulselvi, S.; Mathur, P.; Trikha, A.; Arava, S.; Das, P.; et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci. Rep. 2022, 12, 4058. [Google Scholar] [CrossRef]
- Wettstein, L.; Kirchhoff, F.; Munch, J. The transmembrane protease TMPRSS2 as a therapeutic target for COVID-19 treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ratia, K.; Cooper, L.; Kong, D.; Lee, H.; Kwon, Y.; Li, Y.; Alqarni, S.; Huang, F.; Dubrovskyi, O.; et al. Design of SARS-CoV-2 PLpro inhibitors for COVID-19 antiviral therapy leveraging binding cooperativity. J. Med. Chem. 2022, 65, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.R.; Pourhanifeh, M.H.; Hamblin, M.R.; Shahrzad, M.K.; Mirzaei, H. RdRp inhibitors and COVID-19: Is molnupiravir a good option? Biomed. Pharmacother. 2022, 146, 112517. [Google Scholar] [CrossRef] [PubMed]
- Tarighi, P.; Eftekhari, S.; Chizari, M.; Sabernavaei, M.; Jafari, D.; Mirzabeigi, P. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur. J. Pharmacol. 2021, 895, 173890. [Google Scholar] [CrossRef]
- Molhave, M.; Agergaard, J.; Wejse, C. Clinical Management of COVID-19 Patients—An Update. Semin. Nucl. Med. 2022, 52, 4–10. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 2022, 399, 1941–1953. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Huang, J.; Yin, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: A prospective, multicenter, open-label, randomized controlled clinical trial. Front. Pharmacol. 2021, 12, 683296. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef]
- Özlüşen, B.; Kozan, Ş.; Akcan, R.E.; Kalender, M.; Yaprak, D.; Peltek, İ.B.; Keske, Ş.; Gönen, M.; Ergönül, Ö. Effectiveness of favipiravir in COVID-19: A live systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2575–2583. [Google Scholar] [CrossRef]
- Shinkai, M.; Tsushima, K.; Tanaka, S.; Hagiwara, E.; Tarumoto, N.; Kawada, I.; Hirai, Y.; Fujiwara, S.; Komase, Y.; Saraya, T.; et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: A randomized, phase III clinical trial. Infect. Dis. Ther. 2021, 10, 2489–2509. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, M.; Kumar, N.; Aljawder, D.; Abdulrahman, A.; Mohamed, M.W.; Alnashaba, F.; Fayyad, M.A.; Alshaikh, F.; Alsahaf, F.; Saeed, S.; et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci. Rep. 2022, 12, 4925. [Google Scholar] [CrossRef]
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-de-Hoyo, R. The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials. Sci. Rep. 2021, 11, 11022. [Google Scholar] [CrossRef] [PubMed]
- Krumm, Z.A.; Lloyd, G.M.; Francis, C.P.; Nasif, L.H.; Mitchell, D.A.; Golde, T.E.; Giasson, B.I.; Xia, Y. Precision therapeutic targets for COVID-19. Virol. J. 2021, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.T.; Luo, Y.L.; Xia, S.C.; Sun, Q.F.; Ding, J.G.; Zhou, Y.; Chen, W.; Wang, X.F.; Zhang, W.W.; Du, W.J.; et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3390–3396. [Google Scholar] [PubMed]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia 2021, 13, 14. [Google Scholar] [CrossRef]
- Wen, C.Y.; Xie, Z.W.; Li, Y.P.; Deng, X.L.; Chen, X.T.; Cao, Y.; Ou, X.; Lin, W.Y.; Li, F.; Cai, W.P.; et al. The efficacy and safety of lopinavir/ritonavir and arbidol in patients with coronavirus disease 2019. Zhonghua Nei Ke Za Zhi 2020, 59, 605–609. [Google Scholar]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Heybati, K.; Ali, S.; Chang, O.; Silver, Z.; Dhivagaran, T.; Ramaraju, H.B.; Wong, C.Y.; et al. Efficacy of lopinavir-ritonavir combination therapy for the treatment of hospitalized COVID-19 patients: A meta-analysis. Future Virol. 2022, 17, 169–189. [Google Scholar] [CrossRef]
- Elmekaty, E.Z.I.; Alibrahim, R.; Hassanin, R.; Eltaib, S.; Elsayed, A.; Rustom, F.; Mohamed Ibrahim, M.I.; Abu Khattab, M.; Al Soub, H.; Al Maslamani, M.; et al. Darunavir-cobicistat versus lopinavir-ritonavir in the treatment of COVID-19 infection (DOLCI): A multicenter observational study. PLoS ONE 2022, 17, e0267884. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Low, M.C.H.; Kwok, A.C.Y.; Lui, A.Y.C.; Lau, K.T.K.; Au, I.C.H.; Xiong, X.; Chung, M.S.H.; Kwan, M.Y.W.; Lau, E.H.Y.; et al. Slower recovery with early lopinavir/ritonavir use in pediatric COVID-19 patients: A retrospective observational study. Paediatr. Drugs 2022, 24, 269–280. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab. Syndr. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Current status of oral antiviral drug treatments for SARS-CoV-2 infection in non-hospitalized patients. Med. Sci. Monit. 2022, 28, e935952. [Google Scholar] [CrossRef] [PubMed]
- Sharov, A.V.; Burkhanova, T.M.; Taskin Tok, T.; Babashkina, M.G.; Safin, D.A. Computational analysis of molnupiravir. Int. J. Mol. Sci. 2022, 23, 1508. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Molnupiravir: First Approval. Drugs 2022, 82, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Chokkakula, S.; Min, S.C.; Kim, B.K.; Choi, W.S.; Oh, S.; Yun, Y.S.; Kang, D.H.; Lee, O.J.; Kim, E.G.; et al. Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice. Antiviral. Res. 2022, 208, 105430. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet 2022, 400, 1213–1222. [Google Scholar]
- Drozdzal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybycinski, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Los, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updat. 2021, 59, 100794. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, J.C.; Chiu, C.W.; Lee, C.C.; Tsai, P.J.; Hsu, I.L.; Ko, W.C. Oral nirmatrelvir/ritonavir therapy for COVID-19: The dawn in the dark? Antibiotics 2022, 11, 220. [Google Scholar] [CrossRef]
- Ganatra, S.; Dani, S.S.; Ahmad, J.; Kumar, A.; Shah, J.; Abraham, G.M.; McQuillen, D.P.; Wachter, R.M.; Sax, P.E. Oral Nirmatrelvir and Ritonavir in Non-hospitalized Vaccinated Patients with COVID-19. Clin. Infect. Dis. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Zheng, Q.; Ma, P.; Wang, M.; Cheng, Y.; Zhou, M.; Ye, L.; Feng, Z.; Zhang, C. Efficacy and safety of paxlovid for COVID-19: A meta-analysis. J. Infect. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, X.; Yang, X.; Feng, T.; Duan, Z.; Wang, W.; Sun, Z.; Chen, L.; Nie, X.; Zhu, C.; et al. The efficacy of paxlovid in elderly patients infected with SARS-CoV-2 omicron variants: Results of a non-randomized clinical trial. Front. Med. 2022, 9, 980002. [Google Scholar] [CrossRef]
- Lo, H.S.; Hui, K.P.Y.; Lai, H.M.; He, X.; Khan, K.S.; Kaur, S.; Huang, J.; Li, Z.; Chan, A.K.N.; Cheung, H.H.; et al. Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. ACS Cent. Sci. 2021, 7, 792–802. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Noor, S.; Lysiuk, R.; Menzel, A.; Gasmi Benahmed, A.; Bjorklund, G. Chloroquine and hydroxychloroquine in the treatment of COVID-19: The never-ending story. Appl. Microbiol. Biotechnol. 2021, 105, 1333–1343. [Google Scholar] [CrossRef]
- Asrani, P.; Tiwari, K.; Eapen, M.S.; McAlinden, K.D.; Haug, G.; Johansen, M.D.; Hansbro, P.M.; Flanagan, K.L.; Hassan, M.I.; Sohal, S.S. Clinical features and mechanistic insights into drug repurposing for combating COVID-19. Int. J. Biochem. Cell Biol. 2022, 142, 106114. [Google Scholar] [CrossRef] [PubMed]
- Gotera, C. Treatment and research lines for the patient with COVID-19. What do we have and where are we going? Int. Braz. J. Urol. 2020, 46, 125–132. [Google Scholar] [CrossRef]
- Hache, G.; Rolain, J.M.; Gautret, P.; Deharo, J.C.; Brouqui, P.; Raoult, D.; Honore, S. Combination of hydroxychloroquine plus azithromycin as potential treatment for COVID-19 patients: Safety profile, drug interactions, and management of toxicity. Microb. Drug Resist. 2021, 27, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; He, F.; Souho, T.; Lamboni, L.; Ullah, M.W.; Boni, B.O.; Ahmed, A.A.Q.; Mukole, B.M.; Yang, G. Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed. Pharmacother. 2021, 133, 111008. [Google Scholar] [CrossRef] [PubMed]
- Freilich, D.; Victory, J.; Gadomski, A. Hydroxychloroquine/Chloroquine in COVID-19 With focus on hospitalized patients—A systematic review. medRxiv 2022. [Google Scholar] [CrossRef]
- Echeverria-Esnal, D.; Martin-Ontiyuelo, C.; Navarrete-Rouco, M.E.; De-Antonio Cusco, M.; Ferrandez, O.; Horcajada, J.P.; Grau, S. Azithromycin in the treatment of COVID-19: A review. Expert. Rev. Anti. Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifiro, G. Azithromycin in COVID-19 Patients: Pharmacological Mechanism, Clinical Evidence and Prescribing Guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Haydar, D.; Abdel-Latif, A.; Gensel, J.C.; Anstead, M.I.; Pitts, M.G.; Creameans, J.; Kopper, T.J.; Peng, C.; Feola, D.J. Immunomodulatory effects of azithromycin revisited: Potential applications to COVID-19. Front. Immunol. 2021, 12, 574425. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Ulrik, C.S.; Lapperre, T.S.; Bojesen, R.D.; Eklof, J.; Browatzki, A.; Wilcke, J.T.; Gottlieb, V.; Hakansson, K.E.J.; Tidemandsen, C.; et al. Azithromycin and hydroxychloroquine in hospitalised patients with confirmed COVID-19: A randomised double-blinded placebo-controlled trial. Eur. Respir. J. 2022, 59, 2100752. [Google Scholar] [CrossRef]
- Lamback, E.B.; Oliveira, M.A.; Haddad, A.F.; Vieira, A.F.M.; Neto, A.L.F.; Maia, T.D.S.; Chrisman, J.R.; Spineti, P.P.M.; Mattos, M.A.; Costa, E. Hydroxychloroquine with azithromycin in patients hospitalized for mild and moderate COVID-19. Braz. J. Infect. Dis. 2021, 25, 101549. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Kamal, T.B.; Sarker, M.M.R.; Zhou, J.R.; Rahman, S.M.A.; Mohamed, I.N. Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: Position standing in 2021. Front. Pharmacol. 2021, 12, 659577. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Rommasi, F.; Nasiri, M.J.; Mirsaeidi, M. Immunomodulatory agents for COVID-19 treatment: Possible mechanism of action and immunopathology features. Mol. Cell. Biochem. 2022, 477, 711–726. [Google Scholar] [CrossRef]
- Barkas, F.; Christaki, E.; Liberopoulos, E.; Kosmidou, M.; Milionis, H. Anakinra in COVID-19: A step closer to the cure. Eur. J. Intern. Med. 2022, 96, 113–114. [Google Scholar] [CrossRef]
- Kharazmi, A.B.; Moradi, O.; Haghighi, M.; Kouchek, M.; Manafi-Rasi, A.; Raoufi, M.; Shoaei, S.D.; Hadavand, F.; Nabavi, M.; Miri, M.M.; et al. A randomized controlled clinical trial on efficacy and safety of anakinra in patients with severe COVID-19. Immun. Inflamm. Dis. 2022, 10, 201–208. [Google Scholar] [CrossRef]
- Naveed, Z.; Sarwar, M.; Ali, Z.; Saeed, D.; Choudhry, K.; Sarfraz, A.; Sarfraz, Z.; Felix, M.; Cherrez-Ojeda, I. Anakinra treatment efficacy in reduction of inflammatory biomarkers in COVID-19 patients: A meta-analysis. J. Clin. Lab. Anal. 2022, 36, e24434. [Google Scholar] [CrossRef]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Calabrese, C.; Garofalo, E.; Bruni, A.; Vatrella, A.; Pelaia, G. Therapeutic role of tocilizumab in SARS-CoV-2-induced cytokine storm: Rationale and current evidence. Int. J. Mol. Sci. 2021, 22, 3059. [Google Scholar] [CrossRef] [PubMed]
- Vela, D.; Vela-Gaxha, Z.; Rexhepi, M.; Olloni, R.; Hyseni, V.; Nallbani, R. Efficacy and safety of tocilizumab versus standard care/placebo in patients with COVID-19; a systematic review and meta-analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 2022, 88, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Millan, M.A.; Mesa-Plaza, N.; Guerrero-Santillan, M.; Morales-Ortega, A.; Bernal-Bello, D.; Farfan-Sedano, A.I.; Garcia de Viedma-Garcia, V.; Velazquez-Rios, L.; Frutos-Perez, B.; De Ancos-Aracil, C.L.; et al. Prognostic factors and combined use of tocilizumab and corticosteroids in a Spanish cohort of elderly COVID-19 patients. J. Med. Virol. 2022, 94, 1540–1549. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Tirpude, N.V.; Sharma, S.; Padwad, Y.S.; Kumar, S. Pharmaco-immunomodulatory interventions for averting cytokine storm-linked disease severity in SARS-CoV-2 infection. Inflammopharmacology 2022, 30, 23–49. [Google Scholar] [CrossRef]
- Quek, E.; Tahir, H.; Kumar, P.; Hastings, R.; Jha, R. Treatment of COVID-19: A review of current and prospective pharmacotherapies. Br. J. Hosp. Med. 2021, 82, 1–9. [Google Scholar] [CrossRef]
- Iglesias Gomez, R.; Mendez, R.; Palanques-Pastor, T.; Ballesta-Lopez, O.; Borras Almenar, C.; Megias Vericat, J.E.; Lopez-Briz, E.; Font-Noguera, I.; Menendez Villanueva, R.; Roman Iborra, J.A.; et al. Baricitinib against severe COVID-19: Effectiveness and safety in hospitalised pretreated patients. Eur. J. Hosp. Pharm. 2022, 29, e41–e45. [Google Scholar] [CrossRef]
- Recovery Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Akbarzadeh-Khiavi, M.; Torabi, M.; Rahbarnia, L.; Safary, A. Baricitinib combination therapy: A narrative review of repurposed Janus kinase inhibitor against severe SARS-CoV-2 infection. Infection 2022, 50, 295–308. [Google Scholar] [CrossRef]
- Akter, F.; Araf, Y.; Hosen, M.J. Corticosteroids for COVID-19: Worth it or not? Mol. Biol. Rep. 2022, 49, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2021, 894, 173854. [Google Scholar] [CrossRef] [PubMed]
- Vecchie, A.; Batticciotto, A.; Tangianu, F.; Bonaventura, A.; Pennella, B.; Abenante, A.; Corso, R.; Grazioli, S.; Mumoli, N.; Para, O.; et al. High-dose dexamethasone treatment for COVID-19 severe acute respiratory distress syndrome: A retrospective study. Intern. Emerg. Med. 2021, 16, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, L.; Palumbo, F.P.; Ardita, G.; Antignani, P.L.; Arosio, E.; Failla, G. Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J. Vasc. Surg. Venous. Lymphat. Disord. 2020, 8, 711–716. [Google Scholar] [CrossRef]
- Braz-de-Melo, H.A.; Faria, S.S.; Pasquarelli-do-Nascimento, G.; Santos, I.O.; Kobinger, G.P.; Magalhaes, K.G. The use of the anticoagulant heparin and corticosteroid dexamethasone as prominent treatments for COVID-19. Front. Med. 2021, 8, 615333. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, P. Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorg. Chem. 2022, 121, 105663. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, S.; Gan, L.; Wang, Z.; Peng, S.; Li, Q.; Liu, H.; Liu, X.; Wang, Z.; Shi, Q.; et al. Use of non-steroidal anti-inflammatory drugs and adverse outcomes during the COVID-19 pandemic: A systematic review and meta-analysis. EClinicalMedicine 2022, 46, 101373. [Google Scholar] [CrossRef]
- Chen, J.S.; Alfajaro, M.M.; Chow, R.D.; Wei, J.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Nonsteroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J. Virol. 2021, 95, e00014-21. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef]
- Hu, Q.; Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Zhang, Y.W.; Huang, P.; Ge, G.B. The SARS-CoV-2 main protease (M(pro)): Structure, function, and emerging therapies for COVID-19. MedComm 2022, 3, e151. [Google Scholar] [CrossRef]
- Tyndall, J.D.A. S-217622, a 3CL Protease inhibitor and clinical candidate for SARS-CoV-2. J. Med. Chem. 2022, 65, 6496–6498. [Google Scholar] [CrossRef] [PubMed]
- Unoh, Y.; Uehara, S.; Nakahara, K.; Nobori, H.; Yamatsu, Y.; Yamamoto, S.; Maruyama, Y.; Taoda, Y.; Kasamatsu, K.; Suto, T.; et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease Inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022, 65, 6499–6512. [Google Scholar] [CrossRef]
- Neamati, N. Advances toward COVID-19 therapies special issue. J. Med. Chem. 2022, 65, 2713–2715. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.T.; Enguita, F.J.; Taylor, D.; Griffin, R.J.; Priebe, W.; Emmett, M.R.; Sajadi, M.M.; Harris, A.D.; Clement, J.; Dybas, J.M.; et al. Role of miR-2392 in driving SARS-CoV-2 infection. Cell Rep. 2021, 37, 109839. [Google Scholar] [CrossRef] [PubMed]
- McCollum, C.R.; Courtney, C.M.; O’Connor, N.J.; Aunins, T.R.; Ding, Y.; Jordan, T.X.; Rogers, K.L.; Brindley, S.; Brown, J.M.; Nagpal, P.; et al. Nanoligomers targeting human miRNA for the treatment of severe COVID-19 are safe and nontoxic in mice. ACS Biomater. Sci. Eng. 2022, 8, 3087–3106. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Maines, L.W.; Keller, S.N.; Katz Ben-Yair, V.; Fathi, R.; Plasse, T.F.; Levitt, M.L. Recent progress in the development of opaganib for the treatment of COVID-19. Drug Des. Devel. Ther. 2022, 16, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhao, C.; Liu, Y.; Zhang, C.; Yang, G.; Yang, J.; Wang, Z.; Wang, C.; Tu, C.; Guo, Z.; et al. RNA G-quadruplex formed in SARS-CoV-2 used for COVID-19 treatment in animal models. Cell Discov. 2022, 8, 86. [Google Scholar] [CrossRef]

| Structure | Name | Activity |
|---|---|---|
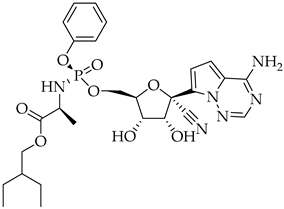 | Remdesivir | Antiviral viral RdRp inhibitor |
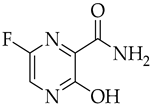 | Favipiravir | Antiviral viral RdRp inhibitor |
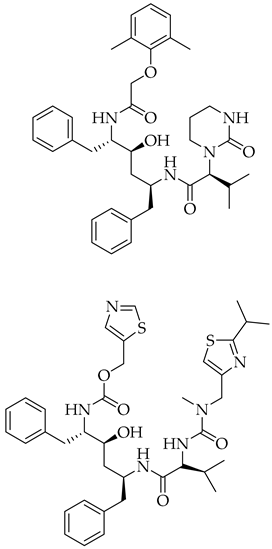 | Lopinavir + Ritonavir | Antiviral viral protease inhibitor |
 | Molnupiravir | Antiviral viral RdRp inhibitor |
 | Paxlovid (ritonavir + nirmatrelvir) | Antiviral viral protease inhibitor |
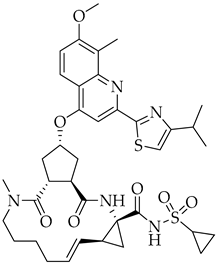 | Simeprevir | Antiviral NS3/4A protease inhibitor |
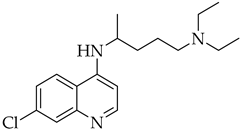 | Chloroquine | Antimalarial increase in endosomal pH → block of the uncoating of the virus |
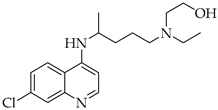 | Hydroxychloroquine | Antimalarial increase in endosomal pH → block of the uncoating of the virus |
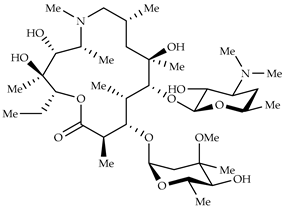 | Azithromycin | Antimicrobial increase in endosomal pH → block of the uncoating of the virus |
 | Anakinra | Interleukin-1 inhibitor |
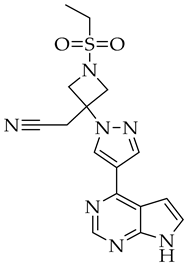 | Baricitinib | JAK inhibitor |
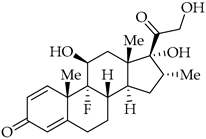 | Dexamethasone | Corticosteroid |
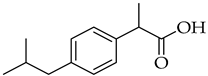 | Ibuprofen | NSAID |
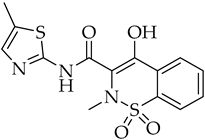 | Meloxicam | NSAID |
| Structure | Name | Activity |
|---|---|---|
 | S-217622 | SARS-CoV-2 3CL protease inhibitor |
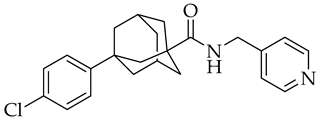 | Opaganib | Sphingosine kinase-2, dihydroceramide desaturase and glucosylceramide synthase inhibitor |
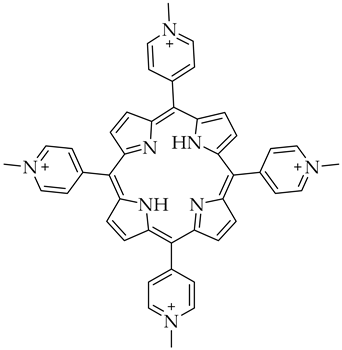 | 5,10,15,20-tetrakis-(N-methyl-4-pyridyl)porphine (TMPyP4) | G-quadruplexes specific ligand |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; Iacopetta, D.; Sinicropi, M.S.; Andreu, I.; Mariconda, A.; Saturnino, C.; Giuzio, F.; Longo, P.; Aquaro, S.; Catalano, A. Drugs for COVID-19: An Update. Molecules 2022, 27, 8562. https://doi.org/10.3390/molecules27238562
Ceramella J, Iacopetta D, Sinicropi MS, Andreu I, Mariconda A, Saturnino C, Giuzio F, Longo P, Aquaro S, Catalano A. Drugs for COVID-19: An Update. Molecules. 2022; 27(23):8562. https://doi.org/10.3390/molecules27238562
Chicago/Turabian StyleCeramella, Jessica, Domenico Iacopetta, Maria Stefania Sinicropi, Inmaculada Andreu, Annaluisa Mariconda, Carmela Saturnino, Federica Giuzio, Pasquale Longo, Stefano Aquaro, and Alessia Catalano. 2022. "Drugs for COVID-19: An Update" Molecules 27, no. 23: 8562. https://doi.org/10.3390/molecules27238562
APA StyleCeramella, J., Iacopetta, D., Sinicropi, M. S., Andreu, I., Mariconda, A., Saturnino, C., Giuzio, F., Longo, P., Aquaro, S., & Catalano, A. (2022). Drugs for COVID-19: An Update. Molecules, 27(23), 8562. https://doi.org/10.3390/molecules27238562












