All-Polymer Piezo-Composites for Scalable Energy Harvesting and Sensing Devices
Abstract
1. Introduction
2. Results and Discussion
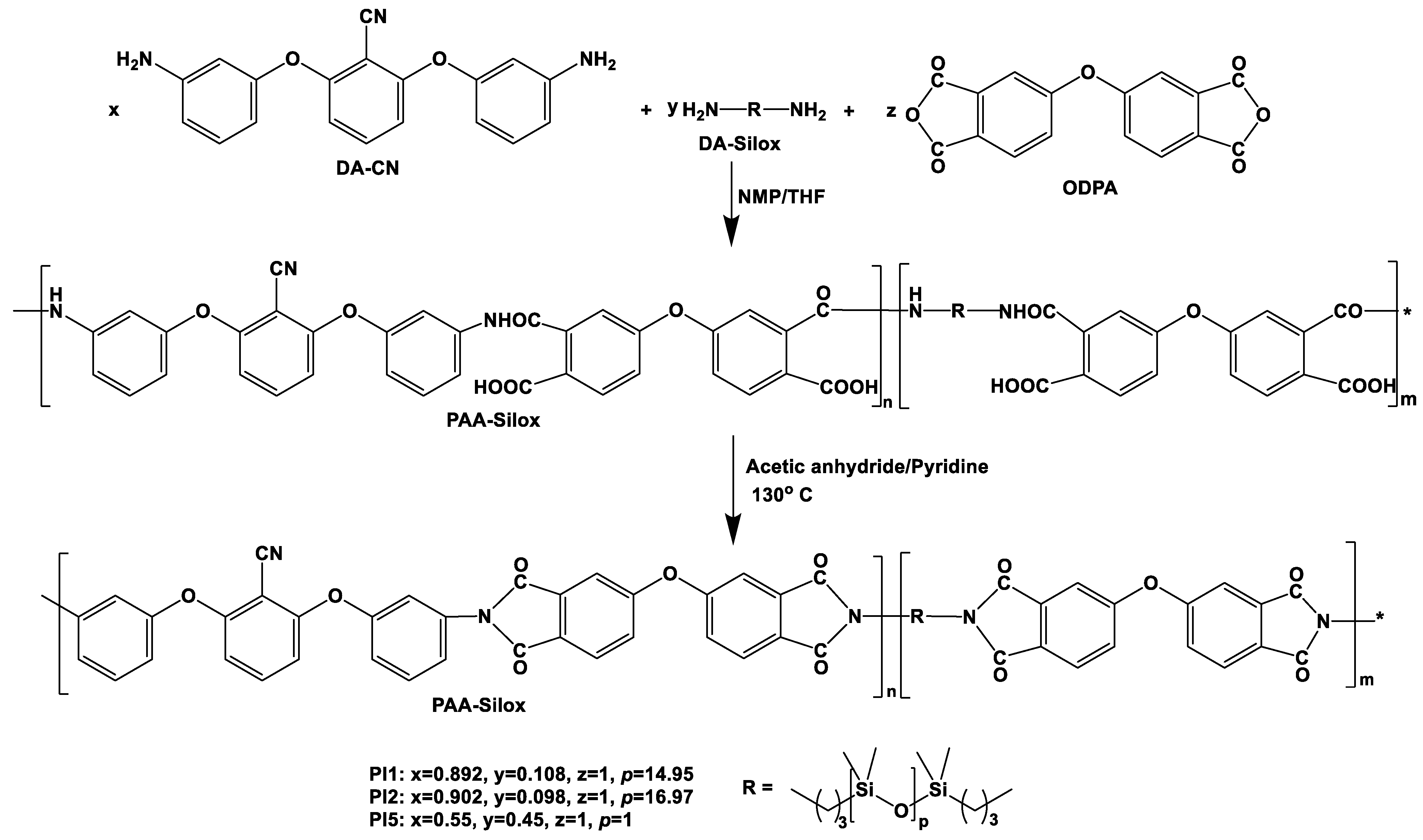
2.1. Synthesis of Poly(siloxane-imide) Copolymers
2.2. Preparation of Elastomeric Composites
2.3. Characterization of the Elastomeric Molecular Composites
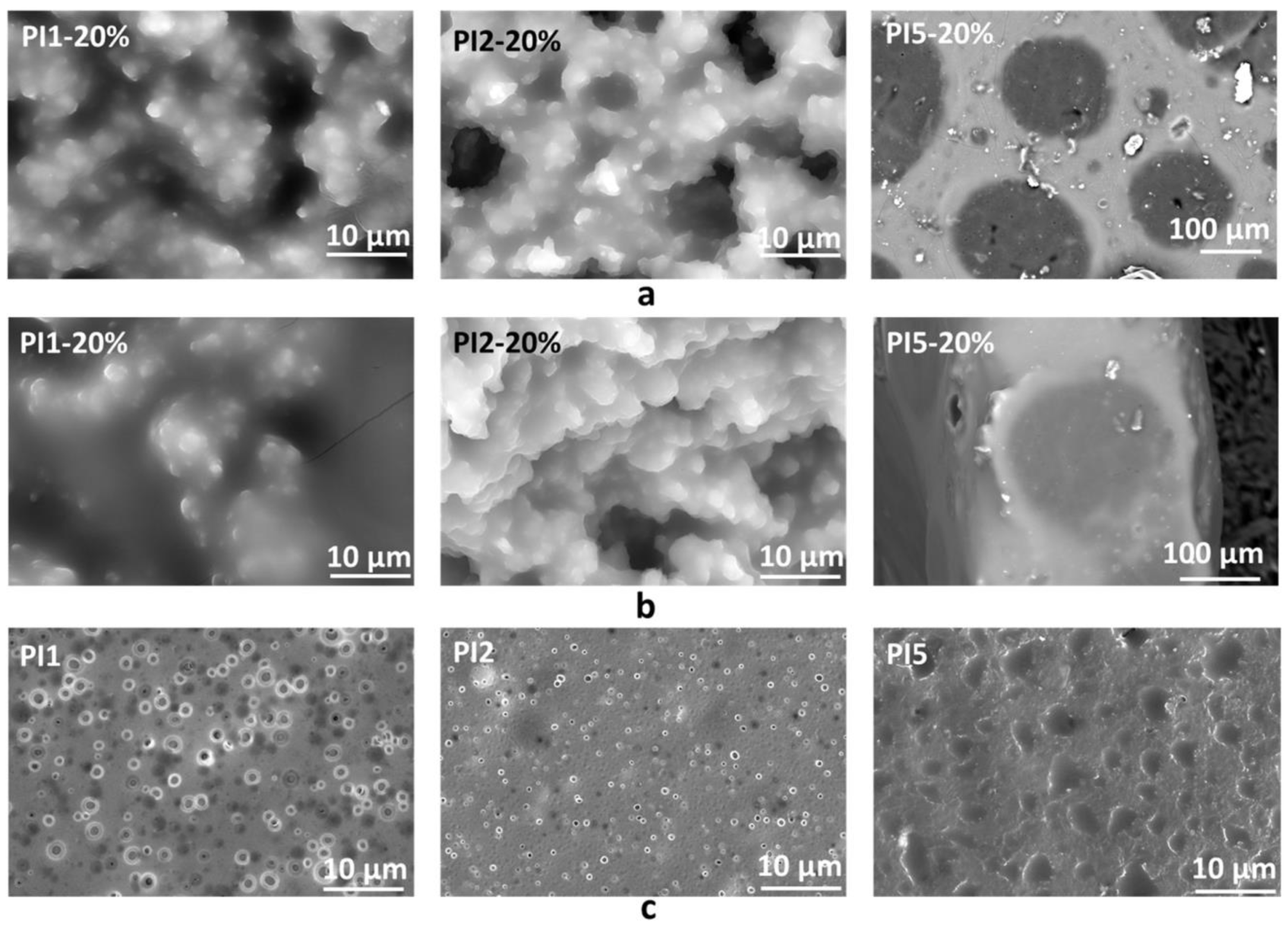
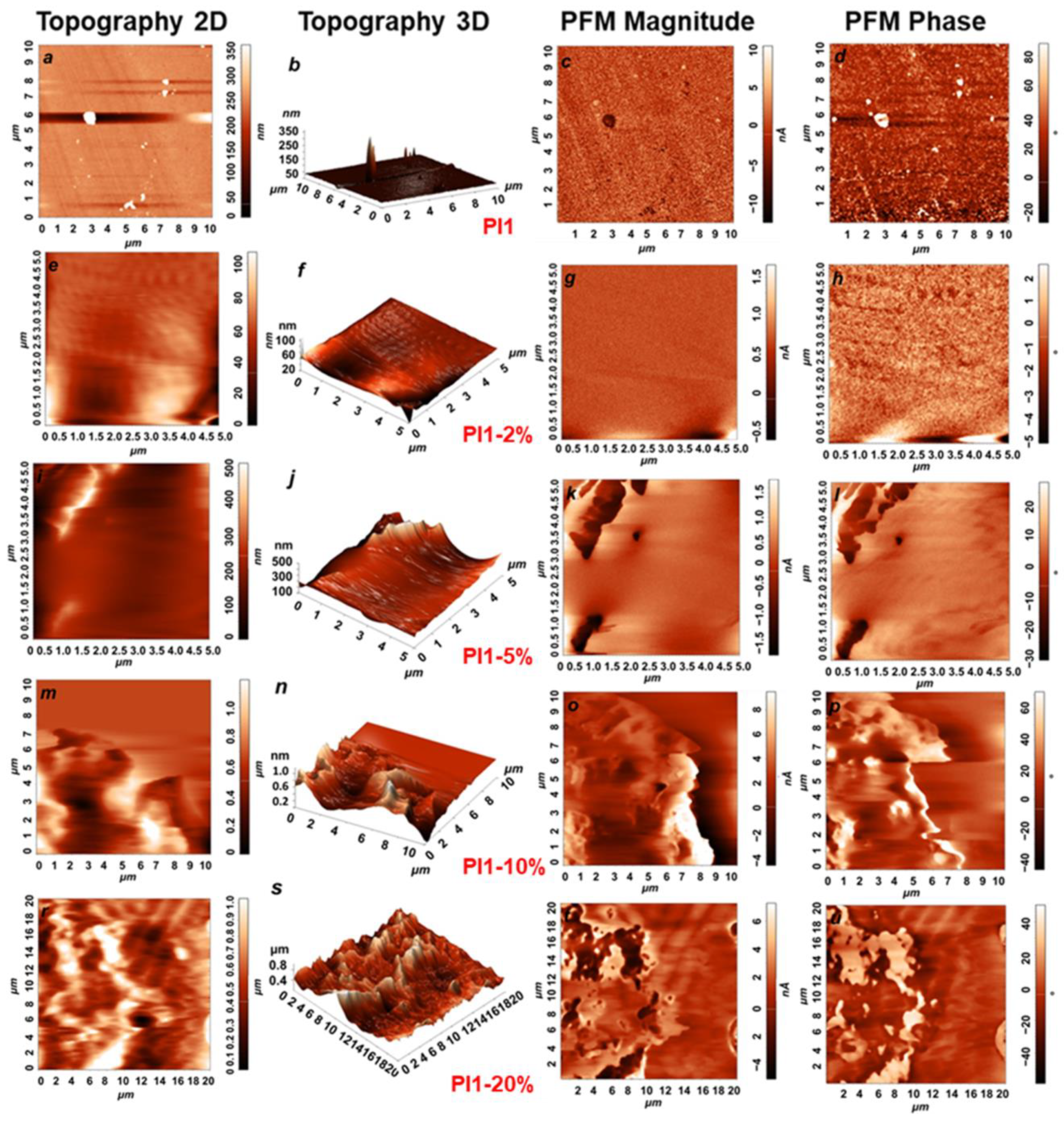
2.3.1. Scanning Electron Microscopy
2.3.2. Dynamic Water Vapor Sorption
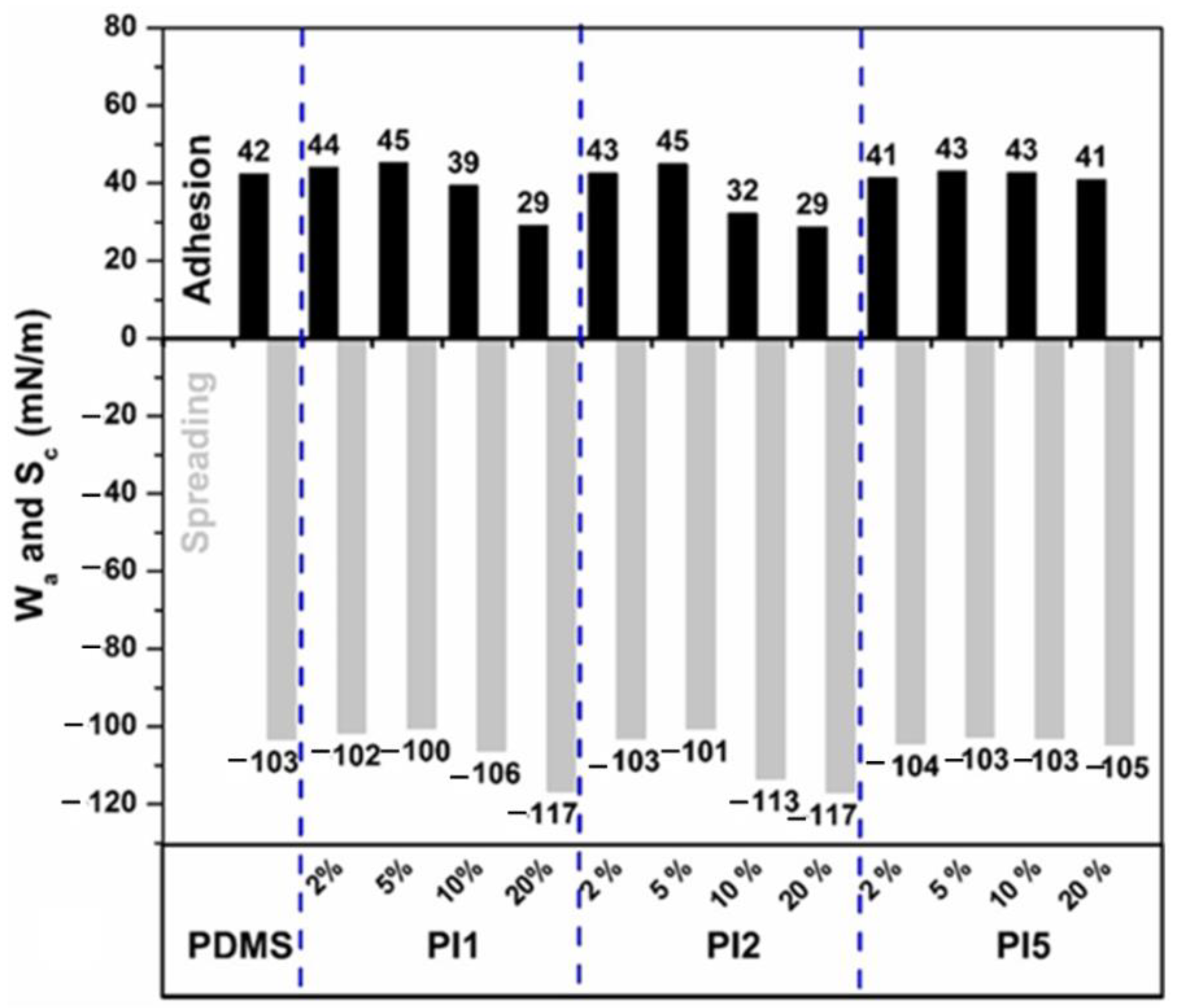
2.3.3. Static Contact Angle and Surface Energy
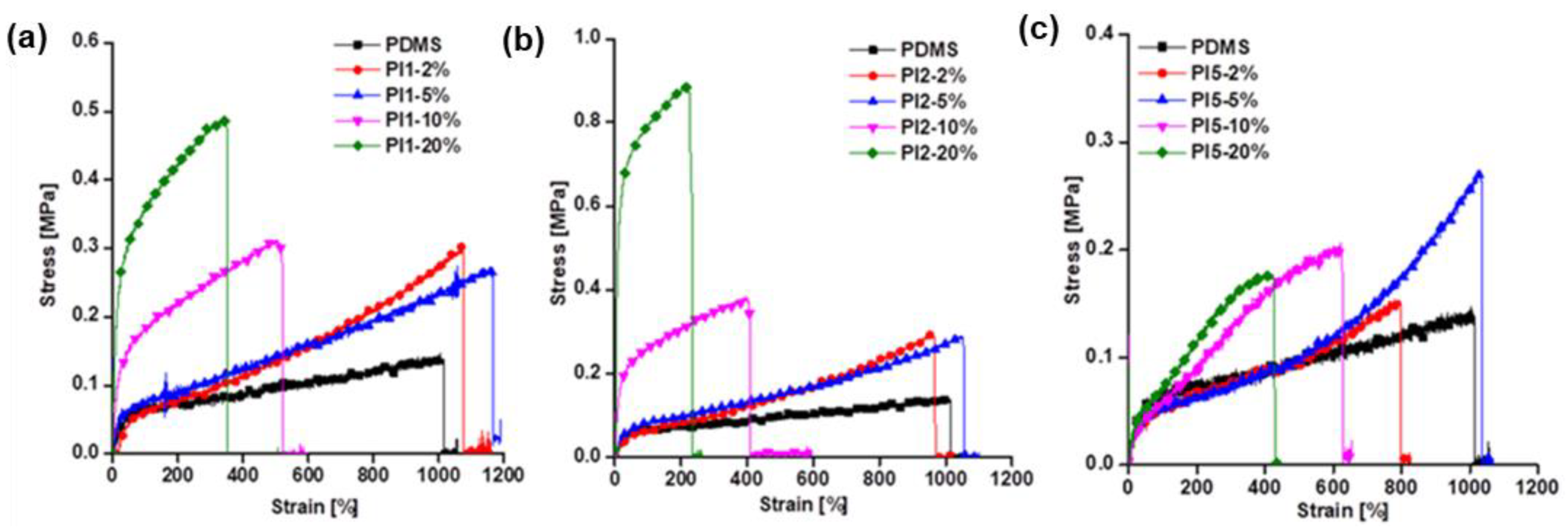
2.3.4. Mechanical Tests
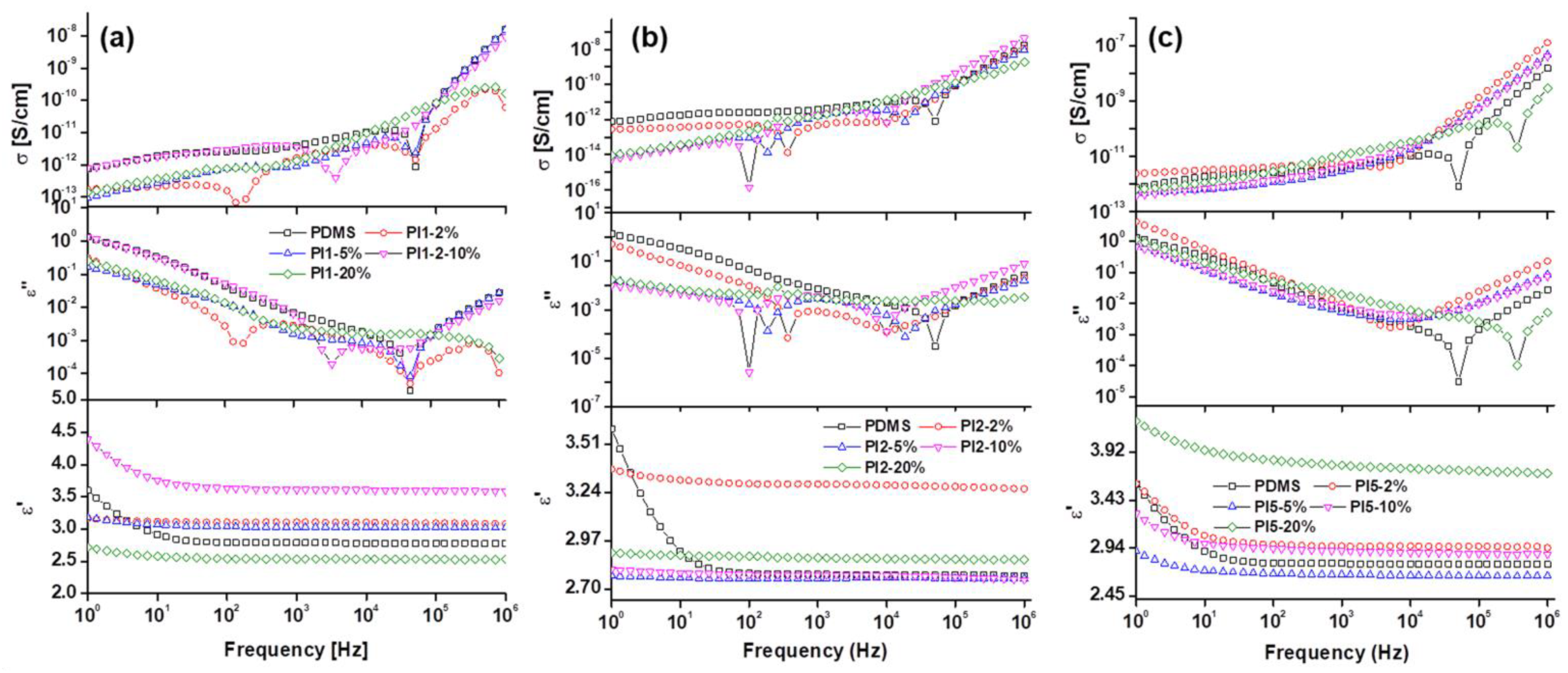
2.3.5. Dielectric Spectroscopy
2.4. Evaluation of Some Functional Capabilities of Elastomeric Composites
2.4.1. Electromechanical Actuation
2.4.2. Piezoelectric Response
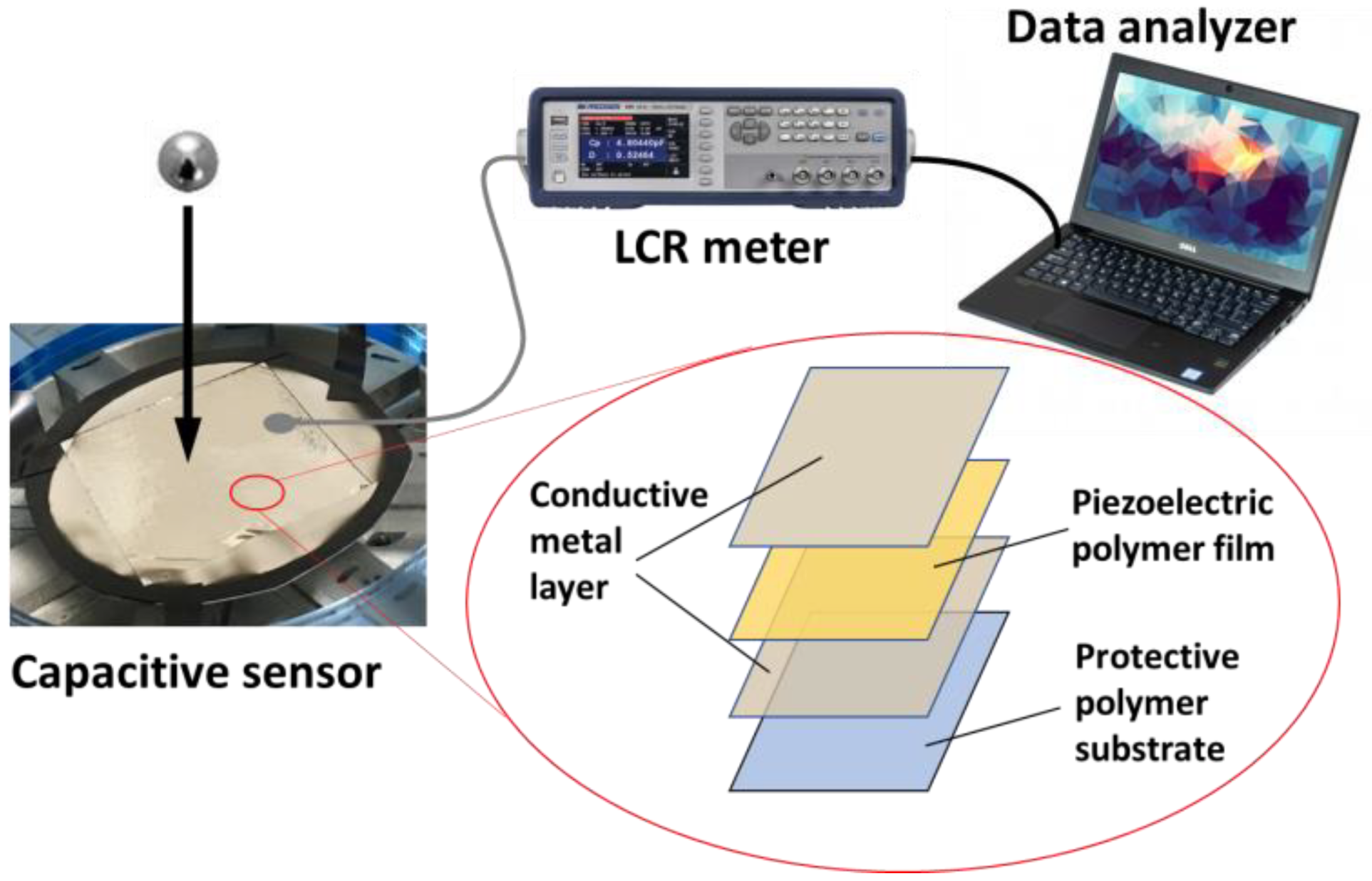
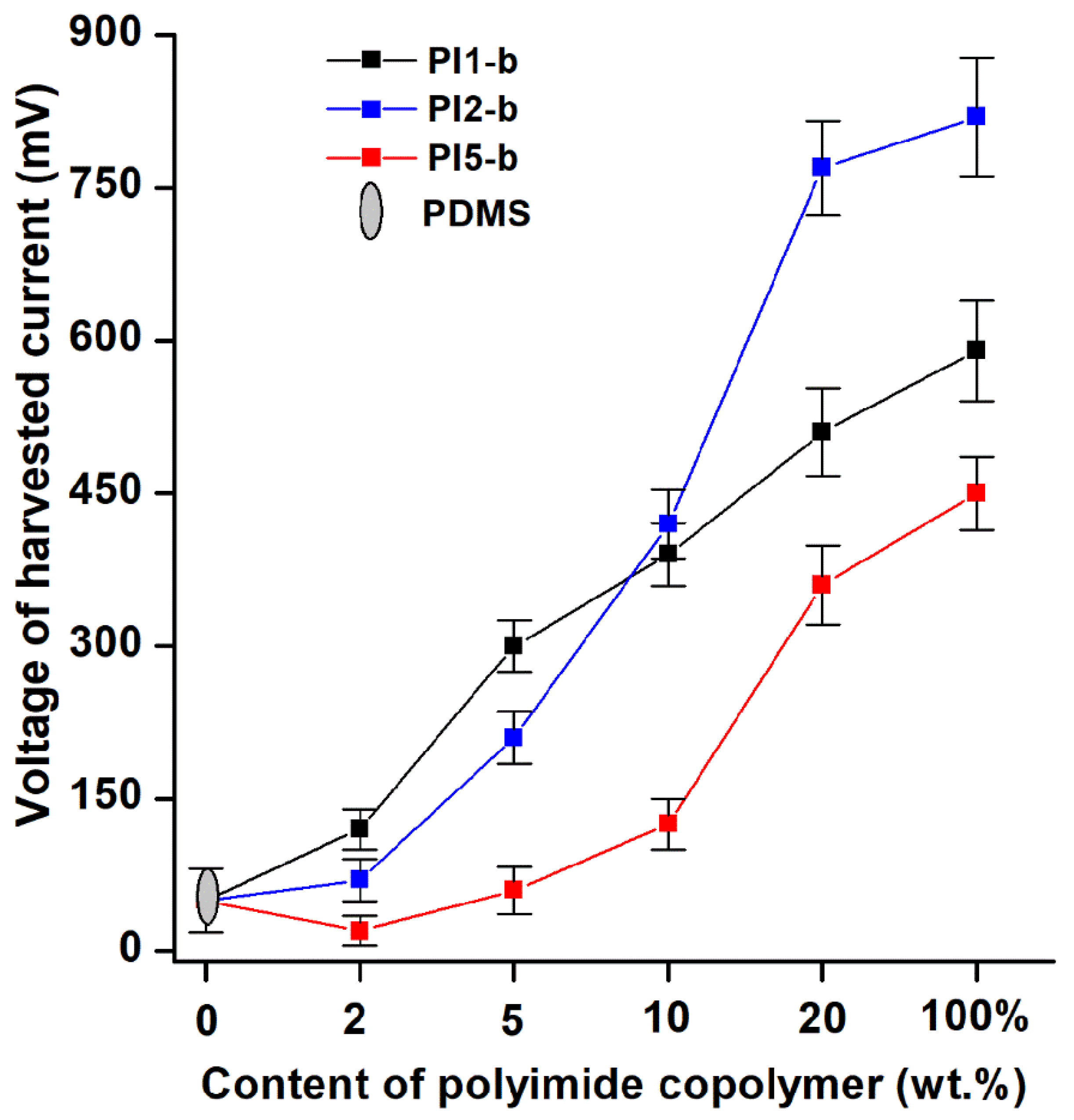
2.4.3. Pressure Sensors and Energy Harvesting Data Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods of Characterization
3.3. Procedures
3.3.1. Synthesis of Poly(siloxane-imide) Copolymers PI1, PI2, and PI5
3.3.2. Preparation of Elastomeric Composites
3.3.3. Preparation of Electroactive Piezoelectric Surfaces and Humidity Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chakrabarty, A.; Çağın, T. Thermo-mechanical properties of a piezoelectric polyimide carbon nanotube composite: Assessment of composite theories. Comput. Mater. Sci. 2014, 92, 185–191. [Google Scholar] [CrossRef]
- Nassar, J.M.; Cordero, M.D.; Kutbee, A.T.; Karimi, M.A.; Torres Sevilla, G.A.; Hussain, A.M.; Shamim, A.; Hussain, M.M. Paper skin multisensory platform for simultaneous environmental monitoring. Adv. Mater. Technol. 2016, 1, 1600004. [Google Scholar] [CrossRef]
- Chen, D.; Pei, Q. Electronic muscles and skins: A review of soft sensors and actuators. Chem. Rev. 2017, 117, 11239–11268. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Tan, Y.J.; Yao, H.; Li, S.; See, H.H.; Hon, M.; Ng, K.A.; Xiong, B.; Ho, J.S.; Tee, B.C.K. A neuro-inspired artificial peripheral nervous system for scalable electronic skins. Sci. Robot. 2019, 4, eaax2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Cho, S.; Shin, Y.-E.; Lee, H.; Kim, J.; Myoung, J.; Cho, S.; Kang, S.; Baig, C.; et al. Flexible ferroelectric sensors with ultrahigh pressure sensitivity and linear response over exceptionally broad pressure range. ACS Nano 2018, 12, 4045–4054. [Google Scholar] [CrossRef]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef]
- Tee, B.C.-K.; Chortos, A.; Berndt, A.; Nguyen, A.K.; Tom, A.; McGuire, A.; Lin, Z.C.; Tien, K.; Bae, W.-G.; Wang, H.; et al. A skin-inspired organic digital mechanoreceptor. Science 2015, 350, 313–316. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Lee, Y.; Lee, H.S.; Ko, H. Fingertip skin-inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 2015, 1, e1500661. [Google Scholar] [CrossRef]
- Field, T. Touch; MIT Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Tawil, D.S.; Rye, D.; Velonaki, M. Interpretation of the modality of touch on an artificial arm covered with an EIT-based sensitive skin. Int. J. Robot. Res. 2012, 31, 1627–1642. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, H.; Liu, Y.; Qi, D.; Liu, Z.; Wang, H.; Yu, J.; Sherburne, M.; Wang, Z.; Chen, X. Skin-inspired haptic memory arrays with an electrically reconfigurable architecture. Adv. Mater. 2016, 28, 1559–1566. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Ho, J.C.; Ko, H.; Gillies, A.G.; Leu, P.W.; Fearing, R.S.; Javey, A. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nat. Mater. 2010, 9, 821–826. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef]
- He, H.; Fu, Y.; Zang, W.; Wang, Q.; Xing, L.; Zhang, Y.; Xue, X. A flexible self-powered T-ZnO/PVDF/fabric electronic-skin with multi-functions of tactile-perception, atmosphere-detection and self-clean. Nano Energy 2017, 31, 37–48. [Google Scholar] [CrossRef]
- Yin, F.; Yang, J.; Peng, H.; Yuan, W. Flexible and highly sensitive artificial electronic skin based on graphene/polyamide interlocking fabric. J. Mater. Chem. C 2018, 6, 6840–6846. [Google Scholar] [CrossRef]
- Wagner, C.R.; Lederman, S.J.; Howe, R.D. A tactile shape display using RC servomotors. In Proceedings of the 10th Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Orlando, FL, USA, 24–25 March 2002. [Google Scholar]
- Piezoelectric and ferroelectric properties of P(VDF-TrFE) copolymers and their application to ultrasonic transducers. In Medical Applications of Piezoelectric Polymers Gordon and Breach; Galletti, P.M., De Rossi, D.E., De Reggi, A.S., Eds.; Taylor&Francis: Gordon and Breach, NY, USA, 1988. [Google Scholar]
- Fukada, E. History and recent progress in piezoelectric polymers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1110–1119. [Google Scholar] [CrossRef]
- Fukada, E. Recent developments of polar piezoelectric polymers. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1110–1119. [Google Scholar] [CrossRef]
- Lang, S.B. Guide to the literature of piezoelectricity and pyroelectricity23. Ferroelectrics 2005, 321, 91–204. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Sameoto, D.; Evoy, S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater. Struct. 2014, 23, 033001. [Google Scholar] [CrossRef]
- Ounaies, Z.; Park, C.; Harrison, J.; Lillehei, P. Evidence of piezoelectricity in SWNT-polyimide and SWNT-PZT-polyimide composites. J. Thermoplast. Compos. Mater. 2008, 21, 393–409. [Google Scholar] [CrossRef]
- Harrison, J.S.; Ounaies, Z. Piezoelectric polymers Encyclopedia of Polymer Science and Technology; Wiley: New York, NY, USA, 2002; pp. 474–498. [Google Scholar]
- Hearth, D.R.; Wirth, J.G. Aminophenoxy benzonitriles. US Patent 3763211, 2 October 1973. [Google Scholar]
- Cazacu, M.; Vlad, A.; Simionescu, M.; Racles, C.; Marcu, M. Incorporation of the siloxanes in hydrolytically degradable organic structures. II. Segmented siloxane-imide poly(anhydride)s. Macromol. Sci. Pure Appl. Chem. 2002, 39, 1487–1499. [Google Scholar] [CrossRef]
- Song, K.; Cho, N.-K.; Park, K.; Kim, C.-S. Investigating mechanical behaviours of PDMS films under cyclic loading. Polymers 2022, 14, 2373. [Google Scholar] [CrossRef] [PubMed]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S. Silicone–barium titanate composites with increased electromechanical sensitivity. The effects of the filler morphology. RSC Adv. 2014, 4, 58522–58529. [Google Scholar] [CrossRef]
- Khan, S.; Scholz, D.; Ordonez, J.S.; Stieglitz, T. PDMS gasket underfill for long-term insulation of high-density interconnections in active implantable medical devices. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2941–2944. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, D.-S.; Oyunbaatar, N.-E.; Shanmugasundaram, A.; Kim, E.-S.; Lee, D.-W. On-stage bioreactor platform integrated with nano-patterned and gold-coated PDMS diaphragm for live cell stimulation and imaging. Mater. Sci. Eng. C 2021, 118, 111355. [Google Scholar] [CrossRef]
- Janegova, K.; Sysel, P.; Kulhankova, H.; Perfilov, V.A.; Bernauer, M.; Fila, V. Poly(imide-siloxane) films with controlled thickness. J. Appl. Polym. Sci. 2021, 138, e49893. [Google Scholar] [CrossRef]
- Qi, H.; Wang, X.; Zhu, T.; Li, J.; Xiong, L.; Liu, F. Low dielectric poly(imide siloxane) films enabled by a well-defined disiloxane-linked alkyl diamine. ACS Omega 2019, 4, 22143–22151. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. London. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Contact Angle, Wettability, and Adhesion; Fowkes, F.M., Ed.; American Chemical Society: Washington, DC, USA, 1964; pp. 99–111. [Google Scholar]
- Blanco, D.; Rivera, N.; Oulego, P.; Díaz, M.; González, R.; Battez, A.H. Novel fatty acid anion-based ionic liquids: Contact angle, surface tension, polarity fraction and spreading parameter. J. Mol. Liq. 2019, 288, 110995. [Google Scholar] [CrossRef]
- Dupré, A.; Dupré, P. Théorie Mécanique de la Chaleur; Gautheir-Villars: Paris, France, 1869. [Google Scholar]
- Bao, L.; Fan, H.; Chen, Y.; Yan, J.; Yang, T.; Guo, Y. Effect of surface free energy and wettability on the adhesion property of waterborne polyurethane adhesive. RSC Adv. 2016, 6, 99346–99352. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The correlation between the surface energy, the contact angle and the spreading parameter, and their relevance for the wetting behaviour of DLC with lubricating oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Terzic, I.; Meereboer, L.N.; Acuautla, M.; Portale, G.; Loos, K. Electroactive materials with tunable response based on block copolymer self-assembly. Nat. Commun. 2019, 10, 601. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Chu, T.; Yu, M.; Yang, Y. An electrodeposited lanthanide MOF thin film as a luminescent sensor for carbonate detection in aqueous solution. J. Mater. Chem. C 2014, 2, 8683–8690. [Google Scholar] [CrossRef]
- Yan, J.; Kang, C.; Bian, Z.; Jin, R.; Ma, X.; Gao, L. Supramolecular self-assembly of chiral polyimides driven by repeat units and end groups. New J. Chem. 2017, 41, 14723–14729. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; He, J.; Liu, M.; Hu, G.-H.; Dang, Z.-M. Synthesis, nanostructures and dielectric properties of novel liquid crystalline block copolymers. Polym. Chem. 2014, 5, 2513–2520. [Google Scholar] [CrossRef]
- Bele, A.; Dascalu, M.; Tugui, C.; Stiubianu, G.T.; Varganici, C.-D.; Racles, C.; Cazacu, M.; Skov, A.L. Soft silicone elastomers exhibiting large actuation strains. J. Appl. Polym. Sci. 2022, 139, 52261. [Google Scholar] [CrossRef]
- Tugui, C.; Bele, A.; Tiron, V.; Hamciuc, E.; Varganici, C.D.; Cazacu, M. Dielectric elastomers with dual piezo-electrostatic response optimized through chemical design for electromechanical transducers. J. Mater. Chem. C 2017, 5, 824–834. [Google Scholar] [CrossRef]
- Racles, C.; Dascalu, M.; Bele, A.; Tiron, V.; Asandulesa, M.; Tugui, C.; Vasiliu, A.L.; Cazacu, M. All-silicone elastic composites with counter-intuitive piezoelectric response, designed for electromechanical applications. J. Mater. Chem. C 2017, 5, 6997. [Google Scholar] [CrossRef]
- Iacob, M.; Tugui, C.; Tiron, V.; Bele, A.; Vlad, S.; Vasiliu, T.; Cazacu, M.; Vasiliu, A.L.; Racles, C. Iron oxide nanoparticles as dielectric and piezoelectric enhancers for silicone elastomers. Smart Mater. Struct. 2017, 26, 105046. [Google Scholar] [CrossRef]
- Racles, C.; Ursu, C.; Dascalu, M.; Asandulesa, M.; Tiron, V.; Bele, A.; Tugui, C.; Teodoroff-Onesim, S. Multi-stimuli responsive free-standing films of DR1- grafted silicones. Chem. Eng. J. 2020, 401, 126087. [Google Scholar] [CrossRef]
- Alexe, M.; Gruverman, A. Nanoscale Characterization of Ferroelectric Materials; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Park, C.; Ounaies, Z.; Wise, K.E.; Harrison, J.S. In situ poling and imidization of amorphous piezoelectric polyimides. Polymer 2004, 45, 5417–5425. [Google Scholar] [CrossRef]
- Cazacu, M.; Antohi, M.; Racles, C.; Vlad, A.; Forna, N. Silicone-based Composite for Relining of Removable Dental Prosthesis. J. Compos. Mater. 2009, 43, 2045. [Google Scholar] [CrossRef]
- Carpi, F.; Anderson, I.; Bauer, S.; Frediani, G.; Gallone, G.; Gei, M.; Graaf, C.; Jean-Mistral, C.; Kaal, W.; Kofod, G.; et al. Standards for dielectric elastomer transducers. Smart Mater. Struct. 2015, 24, 105025. [Google Scholar] [CrossRef]
- Iacob, M.; Tiron, V.; Stiubianu, G.-T.; Dascalu, M.; Hernandez, L.; Varganici, C.-D.; Tugui, C.; Cazacu, M. Bentonite as an active natural filler for silicone leading to piezoelectric-like response material. J. Mater. Res. Technol. 2022, 17, 79–94. [Google Scholar] [CrossRef]
- Stiubianu, G.; Bele, A.; Cazacu, M.; Racles, C.; Vlad, S.; Ignat, M. Dielectric silicone elastomers with mixed ceramic nanoparticles. Mater. Res. Bull. 2015, 71, 67–74. [Google Scholar] [CrossRef]
- Pulyalina, A.; Tian, N.; Senchukova, A.; Faykov, I.; Ryabikova, M.; Novikov, A.; Saprykina, N.; Polotskaya, G. Application of cyclized polyacrylonitrile for ultrafiltration membrane fouling mitigation. Membranes 2022, 12, 489. [Google Scholar] [CrossRef]
- Park, J.; Kang, Y.-C. Effect of radio frequency power on the physicochemical properties of MoS2 films obtained by rf magnetron sputtering. Bull. Korean Chem. Soc. 2016, 37, 1326–1330. [Google Scholar] [CrossRef]
- Choi, S.; Kang, J.; Park, J.; Kang, Y.-C. Tin nitride thin films fabricated by reactive radio frequency magnetron sputtering at various nitrogen gas ratios. Thin. Solid. Films. 2014, 571, 84–89. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, D.; Zheng, C.; Yao, Y.; Long, Z. Facile fabrication of biomimetic superoleophobic composite coating via Schiff base reaction and self-assembly. Prog. Org. Coatings. 2020, 142, 105568. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fujita, H.; Kimura, Y.; Hattori, Y.; Kitamura, M. Wide-range work function tuning in gold surfaces modified with fluorobenzenethiols toward application to organic thin-film transistors. Flex. Print. Electron. 2020, 5, 014011. [Google Scholar] [CrossRef]



| Sample | wt% PIa Relative to Siloxane | PDMS, g | PIa, g | TEOS, g |
|---|---|---|---|---|
| PDMS | 0 | 0.5 | 0 | 0.035 |
| PIa-2% | 2 | 0.5 | 0.0125 | 0.035 |
| PIa-5% | 5 | 0.5 | 0.025 | 0.035 |
| PIa-10% | 10 | 0.5 | 0.05 | 0.035 |
| PIa-20% | 20 | 0.5 | 0.1 | 0.035 |
| Sample | Contact Angle (Degrees) | ||
|---|---|---|---|
| Water | Ethylene Glycol | ||
| PDMS | 115 ± 0.37 | 103 ± 0.53 | |
| PI a | 93 ± 1.33 | 69 ± 2.67 | |
| PI1 | 98 ± 1.47 | 98 ± 2.01 | |
| PI2 | 105 ± 3.32 | 88 ± 0.71 | |
| PI5 | 91 ± 1.72 | 71 ± 1.33 | |
| PI1 | 2% 5% 10% 20% | 113 ± 1.52 112 ± 0.59 117 ± 0.30 127 ± 4.07 | 107 ± 0.76 106 ± 1.55 110 ± 0.97 127 ± 2.12 |
| PI2 | 2% 5% 10% 20% | 115 ± 1.84 112 ± 2.23 124 ± 3.43 127 ± 3.19 | 107 ± 0.58 105 ± 2.98 113 ± 1.32 125 ± 3.82 |
| PI5 | 2% 5% 10% 20% | 116 ± 0.28 114 ± 1.17 114 ± 1.13 116 ± 0.57 | 110 ± 0.28 109 ± 0.67 109 ± 0.55 109 ± 1.24 |
| Sample | Sm,% a | Y, MPa b | Tnm, MPa c | UTT, J/m3 d |
|---|---|---|---|---|
| PDMS | 1015 | 0.23 | 0.14 | 9.6 |
| PI1-2% | 1075 | 0.23 | 0.3 | 16.3 |
| PI1-5% | 1165 | 0.54 | 0.27 | 18.3 |
| PI1-10% | 500 | 0.88 | 0.3 | 12.1 |
| PI1-20% | 340 | 2.24 | 0.48 | 13.5 |
| PI2-2% | 960 | 0.22 | 0.29 | 14.4 |
| PI2-5% | 1053 | 0.3 | 0.28 | 16.9 |
| PI2-10% | 400 | 1.4 | 0.38 | 12.1 |
| PI2-20% | 220 | 6.6 | 0.88 | 17.4 |
| PI5-2% | 800 | 0.19 | 0.15 | 7.1 |
| PI5-5% | 1032 | 0.2 | 0.27 | 12.5 |
| PI5-10% | 623 | 0.2 | 0.2 | 7.8 |
| PI5-20% | 425 | 0.3 | 0.17 | 4.9 |
| Sample | R (nm) | d33 (pm/V) |
|---|---|---|
| PDMS | 0.5 | 0 |
| PI | 2.1 | 2.4 ± 0.5 |
| PI1 | 6 | 6.1 ± 1.5 |
| PI1-2% | 4 | 0.5 ± 0.1 |
| PI1-5% | 59 | 1.0 ± 0.2 |
| PI1-10% | 150 | 1.5 ± 0.4 |
| PI1-20% | 157 | 1.7 ± 0.3 |
| PI2 | 2.6 | 5.5 ± 1 |
| PI2-2% | 9 | 2.5 ± 0.5 |
| PI2-5% | 11 | 2.5 ± 0.5 |
| PI2-10% | 33 | 2.0 ± 0.5 |
| PI2-20% | 68 | 2.5 ± 0.4 |
| PI5 | 2.2 | 2.0 ± 0.5 |
| PI5-2% | 6 | 0.4 ± 0.1 |
| PI5-5% | 8 | 0.5 ± 0.1 |
| PI5-10% | 9 | 1.6 ± 0.3 |
| PI5-20% | 12 | 1.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiubianu, G.-T.; Bele, A.; Bargan, A.; Potolinca, V.O.; Asandulesa, M.; Tugui, C.; Tiron, V.; Hamciuc, C.; Dascalu, M.; Cazacu, M. All-Polymer Piezo-Composites for Scalable Energy Harvesting and Sensing Devices. Molecules 2022, 27, 8524. https://doi.org/10.3390/molecules27238524
Stiubianu G-T, Bele A, Bargan A, Potolinca VO, Asandulesa M, Tugui C, Tiron V, Hamciuc C, Dascalu M, Cazacu M. All-Polymer Piezo-Composites for Scalable Energy Harvesting and Sensing Devices. Molecules. 2022; 27(23):8524. https://doi.org/10.3390/molecules27238524
Chicago/Turabian StyleStiubianu, George-Theodor, Adrian Bele, Alexandra Bargan, Violeta Otilia Potolinca, Mihai Asandulesa, Codrin Tugui, Vasile Tiron, Corneliu Hamciuc, Mihaela Dascalu, and Maria Cazacu. 2022. "All-Polymer Piezo-Composites for Scalable Energy Harvesting and Sensing Devices" Molecules 27, no. 23: 8524. https://doi.org/10.3390/molecules27238524
APA StyleStiubianu, G.-T., Bele, A., Bargan, A., Potolinca, V. O., Asandulesa, M., Tugui, C., Tiron, V., Hamciuc, C., Dascalu, M., & Cazacu, M. (2022). All-Polymer Piezo-Composites for Scalable Energy Harvesting and Sensing Devices. Molecules, 27(23), 8524. https://doi.org/10.3390/molecules27238524













