Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity
Abstract
1. Introduction
2. Experimental
2.1. General Experimental Considerations
2.1.1. Synthesis of 1
2.1.2. Syntheses of 2–3
2.2. X-ray Crystal Structure Determination
3. Results and Discussion
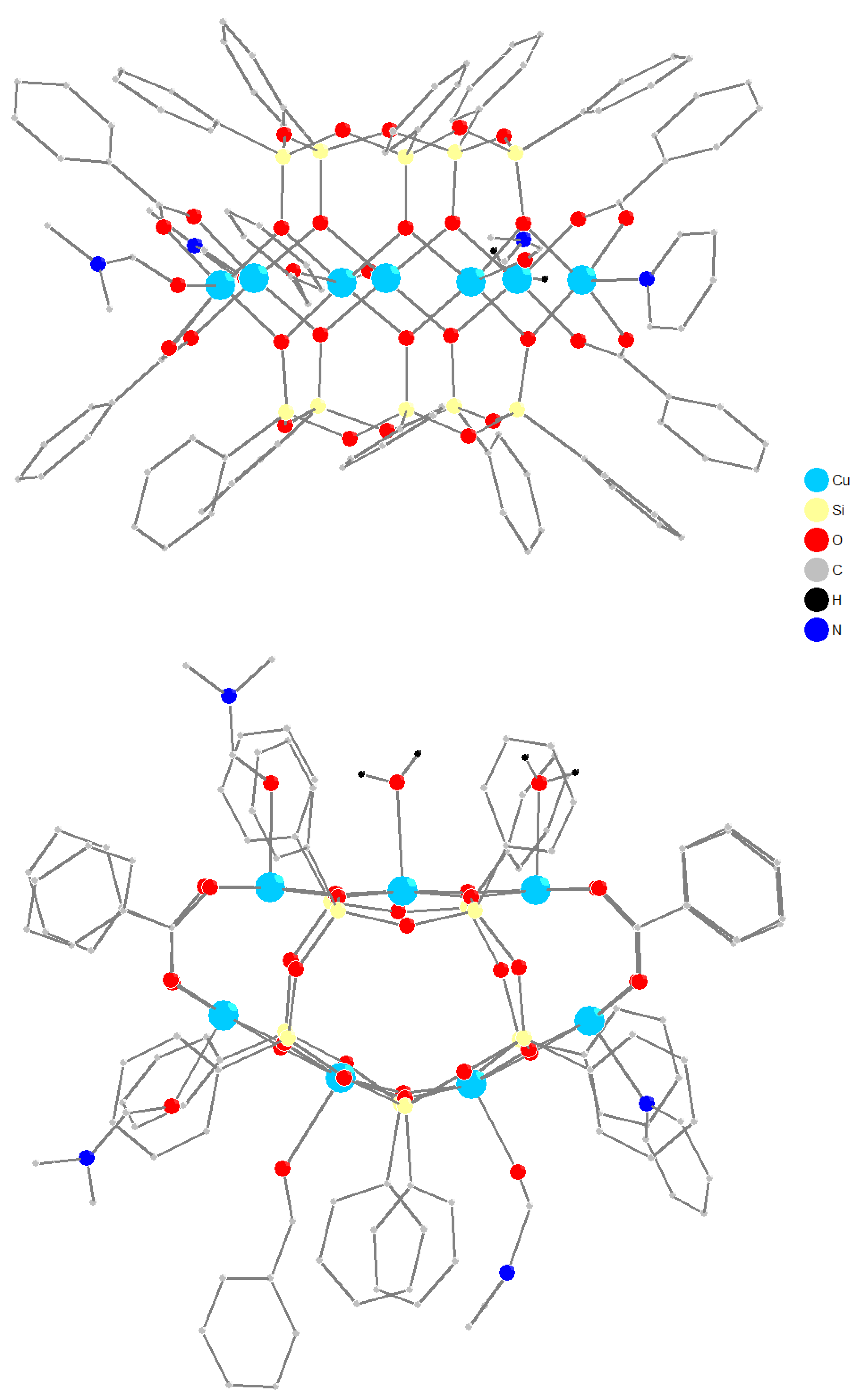
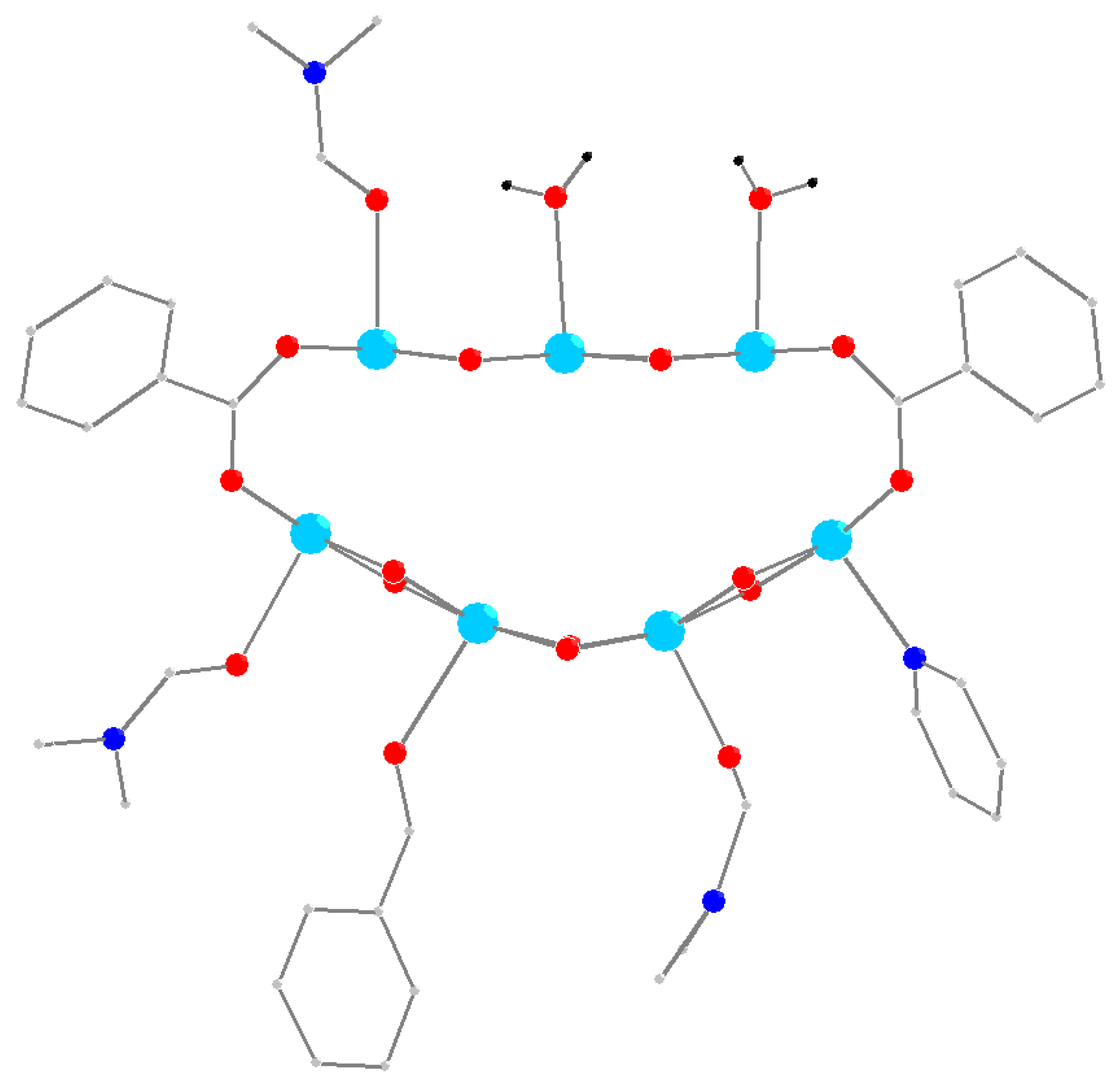
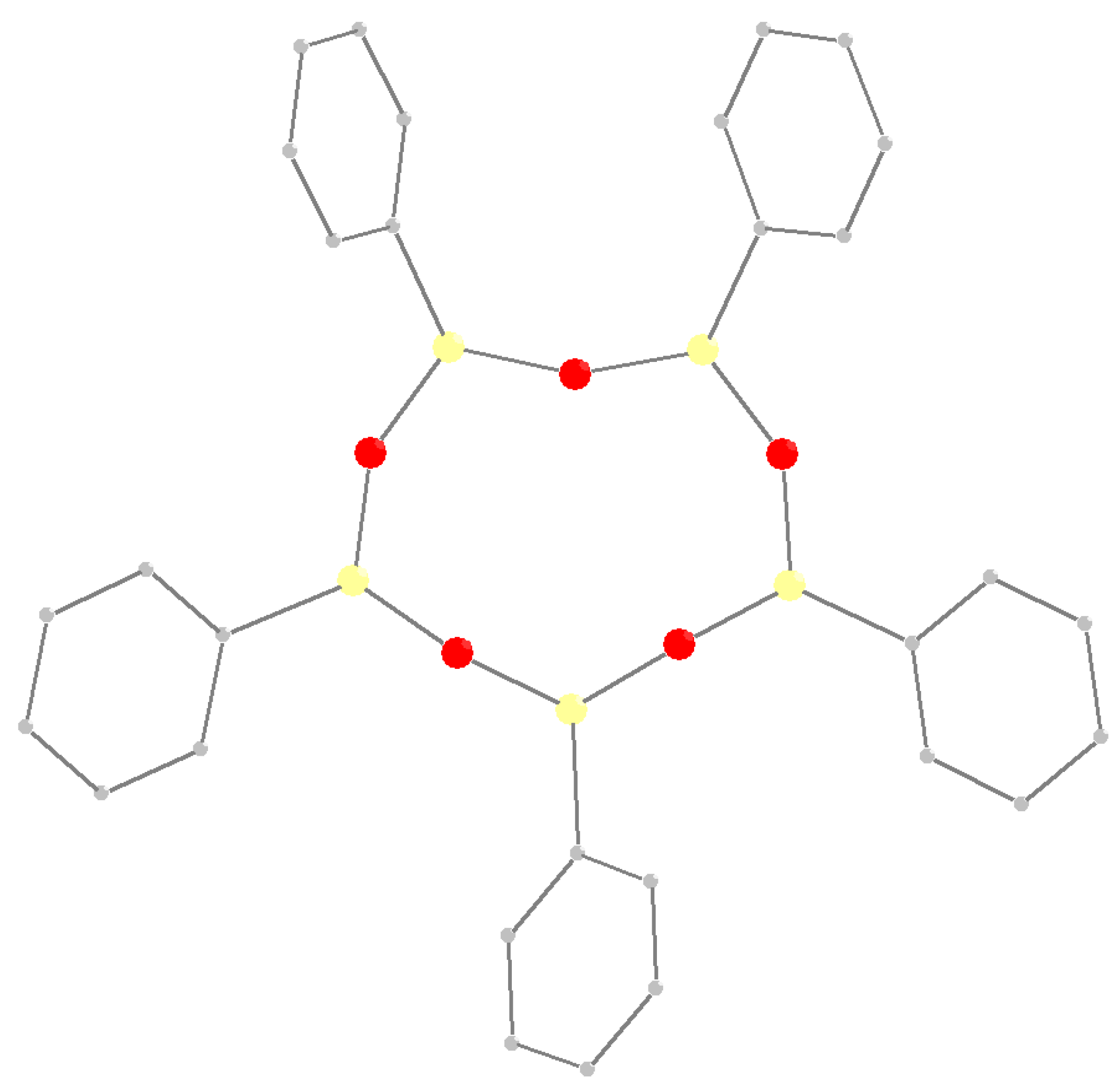
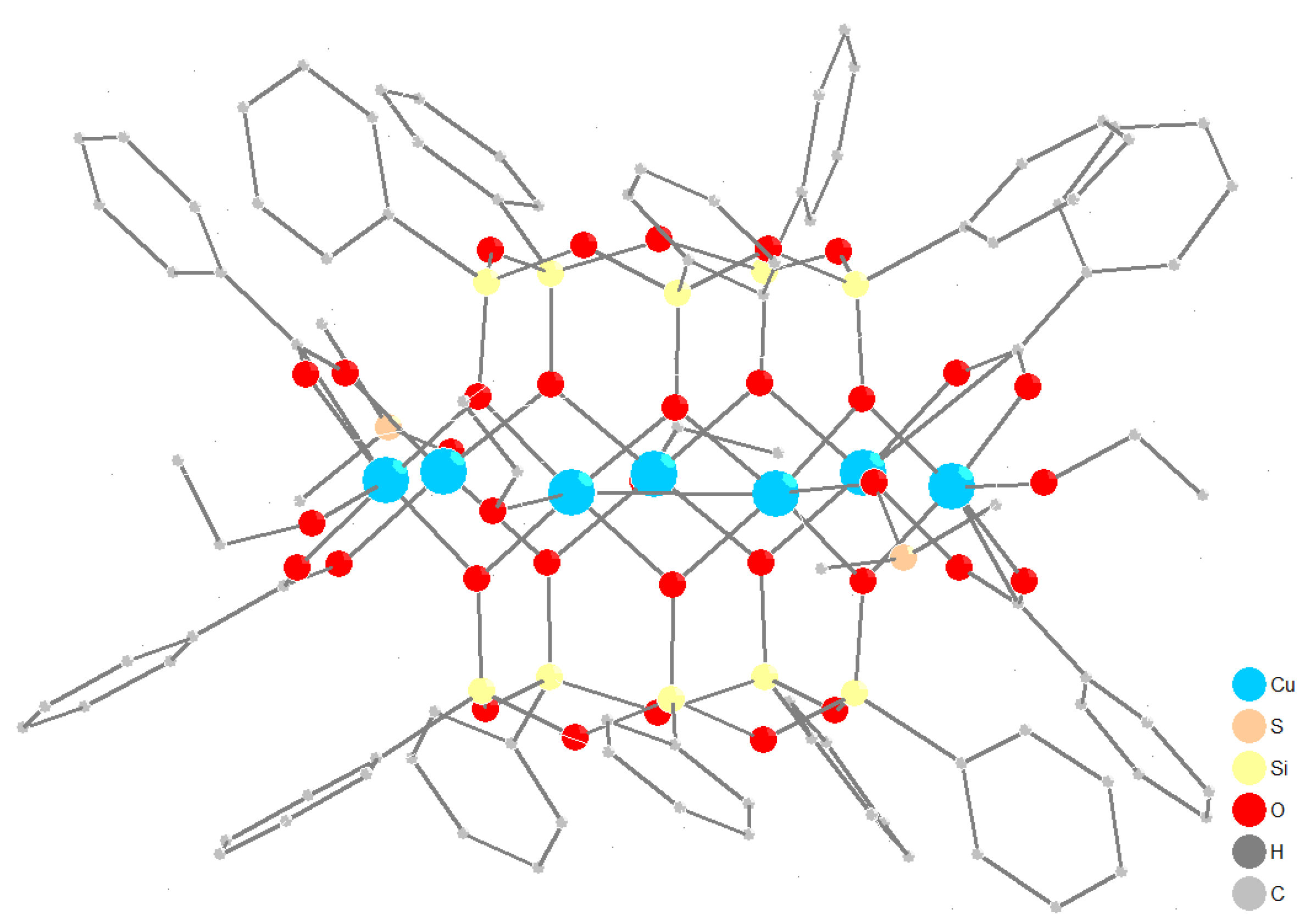
3.1. Synthesis and Structure
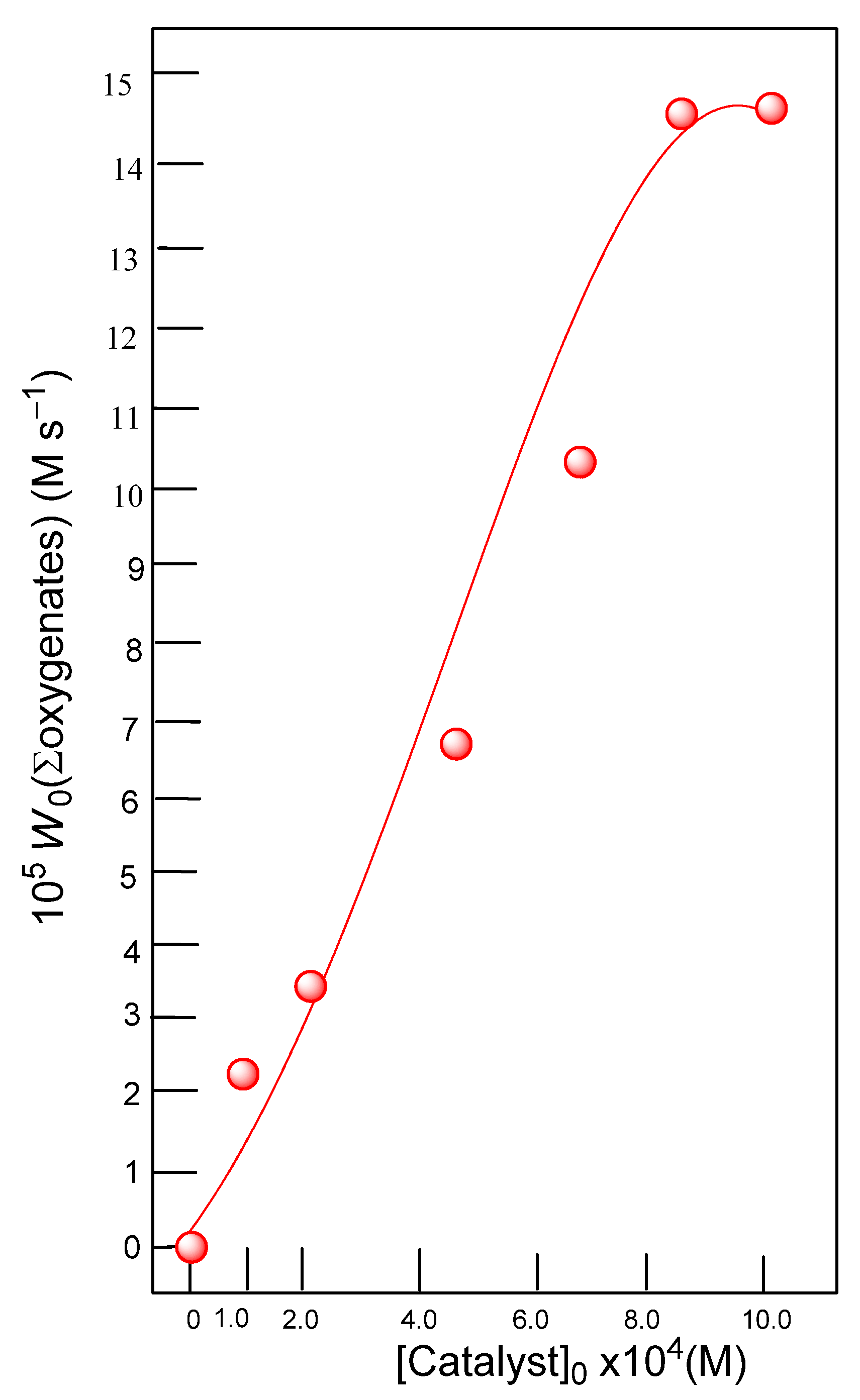
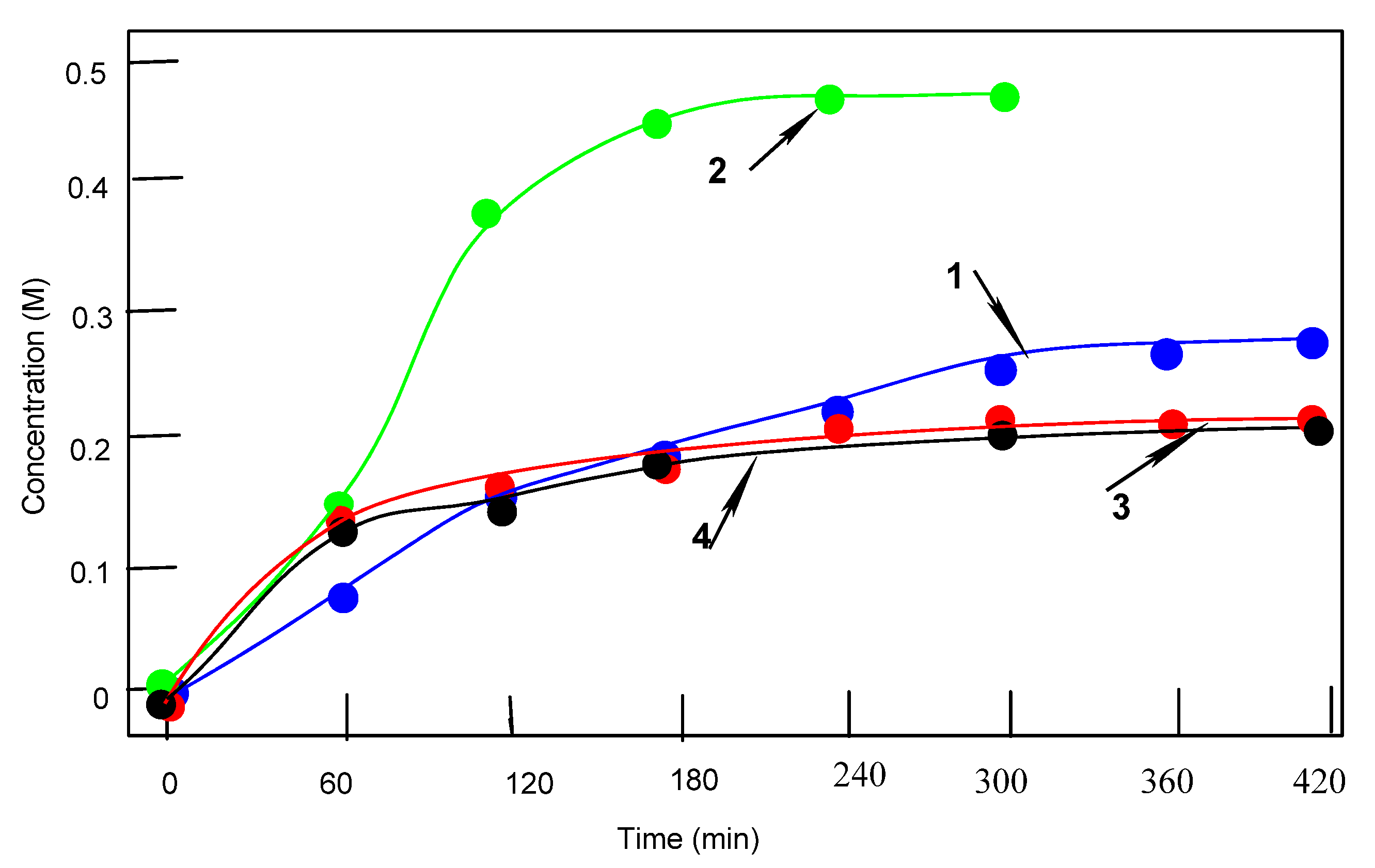
3.2. Complex 3 as a Catalyst in Oxidations with Peroxides
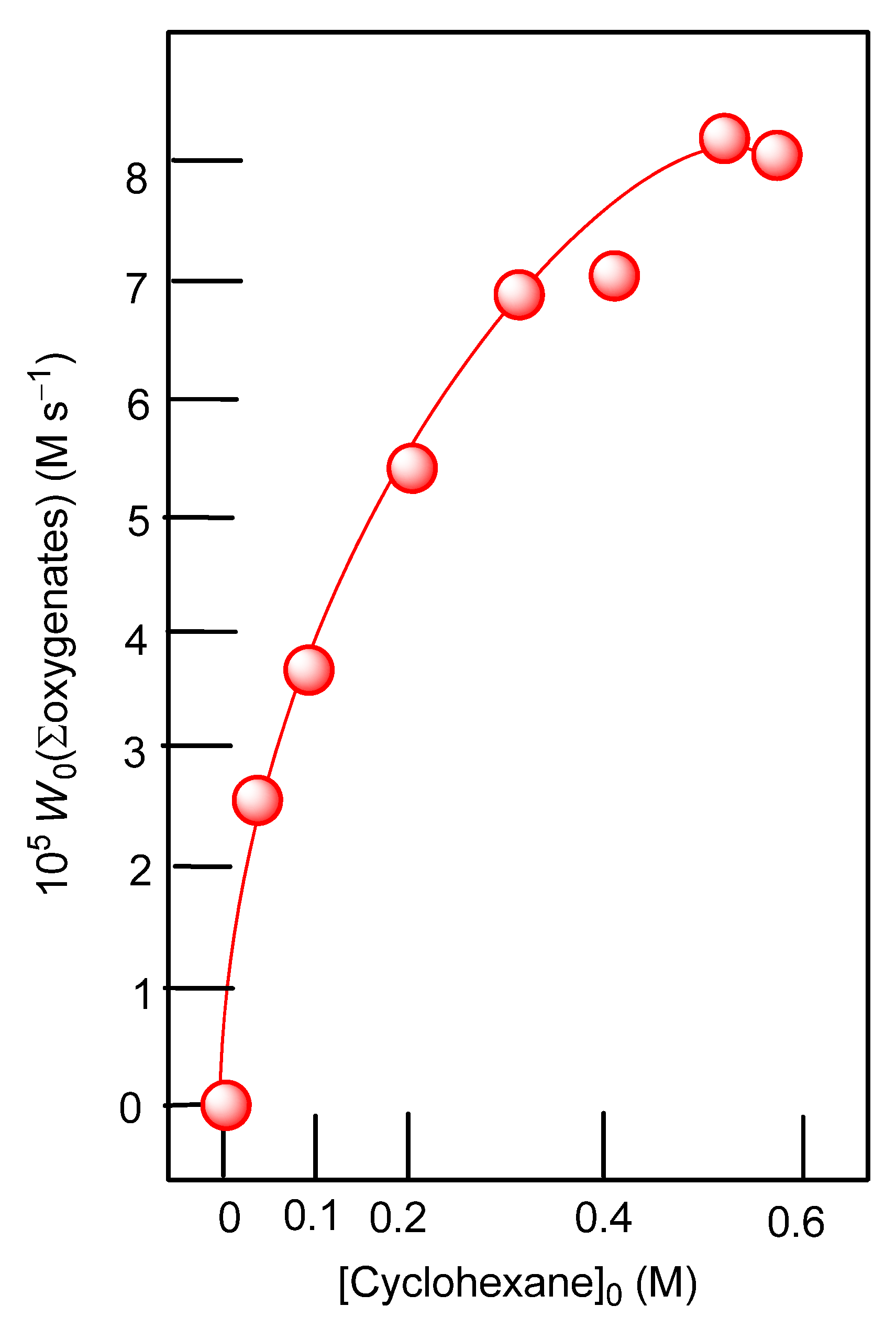
3.3. Oxidation of Alkanes
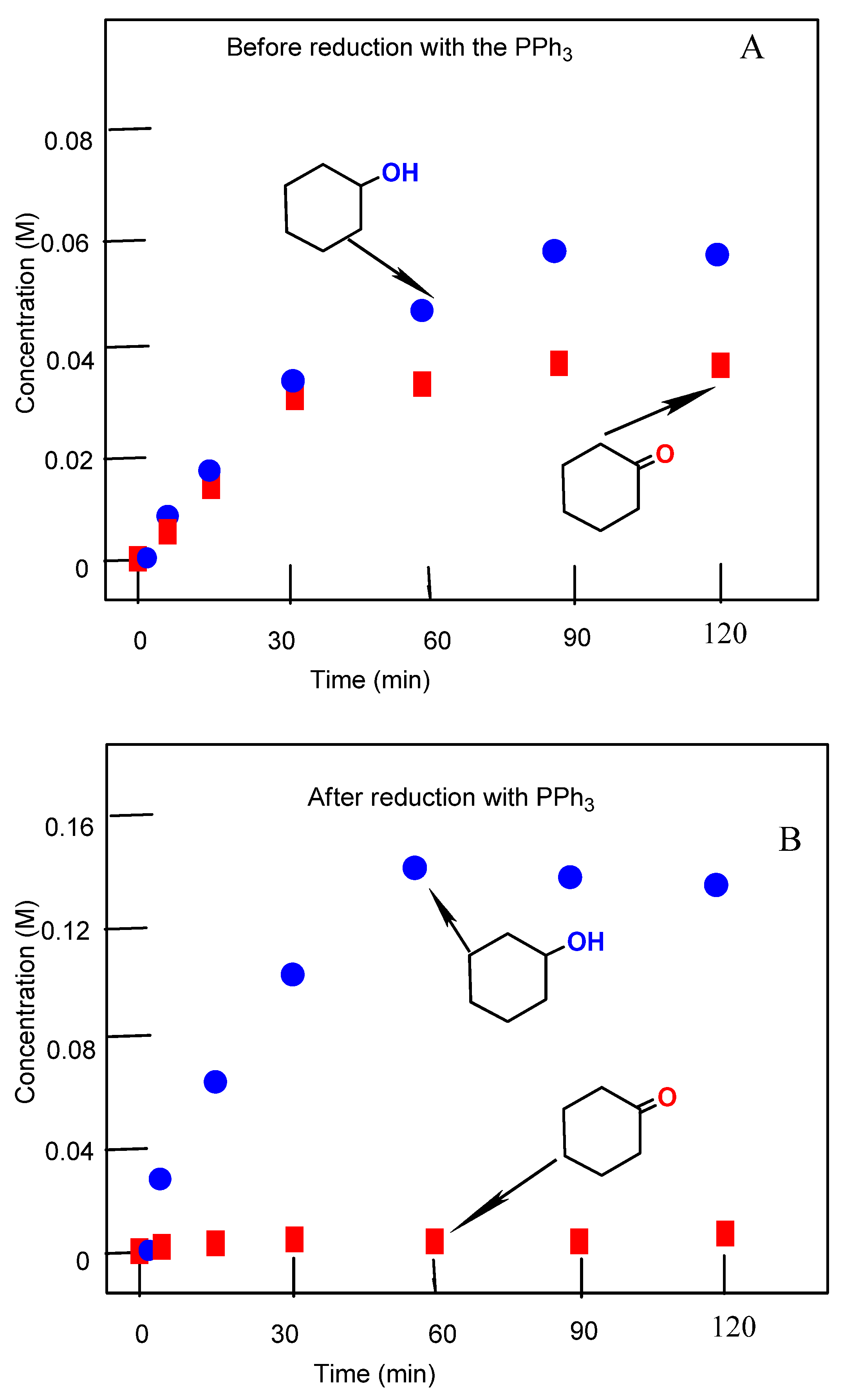
3.4. Oxidation of Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from Silanediols, Disilanols, Silanetriols, and Trisilanols. Chem. Rev. 1996, 96, 2205–2223. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206–207, 321–368. [Google Scholar] [CrossRef]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of Molecular Topology and Metal Nuclearity in Multimetallic Assemblies: Designer Metallosiloxanes Derived from Silanetriols. Chem.—Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. Adv. Organomet. Chem. 2005, 53, 101–153. [Google Scholar]
- Levitsky, M.M.; Zavin, B.G.; Bilyachenko, A. Chemistry of metallasiloxanes. Current trends and new concepts. Russ. Chem. Rev. 2007, 76, 847–866. [Google Scholar] [CrossRef]
- Edelmann, F.T. Metallasilsesquioxanes—Synthetic and Structural Studies. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Darmstadt, Germany, 2003; pp. 383–394. [Google Scholar]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Z.; Han, J. Current Chemistry of Cyclic Oligomeric Silsesquioxanes. Curr. Org. Chem. 2018, 21, 2814–2828. [Google Scholar] [CrossRef]
- Ouyang, J.; Haotian, S.; Liang, Y.; Commisso, A.; Li, D.; Xu, R.; Yu, D. Recent Progress in Metal-containing Silsesquioxanes: Preparation and Application. Curr. Org. Chem. 2018, 21, 2829–2848. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Zubavichus, Y.V.; Korlyukov, A.A.; Khrustalev, V.N.; Shubina, E.S.; Bilyachenko, A.N. Silicon and Germanium-Based Sesquioxanes as Versatile Building Blocks for Cage Metallacomplexes. A Review. J. Clust. Sci. 2019, 30, 1283–1316. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S.; Long, J.; Guari, Y.; Larionova, J. Magnetic cagelike metallasilsesquioxanes. Coord. Chem. Rev. 2019, 398, 213015. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Hou, J.-L.; Wang, Z.; Gupta, R.K.; Jagličić, Z.; Jagodič, M.; Wang, W.-G.; Tung, C.-H.; Sun, D. An Octanuclear Cobalt Cluster Protected by Macrocyclic Ligand: In Situ Ligand-Transformation-Assisted Assembly and Single-Molecule Magnet Behavior. Inorg. Chem. 2020, 59, 5683–5693. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Nigoghossian, K.; Félix, G.; Khrustalev, V.N.; Shubina, E.S.; Long, J.; Guari, Y.; Carlos, L.D.; Bilyachenko, A.N.; Larionova, J. New Magnetic and Luminescent Dy(III) and Dy(III)/Y(III) Based Tetranuclear Silsesquioxane Cages. Eur. J. Inorg. Chem. 2021, 2021, 2696–2701. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, R.; Tang, X.; Jagodič, M.; Jagličić, Z.; Pang, L.; Dou, J.-M.; Gao, Z.-Y.; Feng, H.-Y.; Tung, C.-H.; et al. A Carbonate-Templated Decanuclear Mn Nanocage with Two Different Silsesquioxane Ligands. Inorg. Chem. 2021, 60, 14866–14871. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.; Dronova, M.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’Kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Dorovatovskii, P.V.; et al. Family of Polynuclear Nickel Cagelike Phenylsilsesquioxanes; Features of Periodic Networks and Magnetic Properties. Inorg. Chem. 2017, 56, 12751–12763. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Wang, R.; Bilyachenko, A.; Khrustalev, V.; Jagodič, M.; Jagličić, Z.; Li, Z.; Wang, L.; Tung, C.; Sun, D. Tridecanuclear Gd(III)-silsesquioxane: Synthesis, structure, and magnetic property. ChemPhysMater 2022, 1, 247–251. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Es’kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Shul’pina, L.S.; Ikonnikov, N.S.; et al. First cagelike pentanuclear Co(II)-silsesquioxane. Dalton Trans. 2016, 45, 13663–13666. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, S.; Bisio, C.; Carniato, F. Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions. Processes 2022, 10, 758. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Shubina, E.S.; Felix, G.; Mamontova, E.; Long, J.; Guari, Y.; Larionova, J. New Luminescent Tetranuclear Lanthanide-Based Silsesquioxane Cage-Like Architectures. Chem.—Eur. J. 2020, 26, 16594–16598. [Google Scholar] [CrossRef]
- Sheng, K.; Liu, Y.-N.; Gupta, R.K.; Kurmoo, M.; Sun, D. Arylazopyrazole-functionalized photoswitchable octanuclear Zn(II)-silsesquioxane nanocage. Sci. China Ser. B Chem. 2020, 64, 419–425. [Google Scholar] [CrossRef]
- Nigoghossian, K.; Kulakova, A.N.; Félix, G.; Khrustalev, V.N.; Shubina, E.S.; Long, J.; Guari, Y.; Sene, S.; Carlos, L.D.; Bilyachenko, A.N.; et al. Temperature sensing in Tb3+/Eu3+-based tetranuclear silsesquioxane cages with tunable emission. RSC Adv. 2021, 11, 34735–34741. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Korlyukov, A.A.; Vologzhanina, A.V.; Khrustalev, V.N.; Kulakova, A.N.; Long, J.; Larionova, J.; Guari, Y.; Dronova, M.S.; Tsareva, U.S.; et al. Tuning linkage isomerism and magnetic properties of bi- and tri-metallic cage silsesquioxanes by cation and solvent effects. Dalton Trans. 2017, 46, 12935–12949. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’Pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shul’Pin, G.B. A new “bicycle helmet”-like copper(ii),sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Astakhov, G.S.; Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’Pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Kirillova, M.V.; et al. Exploring Cagelike Silsesquioxane Building Blocks for the Design of Heterometallic Cu4/M4 Architectures. Cryst. Growth Des. 2022, 22, 2146–2157. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High-Cluster Cu9 Cage Silsesquioxanes: Synthesis, Structure, and Catalytic Activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Chen, X.; Liu, H.; Liu, Z.; Xing, T.; Li, A.; Song, H. Metallasilsesquioxane-derived ultrathin porous carbon nanosheet 3D architectures via an “in situ dual templating” strategy for ultrafast sodium storage. J. Mater. Chem. A 2021, 9, 6423–6431. [Google Scholar] [CrossRef]
- Lin, X.; Dong, Y.; Liu, X.; Chen, X.; Li, A.; Song, H. In-situ pre-lithiated onion-like SiOC/C anode materials based on metallasilsesquioxanes for Li-ion batteries. Chem. Eng. J. 2021, 428, 132125. [Google Scholar] [CrossRef]
- Yahyaei, H.; Mohseni, M.; Ghanbari, H.; Messori, M. Synthesis and characterization of polyhedral oligomeric titanized silsesquioxane: A new biocompatible cage like molecule for biomedical application. Mater. Sci. Eng. C 2016, 61, 293–300. [Google Scholar] [CrossRef]
- Zeng, B.; He, K.; Wu, H.; Ye, J.; Zheng, X.; Luo, W.; Xu, Y.; Yuan, C.; Dai, L. Zirconium-Embedded Polyhedral Oligomeric Silsesquioxane Containing Phosphaphenanthrene-Substituent Group Used as Flame Retardants for Epoxy Resin Composites. Macromol. Mater. Eng. 2021, 306, 2100012. [Google Scholar] [CrossRef]
- Abbenhuis, H.C.L. Advances in Homogeneous and Heterogeneous Catalysis with Metal-Containing Silsesquioxanes. Chem. Eur. J. 2000, 6, 25–32. [Google Scholar] [CrossRef]
- Levitskii, M.M.; Smirnov, V.V.; Zavin, B.G.; Bilyachenko, A.N.; Rabkina, A.Y. Metalasiloxanes: New structure formation methods and catalytic properties. Kinet. Catal. 2009, 50, 490–507. [Google Scholar] [CrossRef]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: New York, NY, USA, 2011; Volume 3, pp. 135–166. [Google Scholar]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’Pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Sheng, K.; Si, W.-D.; Wang, R.; Wang, W.-Z.; Dou, J.; Gao, Z.-Y.; Wang, L.-K.; Tung, C.-H.; Sun, D. Keggin-Type Tridecanuclear Europium-Oxo Nanocluster Protected by Silsesquioxanes. Chem. Mater. 2022, 34, 4186–4194. [Google Scholar] [CrossRef]
- Astakhov, G.; Levitsky, M.; Bantreil, X.; Lamaty, F.; Khrustalev, V.; Zubavichus, Y.; Dorovatovskii, P.; Shubina, E.; Bilyachenko, A. Cu(II)-silsesquioxanes as efficient precatalysts for Chan-Evans-Lam coupling. J. Organomet. Chem. 2019, 906, 121022. [Google Scholar] [CrossRef]
- Garg, S.; Unruh, D.K.; Krempner, C. Zirconium and hafnium polyhedral oligosilsesquioxane complexes–green homogeneous catalysts in the formation of bio-derived ethers via a MPV/etherification reaction cascade. Catal. Sci. Technol. 2020, 11, 211–218. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Khrustalev, V.N.; Dronova, M.S.; Gutsul, E.I.; Korlyukov, A.A.; Gelman, D.; Zubavichus, Y.V.; Novichkov, D.A.; Trigub, A.L.; Shubina, E.S.; et al. Cage-like manganesesilsesquioxanes: Features of their synthesis, unique structure, and catalytic activity in oxidative amidations. Inorg. Chem. Front. 2022, 9, 4525–4537. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zueva, A.Y.; Titova, E.M.; Astakhov, G.S.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Korlyukov, A.A.; Shul’pina, L.S.; Shubina, E.S.; et al. A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity. Molecules 2022, 27, 6205. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Gutsul, E.I.; Khrustalev, V.N.; Astakhov, G.S.; Zueva, A.Y.; Zubavichus, Y.V.; Kirillova, M.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Dorovatovskii, P.V.; et al. Acetone Factor in the Design of Cu4-, Cu6-, and Cu9-Based Cage Coppersilsesquioxanes: Synthesis, Structural Features, and Catalytic Functionalization of Alkanes. Inorg. Chem. 2022, 61, 14800–14814. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Levitsky, M.M.; Zubavichus, Y.V.; Khrustalev, V.N.; Titov, A.A.; Dorovatovskii, P.V.; Smol’yakov, A.F.; Shubina, E.S.; Kirillova, M.V.; Kirillov, A.M.; et al. Cu6- and Cu8-Cage Sil- and Germsesquioxanes: Synthetic and Structural Features, Oxidative Rearrangements, and Catalytic Activity. Inorg. Chem. 2021, 60, 8062–8074. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Blaurock, S.; Görls, H.; Edelmann, F.T. The First Niobasilsesquioxanes. Organometallics 2006, 25, 5922–5926. [Google Scholar] [CrossRef]
- Anantharaman, G.; Chandrasekhar, V.; Nehete, U.N.; Roesky, H.W.; Vidovic, D.; Magull, J. New Polyhedral Zinc Siloxanes: Synthesis and X-ray Crystal Structures of Zn8Me7(dioxane)2(O3SiR)3 and [Zn7Me2(THF)5(O3SiR)4][R = (2,6-i-Pr2C6H3)N(SiMe3)]. Organometallics 2004, 23, 2251–2256. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Vologzhanina, A.V.; Eskova, M.A.; Shubina, E.S.; Levitsky, M.M. Unusual penta- and hexanuclear Ni(II)-based silsesquioxane polynuclear complexes. Dalton Trans. 2016, 45, 7320–7327. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Levitsky, M.M.; Korlyukov, A.A.; Shul’Pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Vologzhanina, A.V.; Bilyachenko, A.N.; Dorovatovskii, P.V.; Kozlov, Y.N.; et al. New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts 2019, 9, 701. [Google Scholar] [CrossRef]
- Edelmann, F.T.; Gießmann, S.; Fischer, A. Silsesquioxane Chemistry, 5. Retention of the Cu O core upon formation of the first copper(I) silsesquioxane from tetrameric copper(I) -t-butoxide. Inorg. Chem. Commun. 2000, 3, 658–661. [Google Scholar] [CrossRef]
- Tan, G.; Yang, Y.; Chu, C.; Zhu, H.; Roesky, H.W. Cu24O24Si8R8: Organic Soluble 56-Membered Copper(I) Siloxane Cage and Its Use in Homogeneous Catalysis. J. Am. Chem. Soc. 2010, 132, 12231–12233. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Vologzhanina, A.V.; Shubina, E.S. Tridecanuclear CuII11Na2 Cagelike Silsesquioxanes. Cryst. Growth Des. 2018, 18, 5377–5384. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Vologzhanina, A.V.; Astakhov, G.S.; Gutsul, E.I.; Shubina, E.S.; Levitsky, M.M. High-nuclearity (Cu8-based) cage silsesquioxanes: Synthesis and structural study. Cryst Growth Des. 2018, 18, 2452–2457. [Google Scholar] [CrossRef]
- Schax, F.; Braun, B.; Limberg, C. A Tripodal Trisilanol Ligand and Its Complexation Behavior towards CuI, CuII, and ZnII. Eur. J. Inorg. Chem. 2014, 2014, 2124–2130. [Google Scholar] [CrossRef]
- Bruker. SAINT, v. 8.34A; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Rigaku. CrysAlisPro Software System, v. 1.171.41.106a; Rigaku Oxford Diffraction: Oxford, UK, 2021. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. 2006, D62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. 2011, D67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. 2011, D67, 235–242. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Prigyai, N.; Chanmungkalakul, S.; Ervithayasuporn, V.; Yodsin, N.; Jungsuttiwong, S.; Takeda, N.; Unno, M.; Boonmak, J.; Kiatkamjornwong, S. Lithium-Templated Formation of Polyhedral Oligomeric Silsesquioxanes (POSS). Inorg. Chem. 2019, 58, 15110–15117. [Google Scholar] [CrossRef]
- Laird, M.; Totée, C.; Gaveau, P.; Silly, G.; van der Lee, A.; Carcel, C.; Unno, M.; Bartlett, J.M.; Wong Chi Man, M. Functionalised polyhedral oligomeric silsesquioxane with encapsulated fluoride—First observation of fluxional Si⋯F interactions in POSS. Dalton Trans. 2021, 50, 81–89. [Google Scholar] [CrossRef]
- Ehle, S.; Lorenz, V.; Liebing, P.; Hilfert, L.; Edelmann, F.T. Synthesis and structural characterization of two complex tantalum(V) siloxides. Inorg. Chem. Commun. 2016, 74, 82–85. [Google Scholar] [CrossRef]
- Gießmann, S.; Lorenz, V.; Liebing, P.; Hilfert, L.; Fischer, A.; Edelmann, F.T. Synthesis and structural study of new metallasilsesquioxanes of potassium and uranium. Dalton Trans. 2017, 46, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Korlyukov, A.A.; Levitskii, M.M.; Antipin, M.Y.; Zavin, B.G. New cagelike metallasiloxane containing FeIII ions in different coordination spheres. Russ. Chem. Bull. 2007, 56, 543–545. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Titov, A.A.; Dorovatovskii, P.V.; Shul’pina, L.S.; Lamaty, F.; et al. Ionic Complexes of Tetra- and Nonanuclear Cage Cu(II)-phenylsilsesquioxanes: Synthesis and High Activity in Oxidative Catalysis. ChemCatChem 2017, 9, 4437–4447. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Bilyachenko, A.N.; Kozlov, Y.N.; Shul’pin, G.B. Palanquin-Like Cu4Na4 Silsesquioxane Synthesis (via Oxidation of 1,1-bis(Diphenylphosphino)methane), Structure and Catalytic Activity in Alkane or Alcohol Oxidation with Peroxides. Catalysts 2019, 9, 154. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Vologzhanina, A.V.; Shul’pin, G.B. Heptanuclear Cage Cu(II)-Silsesquioxanes. Features of Synthesis, Structure and Catalytic Activity. Eur. J. Inorg. Chem. 2018, 2018, 2505–2511. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Petrov, A.A.; Korlyukov, A.A.; Shul’pina, L.S.; Khrustalev, V.N.; Dorovatovskii, P.V.; Vologzhanina, A.V.; Tsareva, U.S.; et al. Unusual tri-, hexa- and nonanuclear Cu(II) cage methylsilsesquioxanes: Synthesis, Structures and Catalytic Activity in Oxidations with Peroxides. Inorg. Chem. 2017, 56, 4093–4103. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Shul’pin, G.B. Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N,O-Ligated Cu6 Cage Phenylsilsesquioxane. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Sedykh, E.E.; Levitsky, M.M.; Dorovatovskii, P.V.; Khrustalev, V.N.; Shul’pina, L.S.; Shubina, E.S.; Kozlov, Y.N.; Ikonnikov, N.S.; Bilyachenko, A.N.; et al. The first tris-heteroleptic copper cage, ligated by germsesquioxanes, 2,2′-bipyridines and 3,5-dimethylpyrazolates. Synthesis, structure and unique catalytic activity in oxidation of alkanes and alcohols with peroxides. J. Organometal. Chem. 2019, 899, 120911. [Google Scholar] [CrossRef]
- Bryliakov, K. (Ed.) Frontiers of Green Catalytic Selective Oxidations; Springer: Singapore, 2019; Chapter 1; pp. 1–35. ISBN 978-981-32-9750-0. [Google Scholar] [CrossRef]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S. Metal Complexes Containing Redox-active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts 2019, 9, 1046. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Shul’pina, L.S. Oxidation of Organic Compounds with Peroxides Catalyzed by Polynuclear Metal Compounds, A Review. Catalysts 2021, 11, 186. [Google Scholar] [CrossRef]
- Wójtowicz-Mlochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C-H, C-C and C=C bonds in alkanes, arenes and alkenes: Recent advances. Arkivoc 2017, 2017, 12–58. [Google Scholar] [CrossRef]
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D.; van Eldik, R. New mechanistic aspects of the Fenton reaction. Chem.—Eur. J. 2009, 15, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Goldsmith, C.R. Kinetic Analysis of the Formation and Decay of a Non-Heme Ferric Hydroperoxide Species Susceptible to O–O Bond Homolysis. Inorg. Chem. 2014, 53, 5206–5211. [Google Scholar] [CrossRef]
- Sabbatini, A.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Kopylovich, M.N.; Drew, M.G.B.; Pettinari, C.; Pombeiro, A.J.L. Microwave-assisted and solvent-free peroxidative oxidation of 1-phenylethanol to acetophenone with a CuII-TEMPO catalytic system. Catal. Commun. 2014, 48, 69–72. [Google Scholar] [CrossRef]
- Perraud, O.; Sorokin, A.B.; Dutasta, J.-P.; Martinez, A. Oxidation of cycloalkanes by H2O2 using a copper–hemicryptophane complex as a catalyst. Chem. Commun. 2013, 49, 1288–1290. [Google Scholar] [CrossRef]
- Ünver, H.; Kani, I. Homogeneous oxidation of alcohols in water catalyzed with Cu(II)-triphenyl acetate/bipyridyl complex. Polyhedron 2017, 134, 257–262. [Google Scholar] [CrossRef]
- Calero, R.; Vega, A.; María García, A.; Spodine, E.; Manzur, J. Oxidation and Catalytic Properties of a Binuclear Copper(I) Complex with a Meta-Xylyl Spacer Ligand. J. Chil. Chem. Soc. 2003, 48, 85–88. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, C.B.A.E.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Peroxidative Oxidation of Alkanes and Alcohols under Mild Conditions by Di- and Tetranuclear Copper (II) Complexes of Bis (2-Hydroxybenzylidene) Isophthalohydrazide. Molecules 2018, 23, 2699. [Google Scholar] [CrossRef]
- Nandi, M.; Roy, P. Peroxidative oxidation of cycloalkane by di-, tetra- and polynuclear copper (II) complexes. Indian J. Chem. 2013, 52, 1263–1268. [Google Scholar]
- Choroba, K.; Machura, B.; Kula, S.; Raposo, L.R.; Fernandes, A.R.; Kruszynski, R.; Erfurt, K.; Shul’pina, L.S.; Kozlov, Y.N.; Shul’pin, G.B. Copper(II) complexes with 2,2′:6′,2″-terpyridine, 2,6-di(thiazol-2-yl)pyridine and 2,6-di(pyrazin-2-yl)pyridine substituted with quinolines. Synthesis, structure, antiproliferative activity, and catalytic activity in oxidation of alkanes and alcohols with peroxides. Dalton Trans. 2019, 48, 12656–12673. [Google Scholar] [PubMed]
- Shul’pina, L.S.; Vinogradov, M.M.; Kozlov, Y.N.; Nelyubina, Y.V.; Ikonnikov, N.S.; Shul’pin, G.B. Copper complexes with 1,10-phenanthrolines as efficient catalysts for oxidation of alkanes by hydrogen peroxide. Inorg. Chim. Acta 2020, 51, 2119889. [Google Scholar]
- Kulakova, A.N.; Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; et al. Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts 2018, 8, 484. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’Pina, L.S.; Kulakova, A.N.; Bantreil, X.; Lamaty, F.; Levitsky, M.M.; Gutsul, E.I.; Shubina, E.S.; et al. Heptanuclear Fe5Cu2-Phenylgermsesquioxane containing 2,2′-Bipyridine: Synthesis, Structure, and Catalytic Activity in Oxidation of C–H Compounds. Inorg. Chem. 2017, 57, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Bantreil, X.; Shul’Pina, L.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shubina, E.S.; Lamaty, F.; et al. Hexacoppergermsesquioxanes as complexes with N-ligands: Synthesis, structure and catalytic properties. J. Organomet. Chem. 2019, 884, 17–28. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Golubeva, E.N.; Vinogradov, M.M.; Kozlov, Y.N.; Shul’pin, G.B. EPR and DFT Study of Copper(II) Complex with 1,10-Phenanthroline as Catalyst for Oxidation of Cyclohexanol with tert-Butyl Hydroperoxide. Appl. Magn. Reason. 2022, 53, 887–894. [Google Scholar] [CrossRef]
- Fomenko, I.S.; Afewerki, M.; Gongola, M.I.; Vasilyev, E.S.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B.; Samsonenko, D.G.; Yanshole, V.V.; Nadolinny, V.A.; et al. Novel Copper(II) Complexes with Dipinodiazafluorene Ligands: Synthesis, Structure, Magnetic and Catalytic Properties. Molecules 2022, 27, 4072. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(mu-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen Part 14 from the series “Oxidations by the system hydrogen peroxide–[Mn2L2O3]2+ (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar]
- Shul’pin, G.B.; Gradinaru, J.; Kozlov, Y.N. Alkane hydroperoxidation with hydroperoxides catalysed by copper complexes. Org. Biomol. Chem. 2003, 1, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
| Catalytic System | Total Yield of Oxidation Products (%) | Time (Hours) | TON |
|---|---|---|---|
| Complex 3 (5 × 10−4 M) this work | 32 | 1 | 290 |
| Cu2Fe5-germsesquioxane [89] | 28 | 1 | 260 |
| Cu9Na6-silsesquioxane [26] | 21 | 5.0 | 193 |
| Cu(OAc)2 [97] | 7.6 | 2.0 | |
| Cu(NO3)2•3H2O + HNO3 (results of the authors) | 9 | 2.0 | 80 |
| Cu(NO3)2•3H2O (results of the authors) | 2 | 24.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilyachenko, A.N.; Khrustalev, V.N.; Gutsul, E.I.; Zueva, A.Y.; Korlyukov, A.A.; Shul’pina, L.S.; Ikonnikov, N.S.; Dorovatovskii, P.V.; Gelman, D.; Shubina, E.S.; et al. Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity. Molecules 2022, 27, 8505. https://doi.org/10.3390/molecules27238505
Bilyachenko AN, Khrustalev VN, Gutsul EI, Zueva AY, Korlyukov AA, Shul’pina LS, Ikonnikov NS, Dorovatovskii PV, Gelman D, Shubina ES, et al. Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity. Molecules. 2022; 27(23):8505. https://doi.org/10.3390/molecules27238505
Chicago/Turabian StyleBilyachenko, Alexey N., Victor N. Khrustalev, Evgenii I. Gutsul, Anna Y. Zueva, Alexander A. Korlyukov, Lidia S. Shul’pina, Nikolay S. Ikonnikov, Pavel V. Dorovatovskii, Dmitri Gelman, Elena S. Shubina, and et al. 2022. "Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity" Molecules 27, no. 23: 8505. https://doi.org/10.3390/molecules27238505
APA StyleBilyachenko, A. N., Khrustalev, V. N., Gutsul, E. I., Zueva, A. Y., Korlyukov, A. A., Shul’pina, L. S., Ikonnikov, N. S., Dorovatovskii, P. V., Gelman, D., Shubina, E. S., & Shul’pin, G. B. (2022). Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity. Molecules, 27(23), 8505. https://doi.org/10.3390/molecules27238505









