Recombinant Spider Silk Fiber with High Dimensional Stability in Water and Its NMR Characterization
Abstract
1. Introduction
2. Results and Discussion
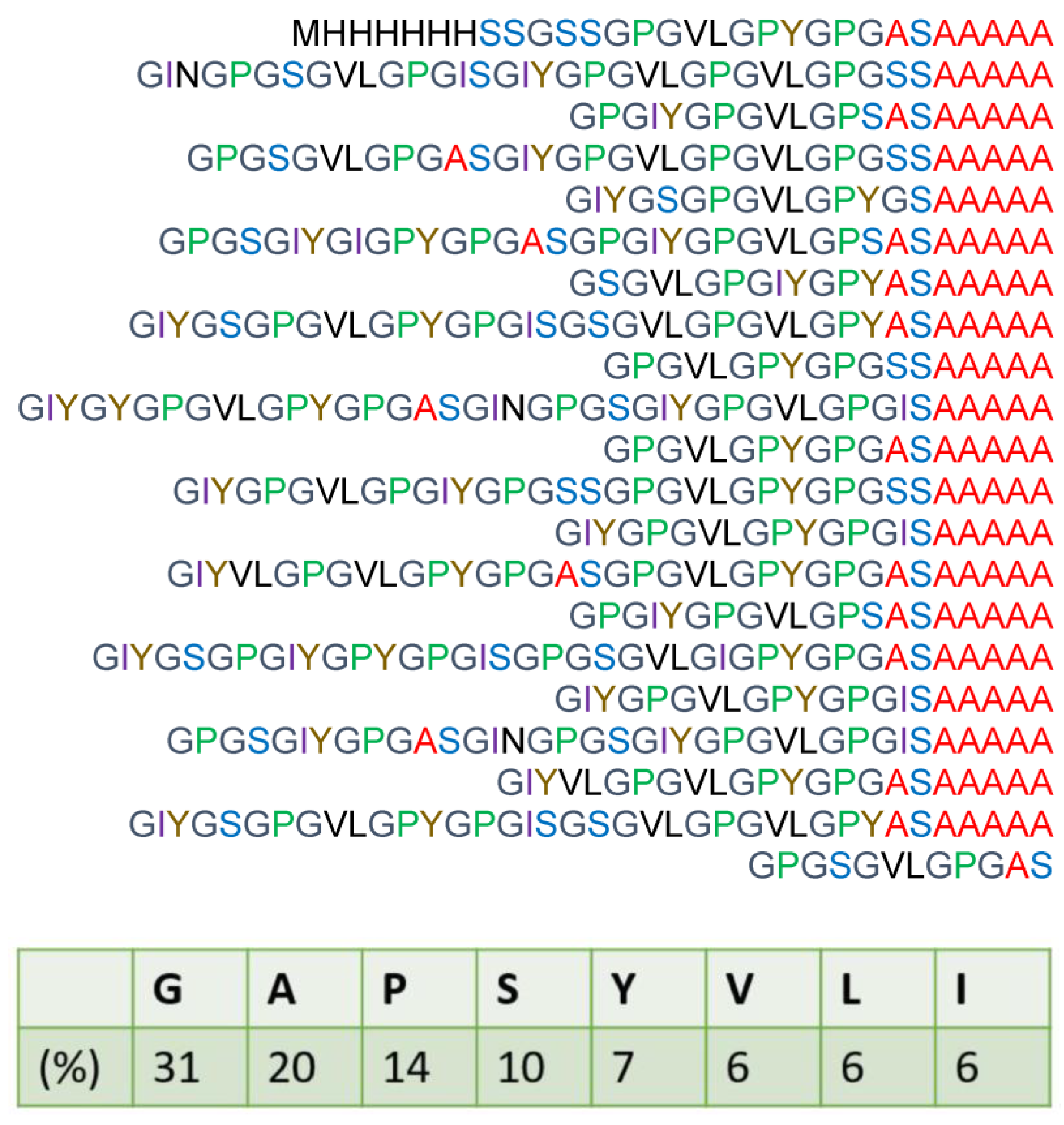
2.1. 13C Solution NMR Spectra of RSSP(VLI) Samples Prepared by Changing the Storage Times in FA
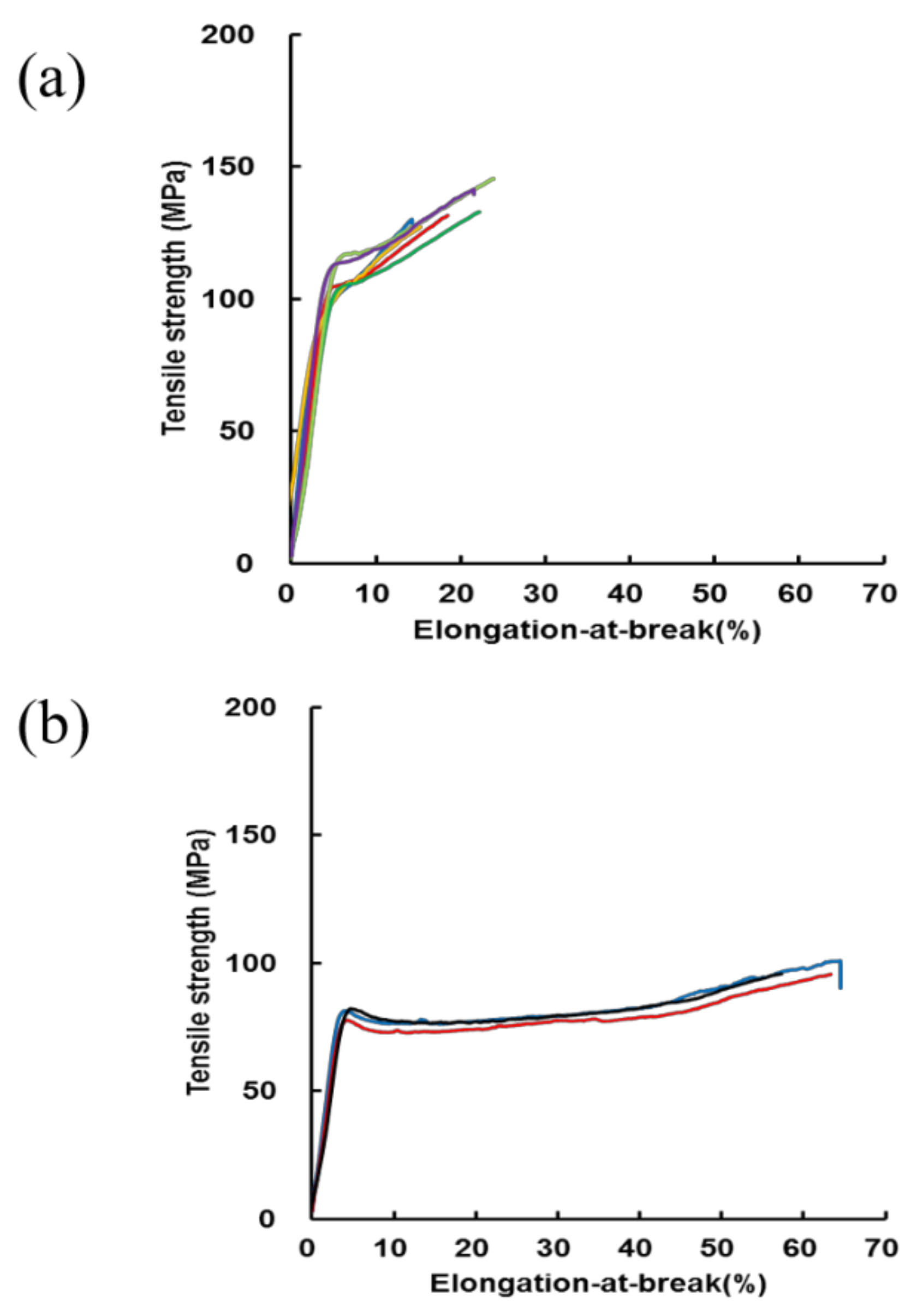
2.2. Stress–Strain Curves of RSSP(VLI) Fiber after Repeated Immersion in Water and Drying of the Fiber
2.3. Dimensional Stability Experiments of Eight Kinds of Silk Fiber Samples
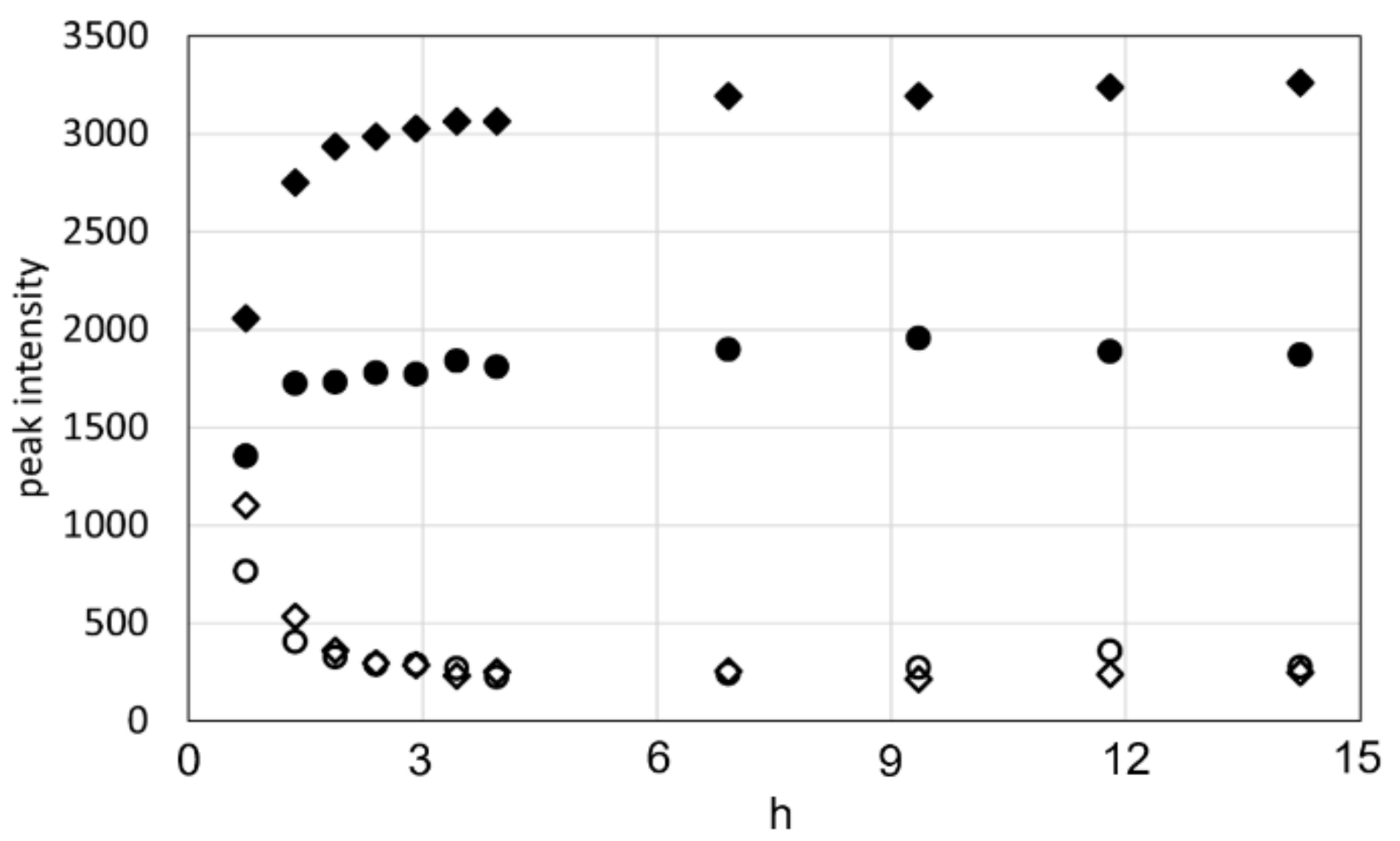
2.4. Water Absorption Experiments of Eight Kinds Using Silk Fiber Samples
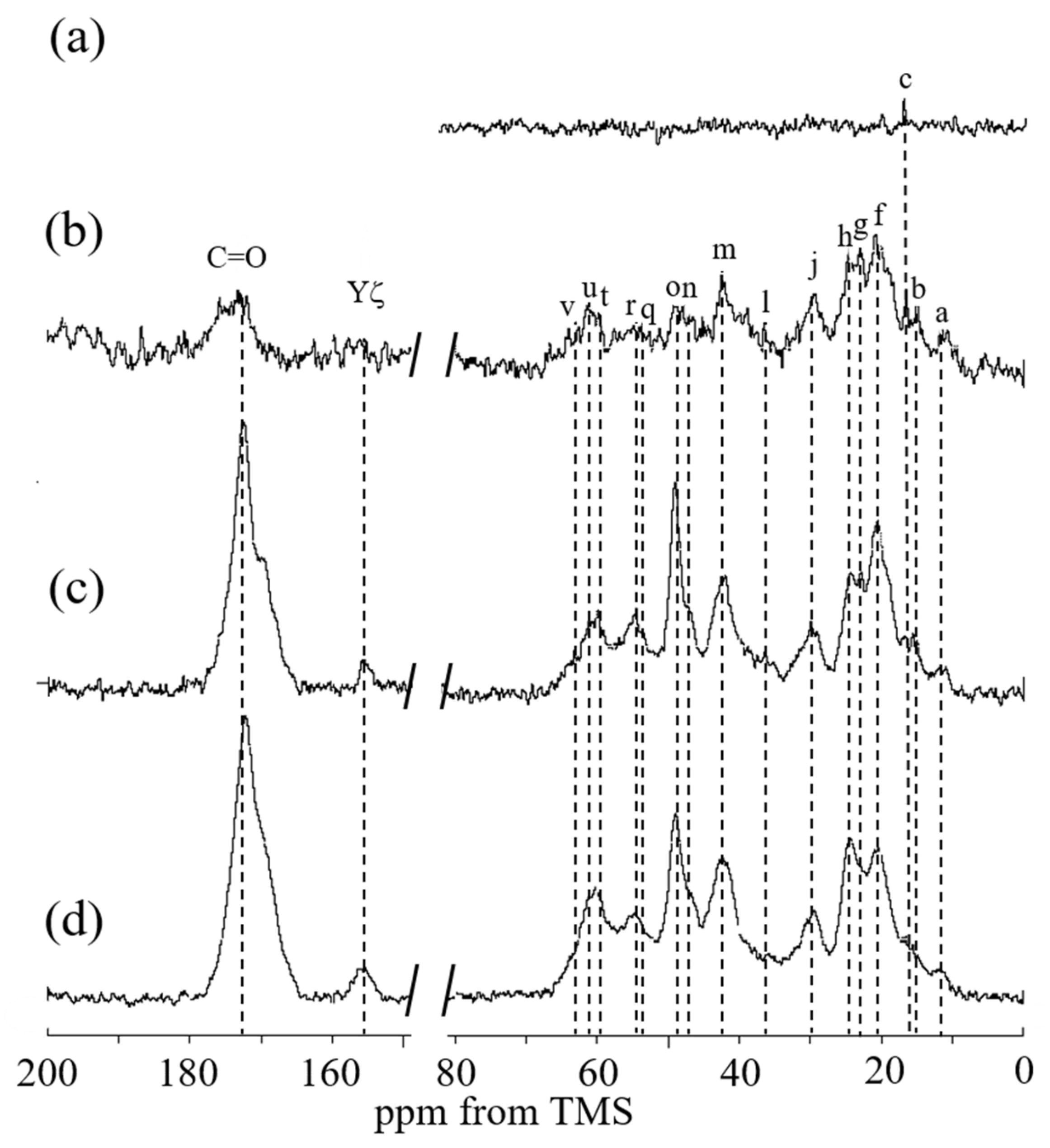
2.5. 13C Solid-State NMR Spectra of RSSP(VLI) Powders and Fibers in the Dry and Hydrated States
| Cα | Cβ | Cγ | Cδ | |
|---|---|---|---|---|
| Gly | 42.0, 42.8 | |||
| m m | ||||
| Ala | 50.0 (r.c.) | 16.6 (r.c) | ||
| p | c | |||
| 48.9 (α) | 20.2 (β) | |||
| o | f | |||
| Pro | 61.4 | 31.3 | 27.0 | 47.1 |
| u | k | i | n | |
| Ser | 53.3 (r.c.), 52.4 (a) | 61.4 (r.c) | ||
| q q* | u | |||
| 55.0 (α) | 63.2 (β) | |||
| r | v | |||
| Tyr | 56.1 | 36.4 | ||
| s | l | |||
| Val | 61.4 | 29.3 | 17.8, 18.5 | |
| u | j | d e | ||
| Leu | 53.3 | 42.8 | 27.0 | 22, 24.6 |
| q | m | i | g h | |
| Ile | 59.2 | 36.4 | 13.9, 24.6 | 11.4 |
| t | l | b h | a |
3. Materials and Methods
3.1. Preparation of RSSP(VLI) Powder Sample
3.2. Preparation of Acetylated RSSP(VLI) and Acetylated RSSP(QQQ) Powder Samples
3.3. 13C Solution NMR Observation of RSSP(VLI) Sample
3.4. Preparation of RSSP(VLI) Fiber, Acetylated RSSP(VLI), and Acetylated RSSP(QQQ) Fibers
3.5. Mechanical Property Measurements of RSSP(VLI) Fibers
3.6. Dimensional Stability Experiments with Eight Kinds of Silk Fiber Samples by Repeated Immersion in Water and Drying
3.7. Water Absorption Experiments of Eight Kinds Using Silk Fiber Samples
3.8. 13C r-INEPT, 13C CP/MAS and 13C DD/MAS NMR Measurements of RSSP(VLI) Powders and Fibers in the Dry and Hydrated States
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gosline, J.M.; DeMont, M.E.; Denny, M.W. The structure and properties of spider silk. Endeavour 1986, 10, 37–43. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Heidebrecht, A.; Scheibel, T. Chapter four. In Recombinant Production of Spider Silk Proteins; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 115–153. [Google Scholar] [CrossRef]
- Asakura, T.; Miller, T. (Eds.) Biotechnology of Silk; Biologically-Inspired Systems; Springer: Dordrecht, The Netherlands, 2014; Volume 5, ISBN 978-94-007-7118-5. [Google Scholar]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D.L. Structure–function–property–design interplay in biopolymers: Spider silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; McArdle, P.; Wang, S.L.; Wilmington, R.L.; Xing, Z.; Greenwood, A.; Cotten, M.L.; Qazilbash, M.M.; Schniepp, H.C. Protein secondary structure in spider silk nanofibrils. Nat. Commun. 2022, 13, 4329. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.N.; Gosline, J.M. The effect of proline on the network structure of major ampullate silks as inferred from their mechanical and optical properties. J. Exp. Biol. 2008, 211, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sponner, A.; Porter, D.; Vollrath, F. Proline and Processing of Spider Silks. Biomacromolecules 2008, 9, 116–121. [Google Scholar] [CrossRef]
- Cohen, N.; Levin, M.; Eisenbach, C.D. On the Origin of Supercontraction in Spider Silk. Biomacromolecules 2021, 22, 993–1000. [Google Scholar] [CrossRef]
- Work, R.W.; Morosoff, N. A Physico-Chemical Study of the Supercontraction of Spider Major Ampullate Silk Fibers. Text. Res. J. 1982, 52, 349–356. [Google Scholar] [CrossRef]
- Elices, M.; Plaza, G.R.; Arnedo, M.A.; Pérez-Rigueiro, J.; Torres, F.G.; Guinea, G.V. Mechanical Behavior of Silk During the Evolution of Orb-Web Spinning Spiders. Biomacromolecules 2009, 10, 1904–1910. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocent, R.; Kaplan, D.L.; Tsukada, M. Acylation of silk and wool with acid anhydrides and preparation of water-repellent fibers. J. Appl. Polym. Sci. 2001, 82, 2832–2841. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Li, T.; Wang, J. Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng. C. 2012, 32, 627–636. [Google Scholar] [CrossRef]
- Xu, M.V.; Lewis, R. Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. USA 1990, 87, 7120–7124. [Google Scholar] [CrossRef]
- Guerette, P.A.; Ginzinger, D.G.; Weber, B.H.; Gosline, J.M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 1996, 272, 112–115. [Google Scholar] [CrossRef]
- Rising, A.; Widhe, M.; Johansson, J.; Hedhammar, M. Spider silk proteins: Recent advances in recombinant production, structure–function relationships and biomedical applications. Cell. Mol. Life Sci. 2011, 68, 169–184. [Google Scholar] [CrossRef]
- Humenik, M.; Smith, A.M.; Scheibel, T. Recombinant Spider Silks—Biopolymers with Potential for Future Applications. Polymers 2011, 3, 640–661. [Google Scholar] [CrossRef]
- Tasei, Y.; Nishimura, A.; Suzuki, Y.; Sato, T.K.; Sugahara, J.; Asakura, T. NMR Investigation about Heterogeneous Structure and Dynamics of Recombinant Spider Silk in the Dry and Hydrated States. Macromolecules 2017, 50, 8117–8128. [Google Scholar] [CrossRef]
- Asakura, T. Structure and Dynamics of Spider Silk Studied with Solid-State Nuclear Magnetic Resonance and Molecular Dynamics Simulation. Molecules 2020, 25, 2634. [Google Scholar] [CrossRef]
- Suzuki, Y.; Higashi, T.; Yamamoto, T.; Okamura, H.; Sato, T.K.; Asakura, T. Presence of β-Turn Structure in Recombinant Spider Silk Dissolved in Formic Acid Revealed with NMR. Molecules 2022, 27, 511. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Matsuda, H.; Aoki, A.; Naito, A. Acetylation and hydration treatment of recombinant spider silk fiber, and their characterization using 13C NMR spectroscopy. Polymer 2022, 243, 124605. [Google Scholar] [CrossRef]
- Asakura, T.; Matsuda, H.; Naito, A.; Abe, Y. Formylation of Recombinant Spider Silk in Formic Acid and Wet Spinning Studied Using Nuclear Magnetic Resonance and Infrared Spectroscopies. ACS Biomater. Sci. Eng. 2022, 8, 2390–2402. [Google Scholar] [CrossRef] [PubMed]
- Koeppel, A.; Holland, C. Progress and Trends in Artificial Silk Spinning: A Systematic Review. ACS Biomater. Sci. Eng. 2017, 3, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Liivak, O.; Calve, S.; Adaska, J.; Ji, G.; Yang, Z.; Grubb, D.; Zax, D.B.; Jelinski, L.W. Regenerated Spider Silk: Processing, Properties, and Structure. Macromolecules 2000, 33, 775–780. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider Silk Fibers Spun from Soluble Recombinant Silk Produced in Mammalian Cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-X.; Qian, Z.-G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef]
- Teulé, F.; Addison, B.; Cooper, A.R.; Ayon, J.; Henning, R.W.; Benmore, C.J.; Holland, G.P.; Yarger, J.L.; Lewis, R.V. Combining flagelliform and dragline spider silk motifs to produce tunable synthetic biopolymer fibers. Biopolymers 2012, 97, 418–431. [Google Scholar] [CrossRef]
- Lin, Z.; Deng, Q.; Liu, X.-Y.; Yang, D. Engineered Large Spider Eggcase Silk Protein for Strong Artificial Fibers. Adv. Mater. 2013, 25, 1216–1220. [Google Scholar] [CrossRef]
- Albertson, A.E.; Teulé, F.; Weber, W.; Yarger, J.L.; Lewis, R.V. Effects of different post-spin stretching conditions on the mechanical properties of synthetic spider silk fibers. J. Mech. Behav. Biomed. Mater. 2014, 29, 225–234. [Google Scholar] [CrossRef]
- Copeland, C.G.; Bell, B.E.; Christensen, C.D.; Lewis, R.V. Development of a Process for the Spinning of Synthetic Spider Silk. ACS Biomater. Sci. Eng. 2015, 1, 577–584. [Google Scholar] [CrossRef]
- Li, X.; Mi, J.; Wen, R.; Zhang, J.; Cai, Y.; Meng, Q.; Lin, Y. Wet-Spinning Synthetic Fibers from Aggregate Glue: Aggregate Spidroin 1 (AgSp1). ACS Appl. Bio Mater. 2020, 3, 5957–5965. [Google Scholar] [CrossRef]
- Wohlrab, S.; Thamm, C.; Scheibel, T. The Power of Recombinant Spider Silk Proteins. In Biotechnology of Silk; Asakura, T., Miller, T., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 179–201. ISBN 978-94-007-7119-2. [Google Scholar]
- Jones, J.A.; Harris, T.I.; Tucker, C.L.; Berg, K.R.; Christy, S.Y.; Day, B.A.; Gaztambide, D.A.; Needham, N.J.C.; Ruben, A.L.; Oliveira, P.F.; et al. More Than Just Fibers: An Aqueous Method for the Production of Innovative Recombinant Spider Silk Protein Materials. Biomacromolecules 2015, 16, 1418–1425. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Eisoldt, L.; Diehl, L.J.; Schmidt, A.; Geffers, M.; Lang, G.; Scheibel, T. Biomimetic Fibers Made of Recombinant Spidroins with the Same Toughness as Natural Spider Silk. Adv. Mater. 2015, 27, 2189–2194. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.-Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q.; et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Salehi, S.; Scheibel, T. Biomimetic spider silk fibres: From vision to reality. Biochemist 2018, 40, 4–7. [Google Scholar] [CrossRef]
- Malay, A.D.; Suzuki, T.; Katashima, T.; Kono, N.; Arakawa, K.; Numata, K. Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Sci. Adv. 2020, 6, eabb6030. [Google Scholar] [CrossRef]
- Zhu, H.; Rising, A.; Johansson, J.; Zhang, X.; Lin, Y.; Zhang, L.; Yi, T.; Mi, J.; Meng, Q. Tensile properties of synthetic pyriform spider silk fibers depend on the number of repetitive units as well as the presence of N- and C-terminal domains. Int. J. Biol. Macromol. 2020, 154, 765–772. [Google Scholar] [CrossRef]
- Hu, C.F.; Qian, Z.-G.; Peng, Q.; Zhang, Y.; Xia, X.-X. Unconventional Spidroin Assemblies in Aqueous Dope for Spinning into Tough Synthetic Fibers. ACS Biomater. Sci. Eng. 2021, 7, 3608–3617. [Google Scholar] [CrossRef]
- Greco, G.; Mirbaha, H.; Schmuck, B.; Rising, A.; Pugno, A.N.M. Artificial and natural silk materials have high mechanical property variability regardless of sample size. Sci. Rep. 2022, 12, 3507. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Lee, K.G.; Park, Y.H. The role of formic acid in solution stability and crystallization of silk protein polymer. Int. J. Biol. Macromol. 2003, 33, 203–213. [Google Scholar] [CrossRef]
- Um, I.C.; Ki, C.S.; Kweon, H.; Lee, K.G.; Ihm, D.W.; Park, Y.H. Wet spinning of silk polymer: II. Effect of drawing on the structural characteristics and properties of filament. Int. J. Biol. Macromol. 2004, 34, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-W.; Tonelli, A.E.; Hudson, S.M. Structural Studies of Bombyx mori Silk Fibroin during Regeneration from Solutions and Wet Fiber Spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, F.; Gu, Z.; Ma, Q.; Hu, X. Exploring the Structural Transformation Mechanism of Chinese and Thailand Silk Fibroin Fibers and Formic-Acid Fabricated Silk Films. Int. J. Mol. Sci. 2018, 19, 3309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiong, Q.; Shan, Y.; Zhang, F.; Lu, S. Porous Silk Scaffold Derived from Formic Acid: Characterization and Biocompatibility. Adv. Mater. Sci. Eng. 2021, 2021, 3245587. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Yang, Y.; Shao, Z. Effect of various dissolution systems on the molecular weight of regenerated silk fibroin. Biomacromolecules 2013, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, F.; Wu, H.; Ming, J.; Fan, Z.; Zuo, B. A novel route to prepare dry-spun silk fibers from CaCl2–formic acid solution. Mater. Lett. 2014, 128, 175–178. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Yue, X.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Regeneration of high-quality silk fibroin fiber by wet spinning from CaCl2–formic acid solvent. Acta Biomater. 2015, 12, 139–145. [Google Scholar] [CrossRef]
- Zhang, F.; You, X.; Dou, H.; Liu, Z.; Zuo, B.; Zhang, X. Facile Fabrication of Robust Silk Nanofibril Films via Direct Dissolution of Silk in CaCl2–Formic Acid Solution. ACS Appl. Mater. Interfaces 2015, 7, 3352–3361. [Google Scholar] [CrossRef]
- Hijirida, D.H.; Do, K.G.; Michal, C.; Wong, S.; Zax, D.; Jelinski, L.W. 13C NMR of Nephila clavipes major ampullate silk gland. Biophys. J. 1996, 71, 3442–3447. [Google Scholar] [CrossRef]
- Yao, J.; Ohgo, K.; Sugino, R.; Kishore, R.; Asakura, T. Structural Analysis of Bombyx mori Silk Fibroin Peptides with Formic Acid Treatment Using High-Resolution Solid-State 13C NMR Spectroscopy. Biomacromolecules 2004, 5, 1763–1769. [Google Scholar] [CrossRef]
- Klunk, W.E.; Pettegrew, J.W. Alzheimer’s β-Amyloid Protein Is Covalently Modified when Dissolved in Formic Acid. J. Neurochem. 1990, 54, 2050–2056. [Google Scholar] [CrossRef]
- Klunk, W.E.; Xu, C.-J.; Pettegrew, J.W. NMR Identification of the Formic Acid-Modified Residue in Alzheimer’s Amyloid Protein. J. Neurochem. 1994, 62, 349–354. [Google Scholar] [CrossRef]
- Zheng, S.; Doucette, A.A. Preventing N- and O-formylation of proteins when incubated in concentrated formic acid. Proteomics 2016, 16, 1059–1068. [Google Scholar] [CrossRef]
- Lenčo, J.; Khalikova, M.A.; Švec, F. Dissolving Peptides in 0.1% Formic Acid Brings Risk of Artificial Formylation. J. Proteome Res. 2020, 19, 993–999. [Google Scholar] [CrossRef]
- Asakura, T.; Matsuda, H.; Naito, A. Acetylation of Bombyx mori silk fibroin and their characterization in the dry and hydrated states using 13C solid-state NMR. Int. J. Biol. Macromol. 2020, 155, 1410–1419. [Google Scholar] [CrossRef]
- Asakura, T.; Isobe, K.; Aoki, A.; Kametani, S. Conformation of Crystalline and Noncrystalline Domains of [3-13C]Ala-, [3-13C]Ser-, and [3-13C]Tyr-Bombyx mori Silk Fibroin in a Hydrated State Studied with 13C DD/MAS NMR. Macromolecules 2015, 48, 8062–8069. [Google Scholar] [CrossRef]
- Asakura, T.; Endo, M.; Tasei, Y.; Ohkubo, T.; Hiraoki, T. Hydration of Bombyx mori silk cocoon, silk sericin and silk fibroin and their interactions with water as studied by 13C NMR and 2H NMR relaxation. J. Mater. Chem. B 1017, 5, 1624–1632. [Google Scholar] [CrossRef]
- Asakura, T.; Endo, M.; Fukuhara, R.; Tasei, Y. 13C NMR characterization of hydrated 13C labeled Bombyx mori silk fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J. Mater. Chem. B 2017, 5, 2152–2160. [Google Scholar] [CrossRef]
- Asakura, T.; Isobe, K.; Kametani, S.; Ukpebor, O.T.; Silverstein, M.C.; Boutis, G.S. Characterization of water in hydrated Bombyx mori silk fibroin fiber and films by 2H NMR relaxation and 13C solid state NMR. Acta Biomater. 2017, 50, 322–333. [Google Scholar] [CrossRef]
- Nishimura, A.; Matsuda, H.; Tasei, Y.; Asakura, T. Effect of Water on the Structure and Dynamics of Regenerated [3-13C] Ser, [3-13C] Tyr, and [3-13C] Ala-Bombyx mori Silk Fibroin Studied with 13C Solid-State Nuclear Magnetic Resonance. Biomacromolecules 2018, 19, 563–575. [Google Scholar] [CrossRef]
- Asakura, T.; Matsuda, H.; Aoki, A.; Kataoka, N.; Imai, A. Conformational change of 13C-labeled 47-mer model peptides of Nephila clavipes dragline silk in poly(vinyl alcohol) film by stretching studied by 13C solid-state NMR and molecular dynamics simulation. Int. J. Biol. Macromol. 1019, 131, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.H.; Michal, C.A.; Jelinski, L.W. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 1996, 271, 84–87. [Google Scholar] [CrossRef]
- Van Beek, J.D.; Hess, S.; Vollrath, F.; Meier, B.H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. USA 2002, 99, 10266–10271. [Google Scholar] [CrossRef]
- Holland, G.P.; Jenkins, J.E.; Creager, M.S.; Lewis, R.V.; Yarger, J.L. Solid-State NMR Investigation of Major and Minor Ampullate Spider Silk in the Native and Hydrated States. Biomacromolecules 2008, 9, 651–657. [Google Scholar] [CrossRef]
- Yamaguchi, E.; Yamauchi, K.; Terry, G.; Asakura, T. Structural analysis of the Gly-rich region in spider dragline silk using stable-isotope labeled sequential model peptides and solid-state NMR. Chem. Commun. 2009, 28, 4176–4178. [Google Scholar] [CrossRef]
- Asakura, T.; Okonogi, M.; Horiguchi, K.; Aoki, A.; Saitô, H.; Knight, D.P.; Williamson, M.P. Two different packing arrangements of antiparallel polyalanine. Angew. Chem. Int. Ed. Eng. 2012, 51, 1212–1215. [Google Scholar] [CrossRef]
- Gray, G.; van der Vaart, A.; Guo, C.; Jones, J.; Onofrei, D.; Cherry, B.; Lewis, R.; Yarger, J.; Holland, G. Secondary structure adopted by the Gly-Gly-X repetitive regions of dragline spider silk. Int. J. Mol. Sci. 2016, 17, 2023–2035. [Google Scholar] [CrossRef]
- Asakura, T.; Nishimura, A.; Tasei, Y. Determination of local structure of 13C selectively labeled 47-mer peptides as a model for Gly-rich region of Nephila clavipes dragline silk using a combination of 13C solid-state NMR and MD simulation. Macromolecules 2018, 51, 3608–3619. [Google Scholar] [CrossRef]
- Asakura, T.; Tasei, Y.; Matsuda, H.; Naito, A. Dynamics of alanine methyl groups in alanine oligopeptides and spider dragline silks with different packing structures as studied by 13C solid-state NMR relaxation. Macromolecules 2018, 51, 6746–6756. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformational Characterization of Bombyx mori Silk Fibroin in the Solid State by High-Frequency 13C Cross Polarization-Magic Angle Spinning NMR, X-Ray Diffraction, and Infrared Spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Asakura, T.; Iwadate, M.; Demura, M.; Williamson, M.P. Structural analysis of silk with 13C NMR chemical shift contour plots. Int. J. Biol. Macromol. 1999, 24, 167–171. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, J.; Masuda, H.; Raghuvansh, K.; Asakura, T. Structural characterization and artificial fiber formation of Bombyx mori silk fibroin in hexafluoro-iso-propanol solvent system. Biopolymers 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Zhu, Z.; Ohgo, K.; Watanabe, R.; Takezawa, T.; Asakura, T. Preparation and characterization of regenerated Bombyx mori silk fibroin fiber containing recombinant cell-adhesive proteins; Nonwoven fiber and monofilament. J. Appl. Polym. Sci. 2008, 109, 2956–2963. [Google Scholar] [CrossRef]
- Zhu, Z.; Kikuchi, Y.; Kojima, K.; Tamura, T.; Kuwabara, N.; Nakamura, T.; Asakura, T. Mechanical Properties of Regenerated Bombyx mori Silk Fibers and Recombinant Silk Fibers Produced by Transgenic Silkworms. J. Biomater. Sci. Polym. Ed. 2010, 21, 395–411. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asakura, T.; Matsuda, H.; Naito, A.; Okamura, H.; Suzuki, Y.; Abe, Y. Recombinant Spider Silk Fiber with High Dimensional Stability in Water and Its NMR Characterization. Molecules 2022, 27, 8479. https://doi.org/10.3390/molecules27238479
Asakura T, Matsuda H, Naito A, Okamura H, Suzuki Y, Abe Y. Recombinant Spider Silk Fiber with High Dimensional Stability in Water and Its NMR Characterization. Molecules. 2022; 27(23):8479. https://doi.org/10.3390/molecules27238479
Chicago/Turabian StyleAsakura, Tetsuo, Hironori Matsuda, Akira Naito, Hideyasu Okamura, Yu Suzuki, and Yunosuke Abe. 2022. "Recombinant Spider Silk Fiber with High Dimensional Stability in Water and Its NMR Characterization" Molecules 27, no. 23: 8479. https://doi.org/10.3390/molecules27238479
APA StyleAsakura, T., Matsuda, H., Naito, A., Okamura, H., Suzuki, Y., & Abe, Y. (2022). Recombinant Spider Silk Fiber with High Dimensional Stability in Water and Its NMR Characterization. Molecules, 27(23), 8479. https://doi.org/10.3390/molecules27238479







