Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′-bi[3,2-b]thienothiophene Derivatives
Abstract
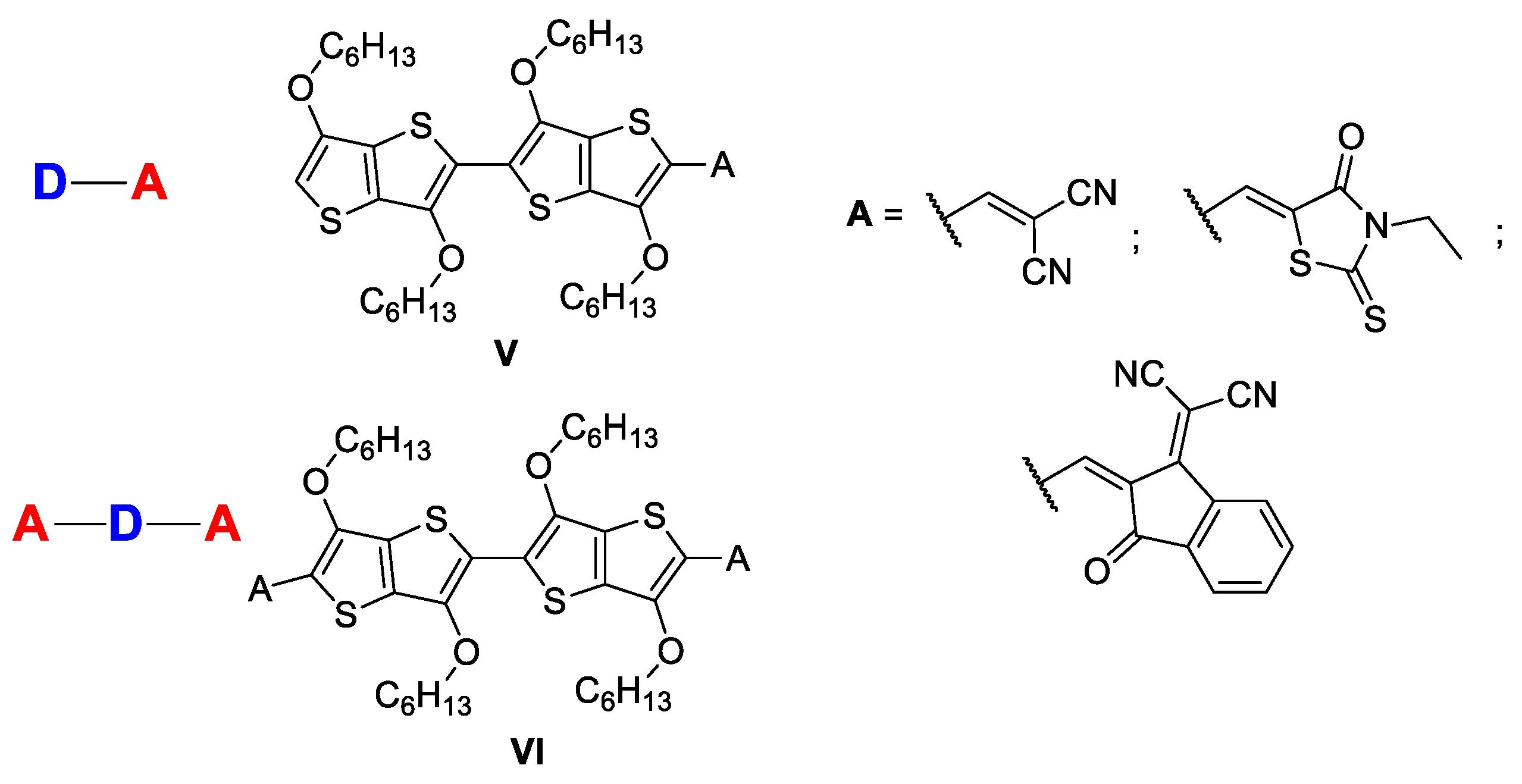
1. Introduction
2. Results and Discussion
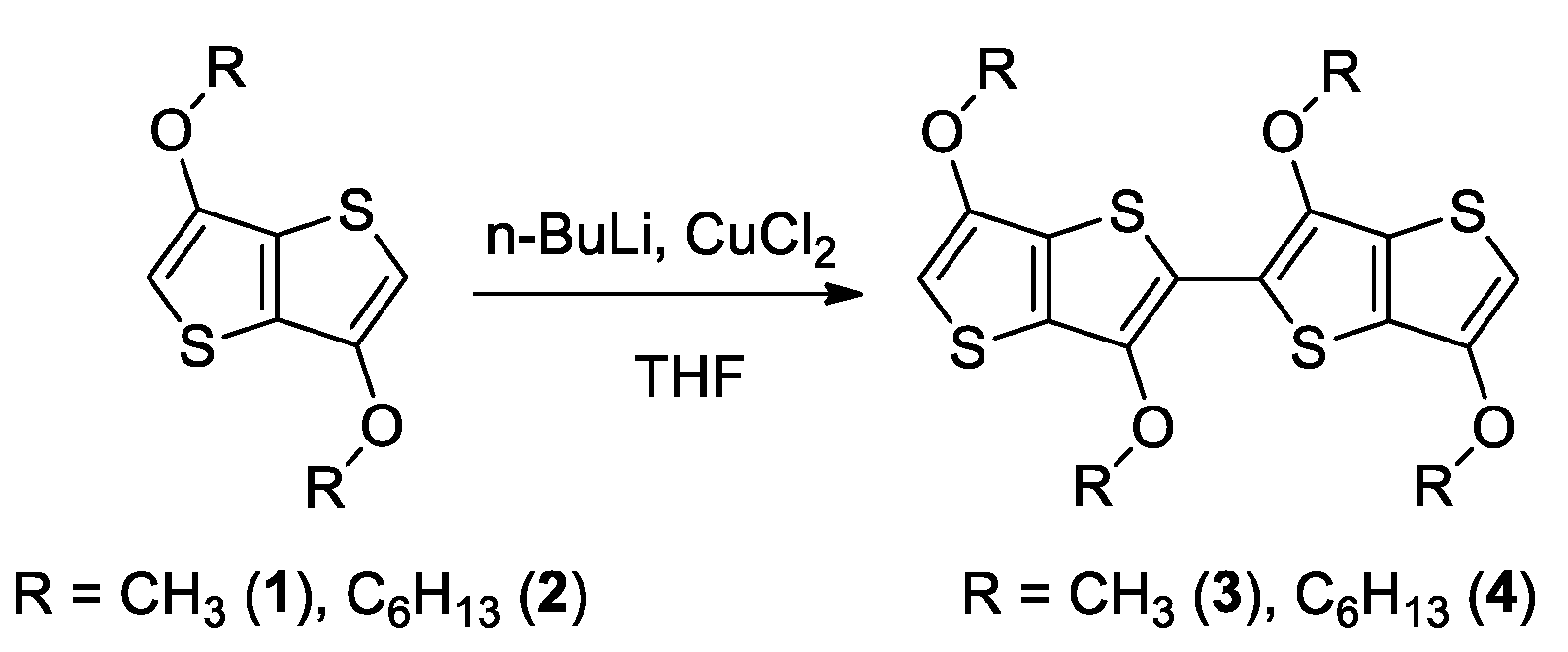
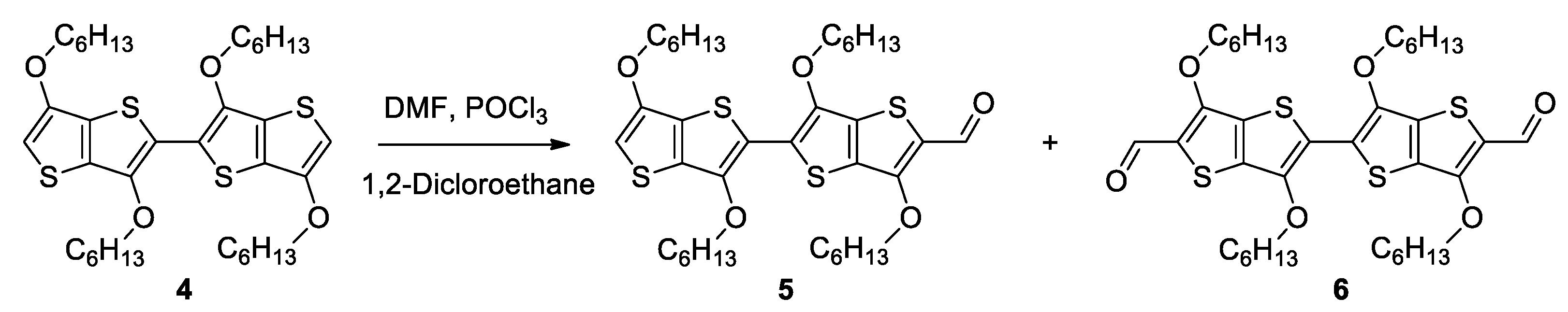
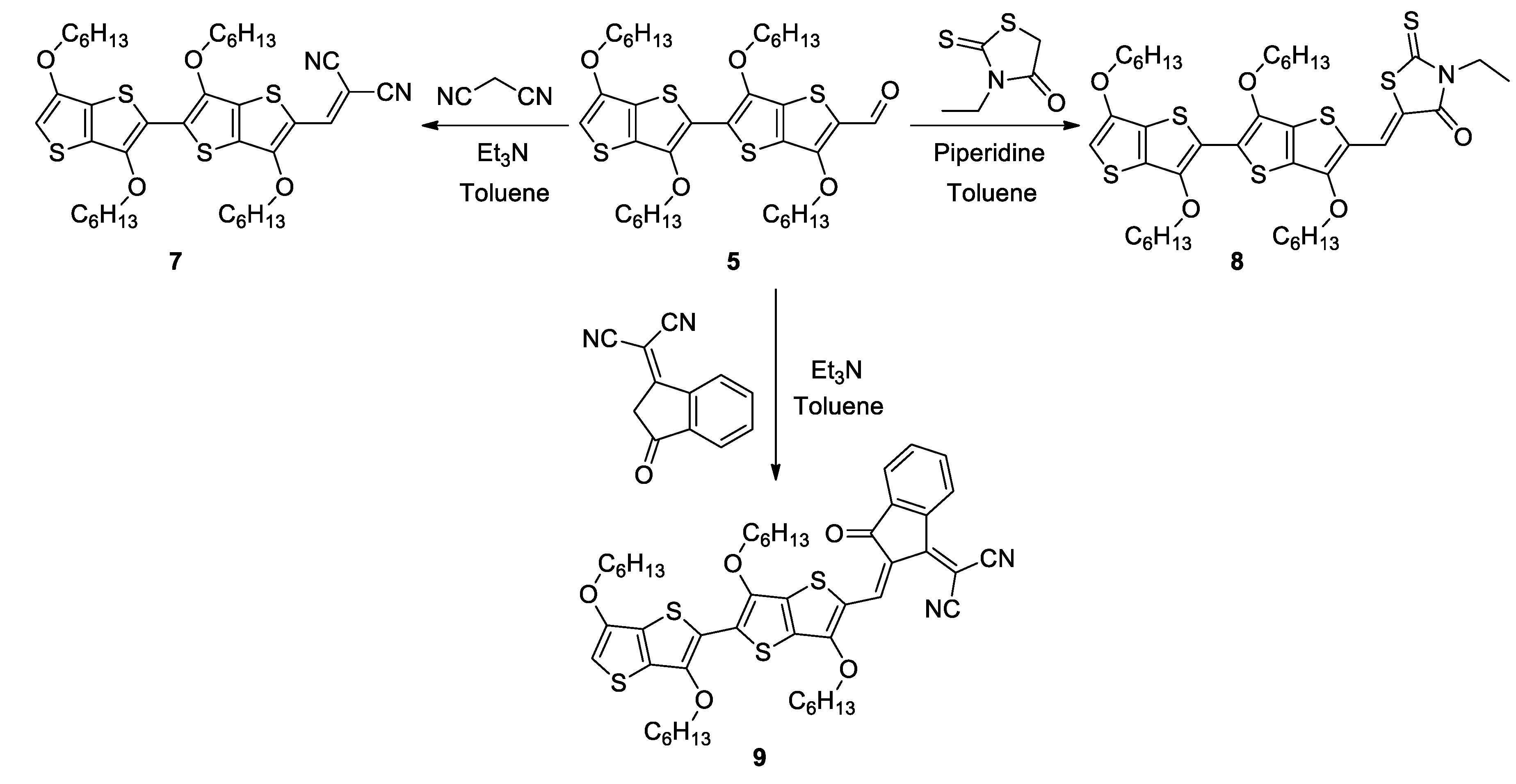
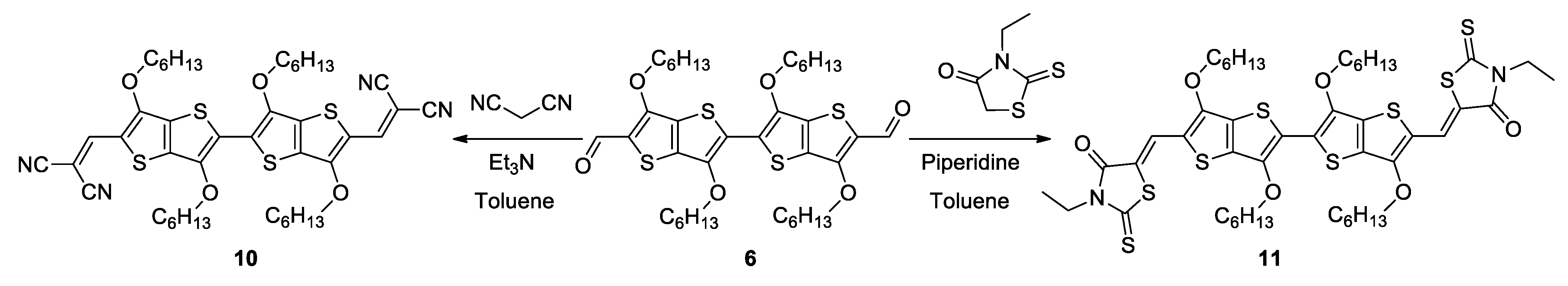
2.1. Synthesis
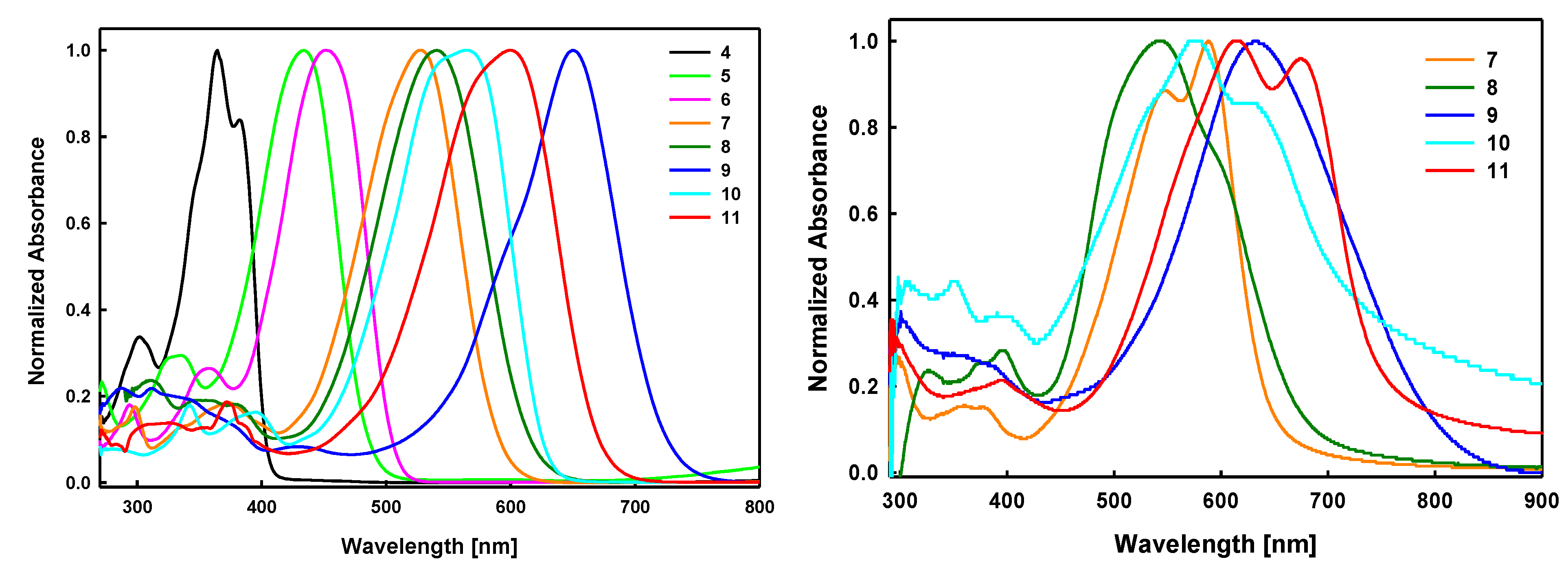
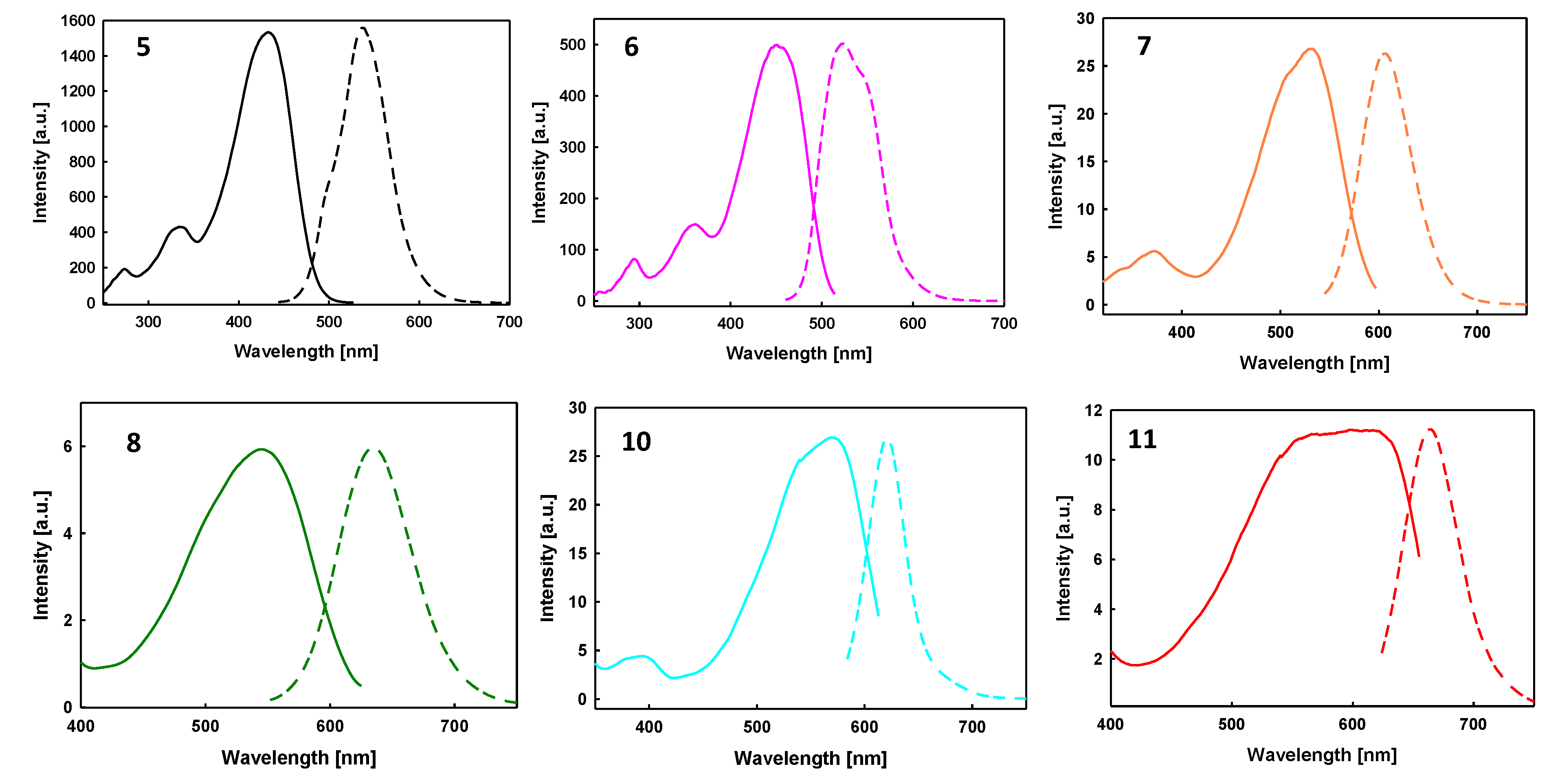
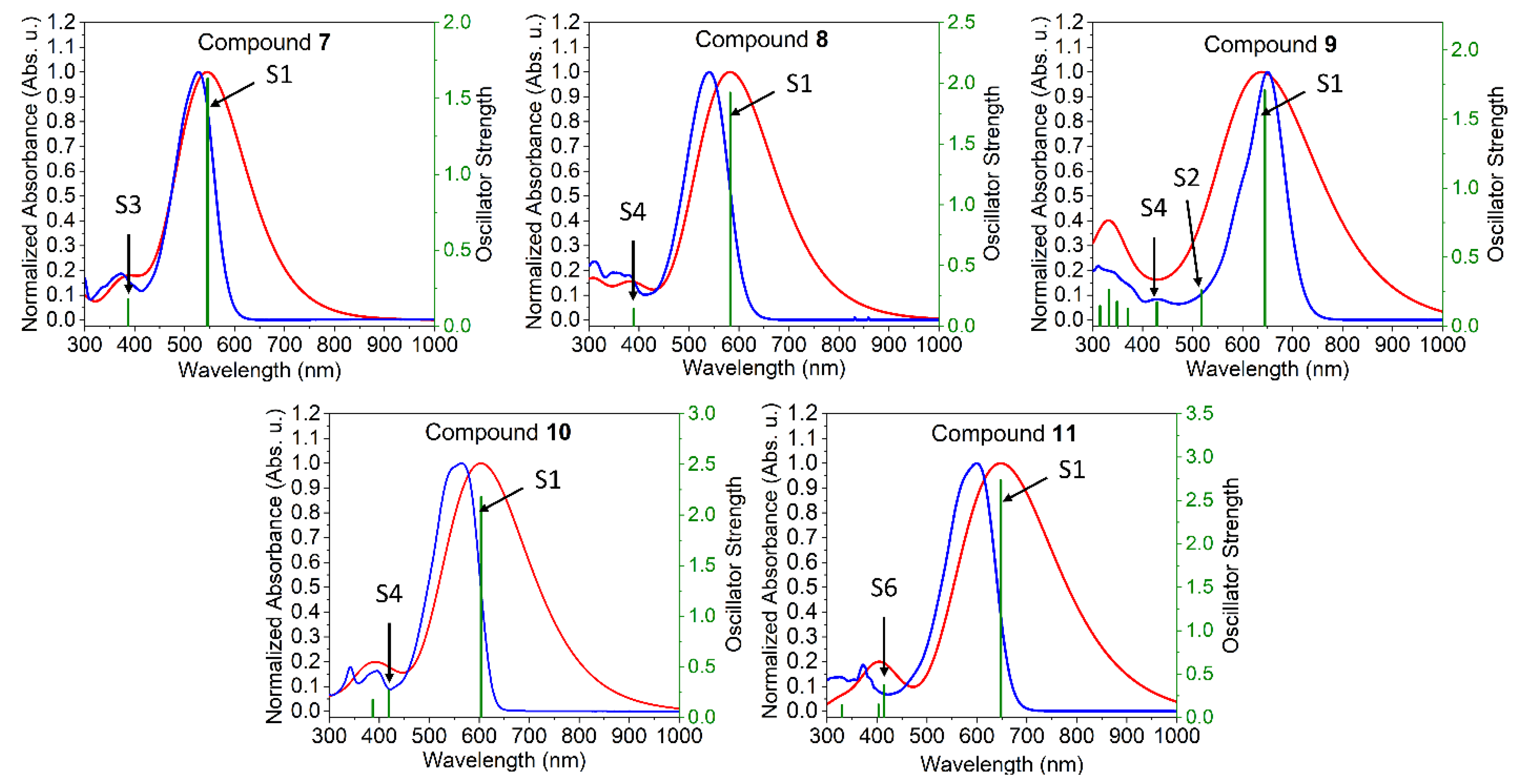
2.2. Optical Properties
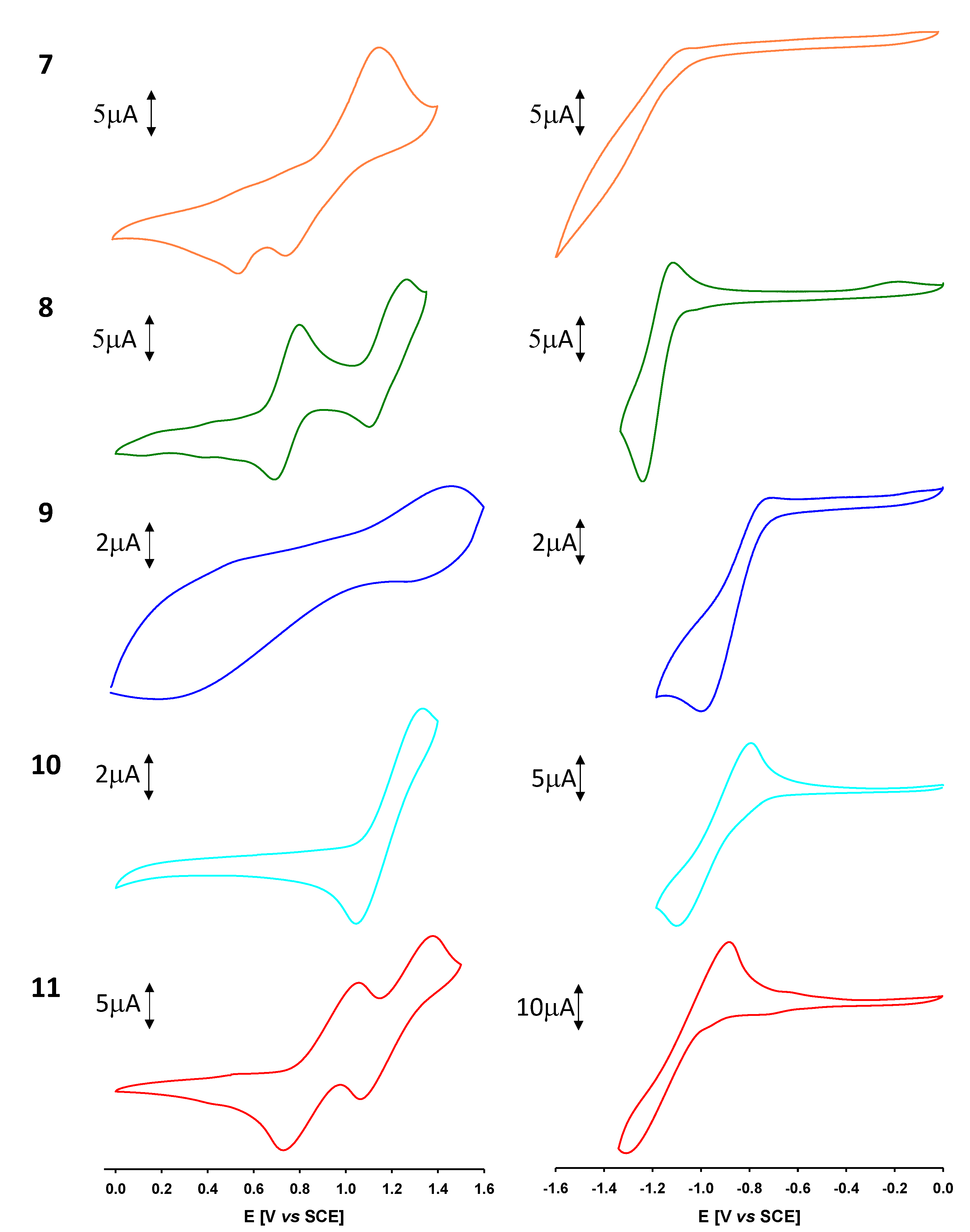
2.3. Electrochemical Properties
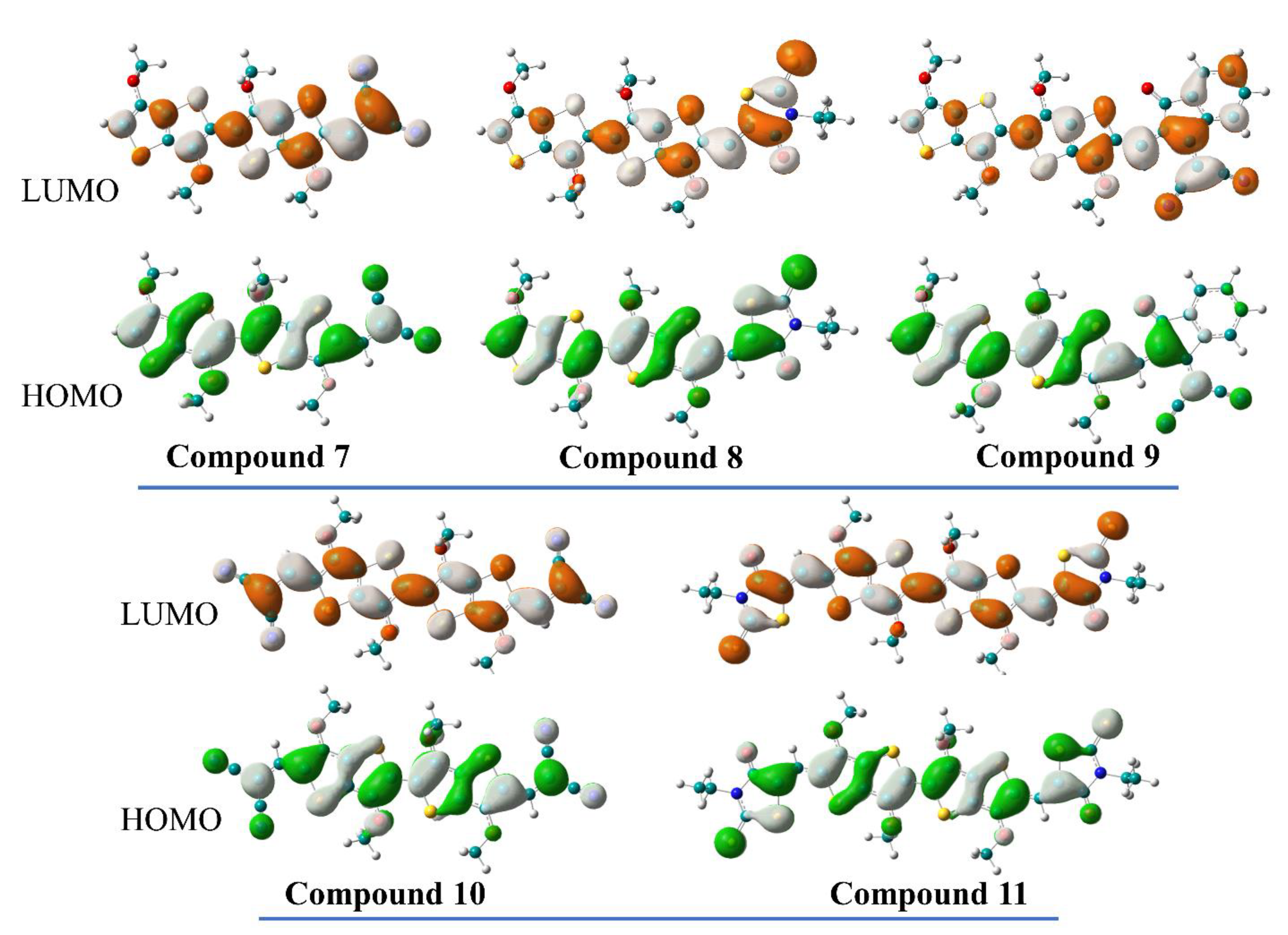
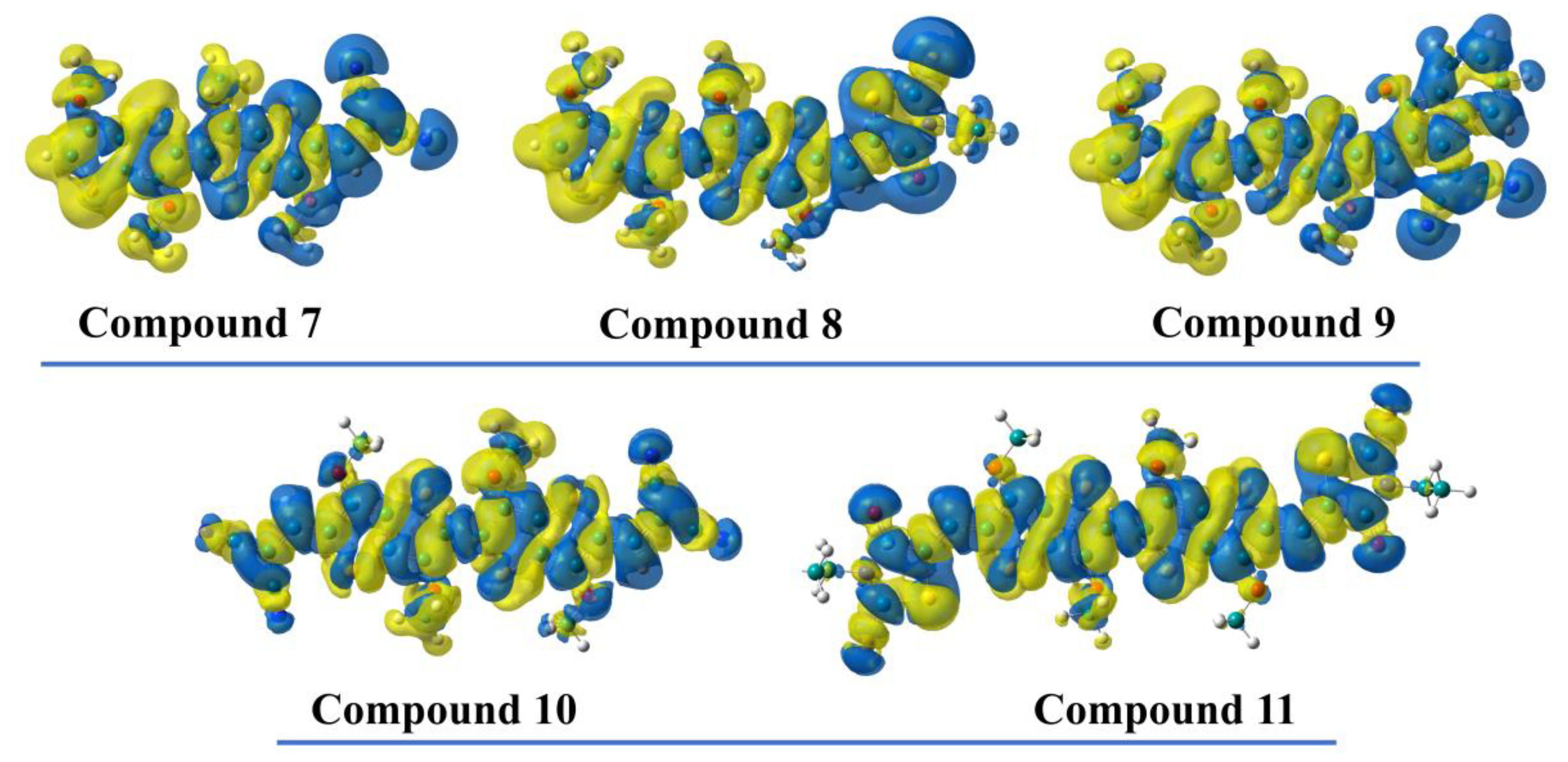
2.4. Theoretical Investigations
3. Materials and Methods
3.1. General Data
3.2. Procedure for the Synthesis of 4
3.3. Procedure for the Synthesis of 5 and 6
3.4. Procedure for the Synthesis of 7 and 10
3.5. Procedure for the Synthesis of 8 and 11
3.6. Procedure for the Synthesis of 9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stanovnik, B. Application of organic azides in the synthesis of heterocyclic systems. Adv. Heterocycl. Chem. 2020, 130, 145–194. [Google Scholar] [CrossRef]
- Heravi, M.M.; Talaei, B. Diketene as Privileged Synthon in the Syntheses of Heterocycles Part 1. Adv. Heterocycl. Chem. 2017, 122, 43–114. [Google Scholar] [CrossRef]
- Heravi, M.M.; Talaei, B. Ketenes as Privileged Synthons in the Syntheses of Heterocyclic Compounds Part 2: Five-Membered Heterocycles. Adv. Heterocycl. Chem. 2015, 114, 147–225. [Google Scholar] [CrossRef]
- Driowya, M.; Bougrin, K.; Benhida, R. Recent advances in microwave-assisted heterocyclic chemistry. Synthesis of three, four and five-membered heterocycles. In Targets in Heterocyclic Systems: Chemistry and Properties; Società Chimica Italiana: Rome, Italy, 2011; Volume 15, pp. 327–422. ISBN 978-88-86208-70-3. [Google Scholar]
- Elgemeie, G.H.; Sayed, S.H. Synthesis and Chemistry of Dithiols. Synthesis 2001, 12, 1747–1771. [Google Scholar] [CrossRef]
- Geng, R.X.; Zhou, C.H. Study on the synthesis of thienothiophene. Chin. J. Org. Chem. 2008, 28, 163–168. [Google Scholar]
- Sommen, G.; Kirsch, G. Thienothiophenes: Synthesis and Applications. Mini-Rev. Org. Chem. 2004, 1, 367–374. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, e1900301. [Google Scholar] [CrossRef]
- Kukolja, S.; Draheim, S.E.; Graves, B.J.; Hunden, D.C.; Pfeil, J.L.; Cooper, R.D.G.; Ott, J.L.; Counter, F.T. Orally absorbable cephalosporin antibiotics. 2. Structure-activity studies of bicyclic glycine derivatives of 7-aminodeacetoxycephalosporanic acid. J. Med. Chem. 1985, 28, 1896–1903. [Google Scholar] [CrossRef]
- Egbertson, M.S.; Cook, J.J.; Bednar, B.; Prugh, J.D.; Bednar, R.A.; Gaul, S.L.; Gould, R.J.; Hartman, G.D.; Homnick, C.F.; Holahan, M.A.; et al. Non-Peptide GPIIb/IIIa Inhibitors. 20. Centrally Constrained Thienothiophene α-Sulfonamides Are Potent, Long Acting in Vivo Inhibitors of Platelet Aggregation. J. Med. Chem. 1999, 42, 2409–2421. [Google Scholar] [CrossRef]
- Williams, T.M.; Hudcosky, R.J.; Hunt, C.A.; Shepard, K.L. The synthesis of substituted 2,3-dihydrothieno[2,3-b]-thiophenesviaintramolecular michael addition. J. Heterocycl. Chem. 1991, 28, 13–16. [Google Scholar] [CrossRef]
- Zhang, X.; Hudson, S.D.; Delongchamp, D.M.; Gundlach, D.J.; Heeney, M.; McCulloch, I. In-Plane Liquid Crystalline Texture of High-Performance Thienothiophene Copolymer Thin Films. Adv. Funct. Mater. 2010, 20, 4098–4106. [Google Scholar] [CrossRef]
- Liedtke, A.; O’Neill, M.; Kelly, S.M.; Kitney, S.P.; Van Averbeke, B.; Boudard, P.; Beljonne, D.; Cornil, J. Optical Properties of Light-Emitting Nematic Liquid Crystals: A Joint Experimental and Theoretical Study. J. Phys. Chem. B 2010, 114, 11975–11982. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.P.; Wong, K.Y.; Jen, A.K.-Y.; Drost, K.J. Functionalized Fused Thiophenes: A New Class of Thermally Stable and Efficient Second-Order Nonlinear Optical Chromophores. Chem. Mater. 1994, 6, 2210–2212. [Google Scholar] [CrossRef]
- Krayushkin, M.M.; Shirinian, V.Z.; Belen’kii, L.I.; Shadronov, A.Y.; Martynkin, A.Y.; Uzhinov, B.M. Synthesis of photochromic derivatives of cyclobutene-1,2-dione. Mendeleev Commun. 2002, 12, 141–143. [Google Scholar] [CrossRef]
- Saidman, S.; Garay, R.; Bessone, J. Kinetic study of 3,6-dimethylthieno[3,2-b]thiophene electropolymerisation. J. Appl. Electrochem. 2001, 31, 839–844. [Google Scholar] [CrossRef]
- Diez, A.S.; Saidman, S.; Garay, R.O. Synthesis of a Thienothiophene Conjugated Polymer. Molecules 2000, 5, 555–556. [Google Scholar] [CrossRef]
- Paik, K.L.; Baek, N.S.; Kim, H.K.; Lee, Y.; Lee, K.J. Synthesis and luminescent properties of novel silicon-based poly(p-phenylene) related polymers containing oxadiazole units for PLED. Thin Solid Film. 2002, 417, 132–135. [Google Scholar] [CrossRef]
- Cai, S.; Tian, G.; Li, X.; Su, J.; Tian, H. Efficient and stable DSSC sensitizers based on substituted dihydroindolo[2,3-b]carbazole donors with high molar extinction coefficients. J. Mater. Chem. A 2013, 1, 11295–11305. [Google Scholar] [CrossRef]
- Lee, M.-W.; Kim, J.-Y.; Lee, D.-H.; Ko, M.J. Novel D-π-A Organic Dyes with Thieno[3,2-b]thiophene-3,4-ethylenedioxythiophene Unit as a π-Bridge for Highly Efficient Dye-Sensitized Solar Cells with Long-Term Stability. ACS Appl. Mater. Interfaces 2014, 6, 4102–4108. [Google Scholar] [CrossRef]
- Cai, N.; Li, R.; Wang, Y.; Zhang, M.; Wang, P. Organic dye-sensitized solar cells with a cobalt redox couple: Influences of π-linker rigidification and dye–bath solvent selection. Energy Environ. Sci. 2013, 6, 139–147. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Mesquita, I.; Andrade, L.; Mendes, A.; Justino, L.L.; Burrows, H.D.; Raposo, M.M.M. Synthesis and characterization of push-pull bithiophene and thieno[3,2-b]thiophene derivatives bearing an ethyne linker as sensitizers for dye-sensitized solar cells. Org. Electron. 2017, 49, 194–205. [Google Scholar] [CrossRef]
- Wei, H.; Chen, W.; Han, L.; Wang, T.; Bao, X.; Li, X.; Liu, J.; Zhou, Y.; Yang, R. A Solution-Processable Molecule using Thieno[3,2-b]thiophene as Building Block for Efficient Organic Solar Cells. Chem. Asian J. 2015, 10, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Kan, B.; Wan, X.; Liu, F.; Ni, W.; Feng, H.; Russell, T.P.; Chen, Y. A solution-processed high performance organic solar cell using a small molecule with the thieno[3,2-b]thiophene central unit. Chem. Commun. 2015, 51, 15268–15271. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wang, Y.; Qiu, N.; Yi, Y.-Q.; Wan, X.; Li, C.; Chen, Y. A Three-dimensional Non-fullerene Small Molecule Acceptor for Solution-processed Organic Solar Cells. Chin. J. Chem. 2017, 35, 1687–1692. [Google Scholar] [CrossRef]
- Shim, H.-S.; Moon, C.-K.; Kim, J.; Wang, C.-K.; Sim, B.; Lin, F.; Wong, K.-T.; Seo, Y.; Kim, J.-J. Efficient Vacuum-Deposited Ternary Organic Solar Cells with Broad Absorption, Energy Transfer, and Enhanced Hole Mobility. ACS Appl. Mater. Interfaces 2016, 8, 1214–1219. [Google Scholar] [CrossRef]
- Patra, D.; Budiawan, W.; Huang, T.-Y.; Wei, K.-H.; Wang, P.-C.; Ho, K.-C.; Al-Hashimi, M.; Chu, C.-W. Enhanced Organic Solar Cell Performance by Lateral Side Chain Engineering on Benzodithiophene-Based Small Molecules. ACS Appl. Energy Mater. 2018, 1, 3684–3692. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Pereira, A.I.; Mendes, A.; Serpa, C.; Pina, J.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Optical and Photovoltaic Properties of Thieno[3,2-b]thiophene-Based Push–Pull Organic Dyes with Different Anchoring Groups for Dye-Sensitized Solar Cells. ACS Omega 2017, 2, 9268–9279. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Su, D.; Shen, F.; Huo, S.; Fu, H.; Zhan, C. Design a thieno[3,2-b]thiophene bridged nonfullerene acceptor to increase open-circuit voltage, short-circuit current-density and fill factor via the ternary strategy. Chin. Chem. Lett. 2019, 31, 1243–1247. [Google Scholar] [CrossRef]
- Wang, W.; Chen, B.; Jiao, X.; Guo, J.; Sun, R.; Guo, J.; Min, J. A new small molecule donor for efficient and stable all small molecule organic solar cells. Org. Electron. 2019, 70, 78–85. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, J.; Hu, Z.; Chen, Y.; Liu, M.; Murtaza, I.; Meng, H. A novel A-D-A small molecule with 1,8-naphthalimide as a potential non-fullerene acceptor for solution processable solar cells. Dye. Pigment. 2017, 142, 39–50. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Lau, T.-K.; Zhu, J.; Wang, J.; Lu, X.; Zhan, X.; Lin, Y. Fused thienobenzene-thienothiophene electron acceptors for organic solar cells. J. Energy Chem. 2018, 37, 58–65. [Google Scholar] [CrossRef]
- Su, J.-M.; Li, Y.-Z.; Chang, Y.-H.; Li, M.-Z.; Qiu, W.-Z.; Liu, S.-W.; Wong, K.-T. Novel thieno[3,2-b]thiophene-embedded small-molecule donors for highly efficient and photostable vacuum-processed organic photovoltaics. Mater. Today Energy 2021, 20, 100633. [Google Scholar] [CrossRef]
- Wang, J.; Xue, P.; Jiang, Y.; Huo, Y.; Zhan, X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 2022, 6, 614–634. [Google Scholar] [CrossRef]
- Biniek, L.; Chochos, C.L.; Leclerc, N.; Hadziioannou, G.; Kallitsis, J.K.; Bechara, R.; Lévêque, P.; Heiser, T. A [3,2-b]thienothiophene-alt-benzothiadiazole copolymer for photovoltaic applications: Design, synthesis, material characterization and device performances. J. Mater. Chem. 2009, 19, 4946–4951. [Google Scholar] [CrossRef]
- Yan, T.; Bin, H.; Sun, C.; Zhang, Z.; Li, Y. Effect of Thieno[3,2-b]thiophene π-bridge on photovoltaic performance of a D-A copolymer of alkoxy-benzodithiophene-alt-fluoro-benzotriazole. Org. Electron. 2018, 55, 106–111. [Google Scholar] [CrossRef]
- Karaman, C.Z.; Göker, S.; Şahin, Ü.; Hacioglu, S.O.; Aslan, S.T.; Hacıefendioğlu, T.; Hizalan, G.; Yıldırım, E.; Çırpan, A.; Toppare, L. Effect of thiophene, 3-hexylthiophene, selenophene, and Thieno[3,2-b]thiophene spacers on OPV device performance of novel 2,1,3-benzothiadiazole based alternating copolymers. J. Electroanal. Chem. 2021, 895, 115483. [Google Scholar] [CrossRef]
- Kini, G.P.; Oh, S.; Abbas, Z.; Rasool, S.; Jahandar, M.; Song, C.E.; Lee, S.K.; Shin, W.S.; So, W.-W.; Lee, J.-C. Effects on Photovoltaic Performance of Dialkyloxy-benzothiadiazole Copolymers by Varying the Thienoacene Donor. ACS Appl. Mater. Interfaces 2017, 9, 12617–12628. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, M.; Li, B.; Jiang, C.; Li, Q. High efficiency organic photovoltaics devices based on isoindigo conjugated polymers with a thieno[3,2-b]thiophene π-bridge. J. Mater. Chem. A 2016, 4, 16064–16072. [Google Scholar] [CrossRef]
- Fan, B.; Xue, X.; Meng, X.; Sun, X.; Huo, L.; Ma, W.; Sun, Y. High-performance conjugated terpolymer-based organic bulk heterojunction solar cells. J. Mater. Chem. A 2016, 4, 13930–13937. [Google Scholar] [CrossRef]
- Ha, J.-W.; Song, C.E.; Kim, H.S.; Ryu, D.H.; Shin, W.S.; Hwang, D.-H. Highly Efficient and Photostable Ternary Organic Solar Cells Enabled by the Combination of Non-Fullerene and Fullerene Acceptors with Thienopyrrolodione-based Polymer Donors. ACS Appl. Mater. Interfaces 2020, 12, 51699–51708. [Google Scholar] [CrossRef]
- Terenti, N.; Giurgi, G.-I.; Crişan, A.P.; Anghel, C.; Bogdan, A.; Pop, A.; Stroia, I.; Terec, A.; Szolga, L.; Grosu, I.; et al. Structure–properties of small donor–acceptor molecules for homojunction single-material organic solar cells. J. Mater. Chem. C 2022, 10, 5716–5726. [Google Scholar] [CrossRef]
- Nan, M.I.; Lakatos, E.; Giurgi, G.-I.; Szolga, L.; Po, R.; Terec, A.; Jungsuttiwong, S.; Grosu, I.; Roncali, J. Mono- and di-substituted pyrene-based donor-π-acceptor systems with phenyl and thienyl π-conjugating bridges. Dye. Pigment. 2020, 181, 108527. [Google Scholar] [CrossRef]
- Demeter, D.; Mohamed, S.; Diac, A.; Grosu, I.; Roncali, J. Small Molecular Donors for Organic Solar Cells Obtained by Simple and Clean Synthesis. ChemSusChem 2014, 7, 1046–1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piron, F.; Leriche, P.; Grosu, I.; Roncali, J. Electropolymerizable 3Dπ-conjugated architectures with ethylenedioxythiophene (EDOT) end-groups as precursors of electroactive conjugated networks. J. Mater. Chem. 2010, 20, 10260–10268. [Google Scholar] [CrossRef][Green Version]
- Terenti, N.; Crisan, A.P.; Jungsuttiwong, S.; Hădade, N.D.; Pop, A.; Grosu, I.; Roncali, J. Effect of the mode of fixation of the thienyl rings on the electronic properties of electron acceptors based on indacenodithiophene (IDT). Dye. Pigment. 2020, 187, 109116. [Google Scholar] [CrossRef]
- Hergué, N.; Frère, P.; Roncali, J. Efficient synthesis of 3,6-dialkoxythieno[3,2-b]thiophenes as precursors of electrogenerated conjugated polymers. Org. Biomol. Chem. 2011, 9, 588–595. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Leriche, P.; Mercier, N.; Roncali, J. Poly(3,6-dimethoxy-thieno[3,2-b]thiophene): A possible alternative to poly(3,4-ethylenedioxythiophene) (PEDOT). Chem. Commun. 2005, 9, 1161–1163. [Google Scholar] [CrossRef][Green Version]
- Diac, A.; Demeter, D.; Allain, M.; Grosu, I.; Roncali, J. Simple and Versatile Molecular Donors for Organic Photovoltaics Prepared by Metal-Free Synthesis. Chem. A Eur. J. 2015, 21, 1598–1608. [Google Scholar] [CrossRef]
- Trasatti, S. The absolute electrode potential: An explanatory note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Crăciun, A.; Focșan, M.; Găină, L.; Aștilean, S. Enhanced one- and two-photon excited fluorescence of cationic (phenothiazinyl)vinyl-pyridinium chromophore attached to polyelectrolyte-coated gold nanorods. Dye. Pigment. 2017, 136, 24–30. [Google Scholar] [CrossRef]

| 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|
| Optical | |||||
| λmax [nm] a | 528 | 540 | 650 | 565 | 600 |
| εmax [M−1cm−1] | 62,500 | 61,700 | 89,300 | 79,800 | 98,000 |
| ∆E [eV] b | 2.08 | 2.00 | 1.71 | 1.97 | 1.85 |
| λmax [nm] c | 550, 588 | 543 | 635 | 577, 633 | 615, 676 |
| Egopt [eV] d | 1.92 | 1.84 | 1.53 | 1.70 | 1.66 |
| Electrochemical | |||||
| Epa1, Epa2 [eV] | 1.16 | 1.08, 1.27 | 1.46 | 1.33 | 1.06, 1.38 |
| Epc1 [eV] | −1.26 | −1.00 | −1.11 | −1.30 | |
| EHOMO [eV] e | −5.55 | −5.32 | −5.25 | −5.75 | −5.48 |
| ELUMO [eV] e | −3.62 | −3.58 | −3.94 | −3.86 | −3.71 |
| Eg [eV] e | 1.93 | 1.74 | 1.31 | 1.89 | 1.77 |
| B3LYP-D3/Def2-TZVP | |||||
| EHOMO [eV] f | −5.52 | −5.38 | −5.46 | −5.82 | −5.42 |
| ELUMO [eV] f | −2.91 | −2.86 | −3.18 | −3.43 | −3.16 |
| Eg [eV] | 2.61 | 2.52 | 2.28 | 2.39 | 2.26 |
| 5 | 6 | 7 | 8 | 10 | 11 | |
|---|---|---|---|---|---|---|
| λexc [nm] | 434 | 450 | 535 | 542 | 574 | 614 |
| λem [nm] | 537 | 524 | 607 | 635 | 623 | 665 |
| Stokes Shift [cm−1] | 4420 | 3138 | 2218 | 2702 | 1370 | 1250 |
| QY [%] | 77 a | 18 a | 10 b | 1 b | 6 b | <1 b |
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|
| τav [ns] a | 2.6 | 0.69 | 1.23 | 0.49 | 0.29 | 0.78 | 0.77 |
| kr [ns−1] b | 0.296 | 0.261 | 0.081 | 0.020 | 0.077 | 0.013 | |
| knr [ns−1] c | 0.088 | 1.188 | 0.732 | 2.020 | 1.205 | 1.286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrian, L.; Giurgi, G.-I.; Stroia, I.; Bogdan, E.; Crişan, A.P.; Hădade, N.D.; Grosu, I.; Terec, A. Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′-bi[3,2-b]thienothiophene Derivatives. Molecules 2022, 27, 8463. https://doi.org/10.3390/molecules27238463
Gabrian L, Giurgi G-I, Stroia I, Bogdan E, Crişan AP, Hădade ND, Grosu I, Terec A. Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′-bi[3,2-b]thienothiophene Derivatives. Molecules. 2022; 27(23):8463. https://doi.org/10.3390/molecules27238463
Chicago/Turabian StyleGabrian, Levi, Gavril-Ionel Giurgi, Ioan Stroia, Elena Bogdan, Andreea Petronela Crişan, Niculina Daniela Hădade, Ion Grosu, and Anamaria Terec. 2022. "Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′-bi[3,2-b]thienothiophene Derivatives" Molecules 27, no. 23: 8463. https://doi.org/10.3390/molecules27238463
APA StyleGabrian, L., Giurgi, G.-I., Stroia, I., Bogdan, E., Crişan, A. P., Hădade, N. D., Grosu, I., & Terec, A. (2022). Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′-bi[3,2-b]thienothiophene Derivatives. Molecules, 27(23), 8463. https://doi.org/10.3390/molecules27238463









