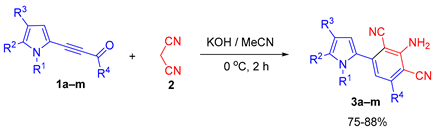
Abstract
An efficient method for the synthesis of pharmaceutically and high-tech prospective 2-(3-amino-2,4-dicyanophenyl)pyrroles (in up to 88% yield) via the reaction of easily available substituted acylethynylpyrroles with malononitrile has been developed. The reaction proceeds in the KOH/MeCN system at 0 °C for 2 h. In the case of 2-acylethynylpyrroles without substituents in the pyrrole ring, the reaction changes direction: instead of the target 2-(3-amino-2,4-dicyanophenyl)pyrroles, the unexpected formation of pyrrolyldienols and products of their intramolecular cyclization, 3-amino-1-acylethylidene-2-cyanopyrrolizines, is observed.
1. Introduction8b 5t
Substituted pyrroles are of special significance as key structural units of many natural products [1,2,3,4], pharmacologically active compounds [5,6,7,8], including drugs based on them [9,10,11,12,13], and high-tech materials [14,15,16,17,18]. Among them, of particular importance are aryl-substituted pyrroles, having diverse practically useful properties [19,20,21,22,23,24,25,26,27]. For instance, many pharmaceuticals, such as anti-hyperlipidemic atorvastatin [9,10], potassium-competitive acid blocker vonoprazan [28], marine-derived natural product with anti-tumor activity neolamellarin [29], fluorescent anion sensors [30,31], BODIPY dyes [32,33,34,35,36,37] and ligands for transition metals [38] were designed based on arylpyrroles. Certain arylpyrrole derivatives are intermediates in the synthesis of heterocyclic compounds such as 5-aryl-1,2-dihydro-1-pyrrolizinones with potential anti-inflammatory and analgesic activities [39] or analogs of anticancer drug Mitomycin C, arylpyrrolizines [40].
The recent syntheses of arylpyrroles are based on the introduction of aryl moieties in the pyrrole ring [41,42,43,44,45,46,47] (for instance, the Suzuki–Miyaura coupling [44,47]) and construction of the pyrrole cycle from acyclic compounds with aryl substituents [48,49,50,51]. The reactions of alkylarylketoximes with acetylene [52] or its precursor, calcium carbide [50], which allow the obtainment of various 2-arylpyrroles, are considered to be of a general character. However, even these reactions do not enable the synthesis of pyrroles with some aryl substituents, (e.g., containing both amino and nitrile groups in the benzene ring, in particular, 2,6-dicyanoaniline). This is, probably, due to the inaccessibility of the starting reagents.
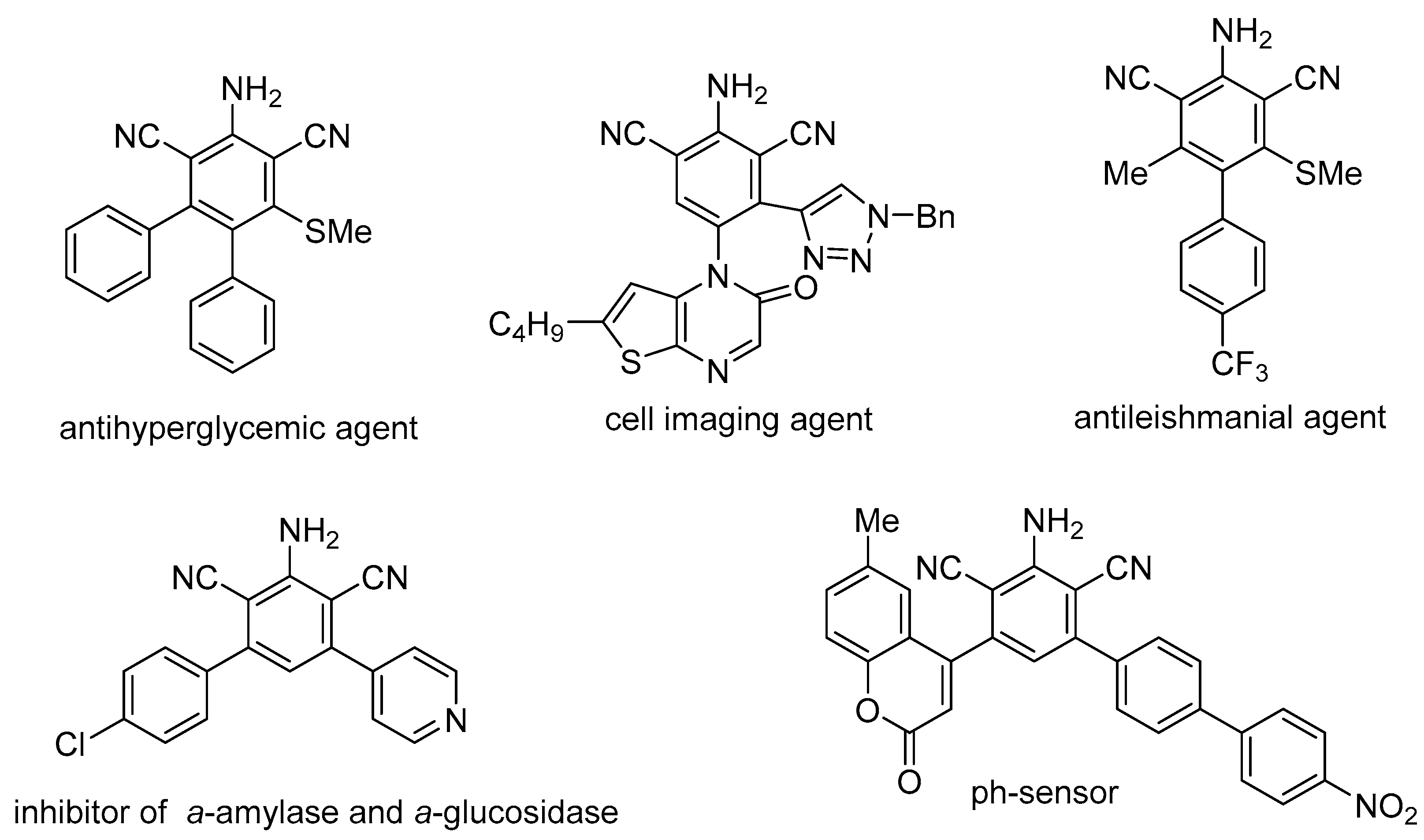
At the same time, the compounds containing 2,6-dicyanoaniline scaffold exhibit various biological activities [53,54,55], strong fluorescence [55,56,57,58,59] and may be used as nonlinear optical materials [55,60,61] and cell imaging agents [55,62] (Figure 1). Furthermore, they serve as building blocks for the synthesis of diverse biologically active compounds and a large number of heterocyclic derivatives, including indoles, quinazolines, fluorenones, indazoles, benzoxazines and benzotriazinones [55].
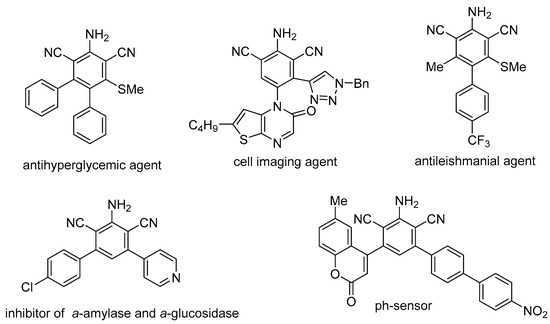
Figure 1.
Biologically active and high-tech 2,6-dicyanoaniline derivatives.
In the light of the foregoing, the synthesis of arylpyrroles with amino and cyano substituents from readily available starting compounds is an important challenge.
Diverse methods for the preparation of 2,6-dicyanoanilines are summarized in review [55].
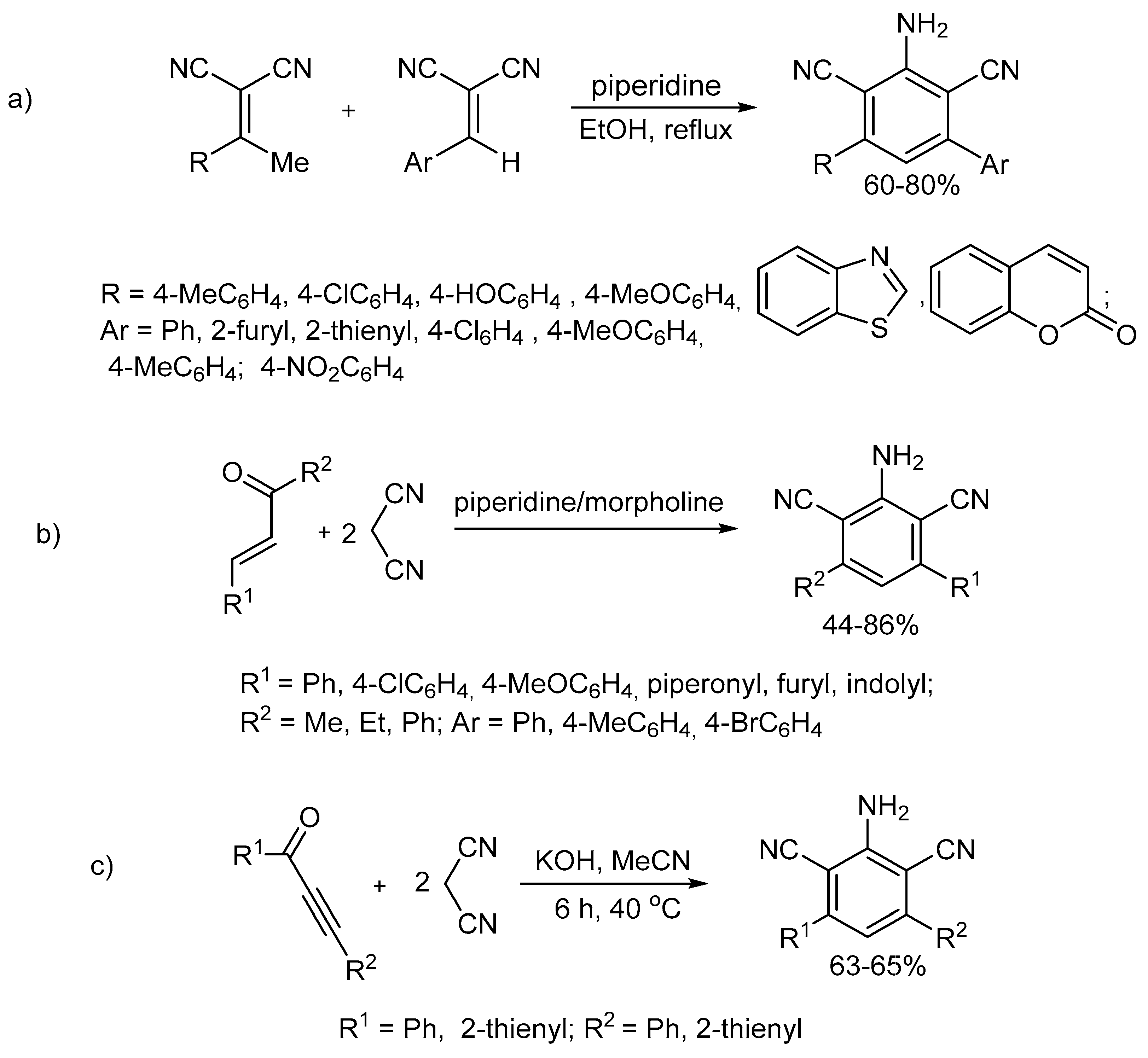
However, only some of them allow 3,5-disubstituted 2,6-dicyanoanilines with hetocyclic (except for pyrrole) substituents to be synthesized (Scheme 1) [55]. These are base-catalyzed reactions of 2-arylidenemalononitrile with 2-(1-arylethylidene)malononitrile (Scheme 1a) and ethylenic ketones with malononitrile (Scheme 1b).
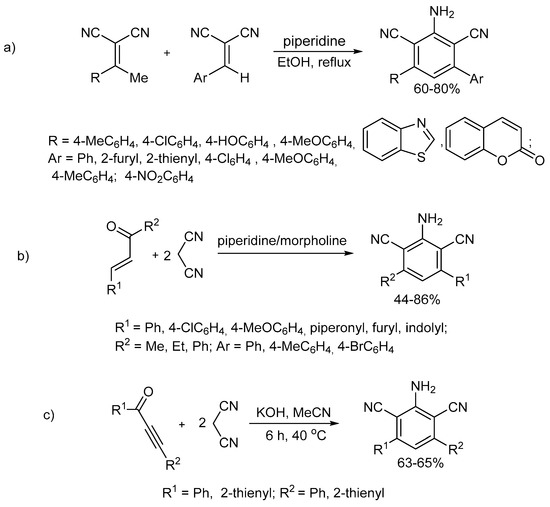
Scheme 1.
Methods for the synthesis of 3,5-disubstituted 2,4-dicyanoanilines with heterocyclic substituents: previous works. The reaction of 2-arylidenemalononitrile with 2-(1-arylethylidene)malononitrile (a). The reaction of ethylenic ketones with malononitrile (b). The reaction of cyclization of acetylenic ketones with malononitrile (c).
Our attention was drawn to the methods based on the cyclization of acetylenic ketones with malononitrile, although they did not give 2,6-dicyanoanilines with heterocyclic substituents [56,63,64]. However, these methods have several shortcomings, such as low reaction selectivity or non-mild reaction conditions (reflux in toluene) and the large span of the yields (16–70%). Later [65], the conditions for the selective formation of 3,5-diaryl-2,6-dicyanoanilines via the same reaction with a yield of up to 87% were found (KOH/MeCN, 40 °C, 6 h) (Scheme 1c). However, the substrate scope of this reaction is limited only to aryl and thienyl substituents. Perhaps this is due to the inaccessibility of acetylenic ketones with other substituents, such as, in particular, acylethynylpyrrolylketones.
Recently, thanks to the discovery and development of room temperature reaction of pyrroles with electrophilic haloacetylenes in the medium of solid metal oxides and salts, acetylenic ketones with pyrrole and aryl or hetaryl substituents in acyl moiety have become readily available [66,67,68,69,70] and widely used as a rewarding platform for design of polyfunctional pyrrole compounds [71,72].
2. Results and Discussion
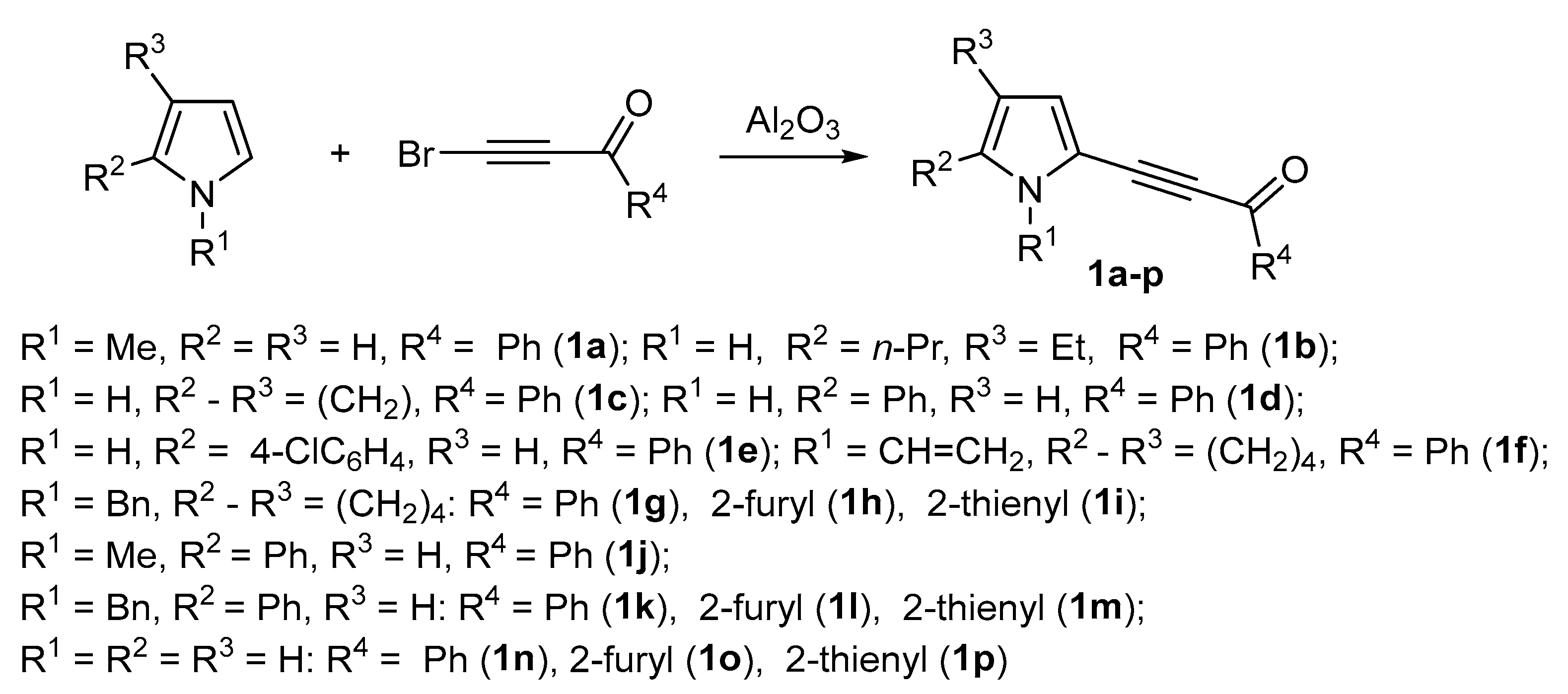
In the present paper, we describe the reaction of acylethynylpyrroles 1a–p, synthesized according to Scheme 2, with malononitrile 2. The study has been undertaken in order to develop an effective method for the synthesis of pyrroles with a 2,6-dicyanoaniline substituent.
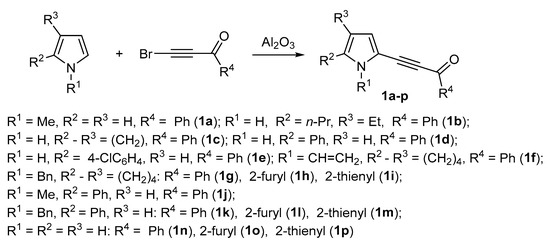
Scheme 2.
Synthesis of 2-acylethynylpyrroles 1a–p.
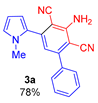
According to our preliminary experiments, under the abovementioned conditions [65] (KOH/MeCN, 40 °C, 6 h), the reaction of 1-methyl-2-(benzoylethynyl)pyrrole 1a with malononitrile 2 (the ratio 1a : 2 : KOH is 1 : 2 : 2) did not afford the expected product, the process was accompanied by resinification. Therefore, in order to find suitable conditions for the construction of 1-methyl-2-(3-amino-2,4-dicyanophenyl)pyrrole 3a, we further carried out the reaction of acylethynylpyrrole 1a with malononitrile 2 at room temperature. However, the tarring of the reaction mixture was also observed in this case. It was possible to obtain pyrrole 3a in 78% yield only at 0 °C for 2 h. It should be emphasized that the reaction is completely selective: no other products except for pyrrole 3a were found in the reaction mixture.
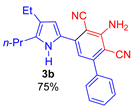
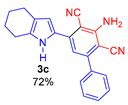
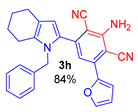
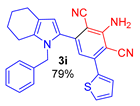
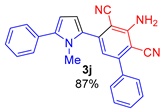
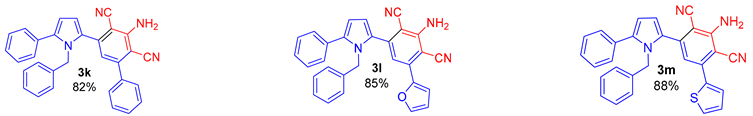
Since the conversion of the starting acylethynylpyrrole 1a was complete under the conditions employed, we used them for the reaction of malononitrile with other substituted acylethynylpyrroles 1b–1m. As a result, a number of previously unknown 2-(3-amino-2,4-dicyanophenyl)pyrroles 3b–3m were synthesized in good to excellent yields (Table 1).

Table 1.
Synthesis of 2-(3-amino-2,4-dicyanophenyl)pyrroles 3a–m from 2-acylethynylpyrroles 1a–m and malononitrile 2.
As follows from Table 1, the reaction is equally effective for acylethynylpyrroles with alkyl and aryl substituents at carbon atoms in the pyrrole ring and also for 4,5,6,7-tetrahydroindole derivatives. The nature of the substituent at the acyl function (aryl or hetaryl), and at the nitrogen atom (H, methyl, benzyl or vinyl) almost does not affect the outcome of the reaction.
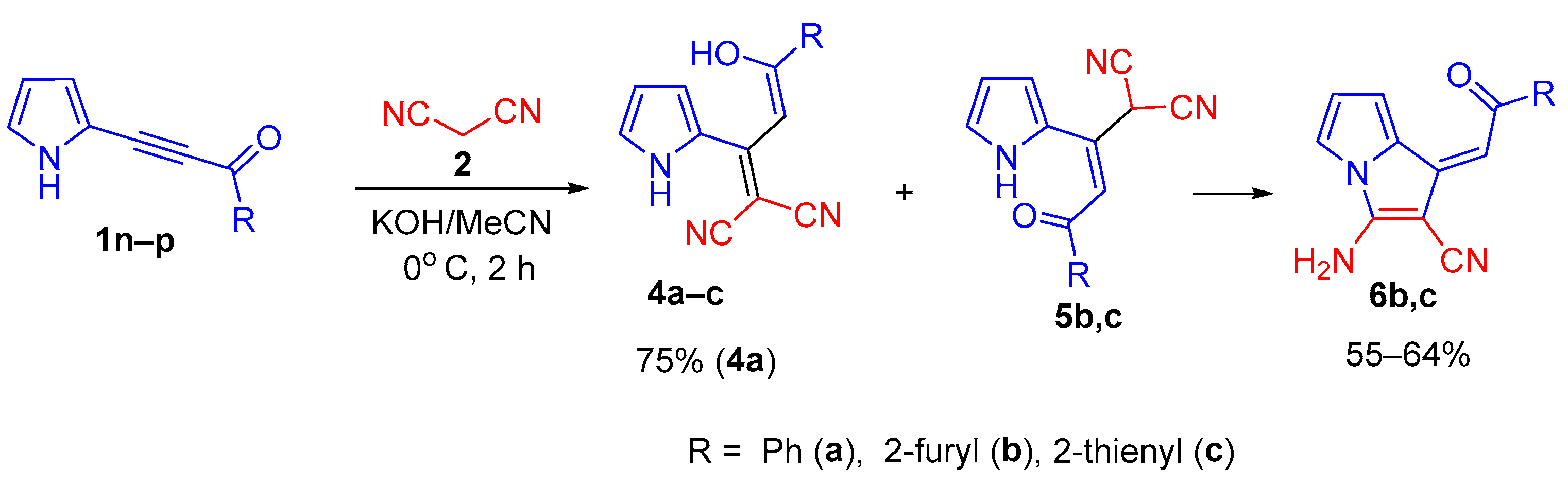
To our surprise, in the case of acylethynylpyrroles 1n–p with unsubstituted pyrrole ring, we encountered quite a different reaction: under the same conditions (KOH/MeCN, the ratio of 1 : 2 : base is 1 : 2 : 2, 0 °C, 2 h), instead of the expected 2-(3-amino-2,4-dicyanophenyl)pyrroles 3, the adducts of malononitrile and acylethynylpyrrole in the enol form, pyrrolyldienols 4a–c, were formed. The latter were isolated either in pure form (in the case of enol 4a) or in the mixtures (in the case of enols 4b,c) with small amounts (10–14%) of their keto tautomers 5b,c (Scheme 3).
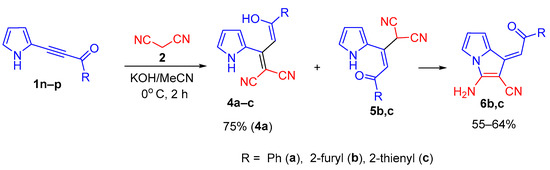
Scheme 3.
Reaction of acylethynylpyrroles 1n–p with malononitrile in the KOH/MeCN system.
It should be emphasized that, according to the 1H NMR data, dienols 4a–c and ketones 5a–c are Z-isomers, the latter being stabilized by intramolecular hydrogen bond between the NH-proton and C = O group (δ NH—15.4 15.9 ppm).
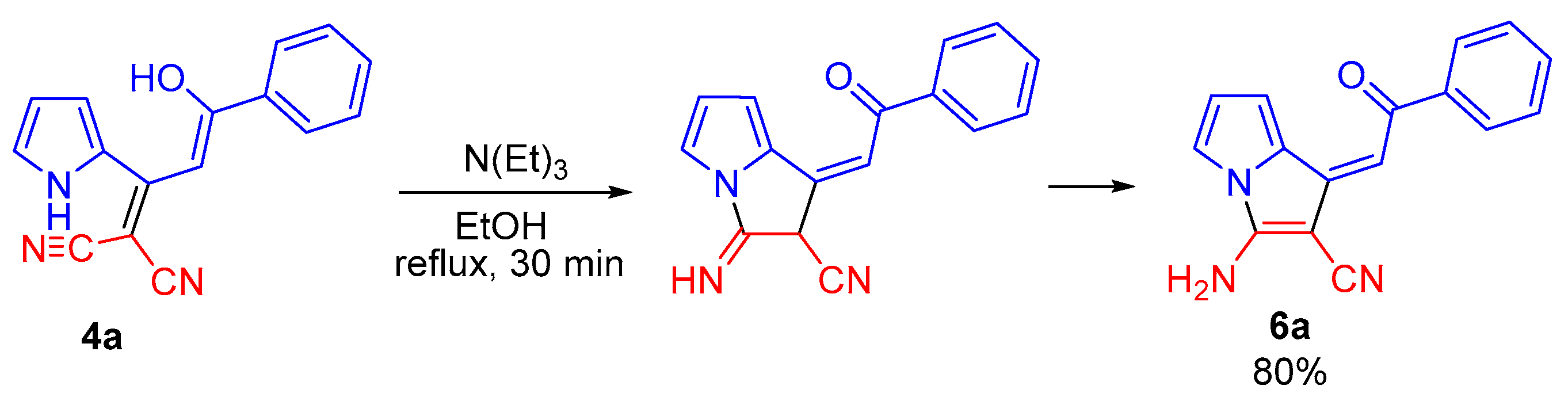
During isolation and drying of the crude product (especially at elevated temperature), the mixture of tautomers 4b,c and 5b,c was selectively transformed to aminopyrrolizines 6b,c in 55 and 64% yields, respectively. The corresponding enol 4a turned out to be stable and was isolated in 75% yield without impurity of keto-tautomer and the corresponding pyrrolizine. Aminopyrrolizine 6a was prepared in 80% yield by refluxing enol 4a in ethanol in the presence of triethylamine (Scheme 4).
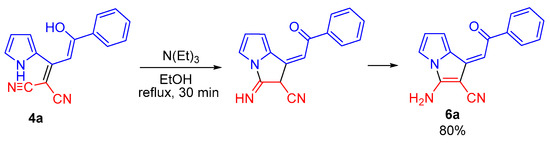
Scheme 4.
Synthesis of pyrrolizine 6a by intramolecular cyclization of enol 4a.
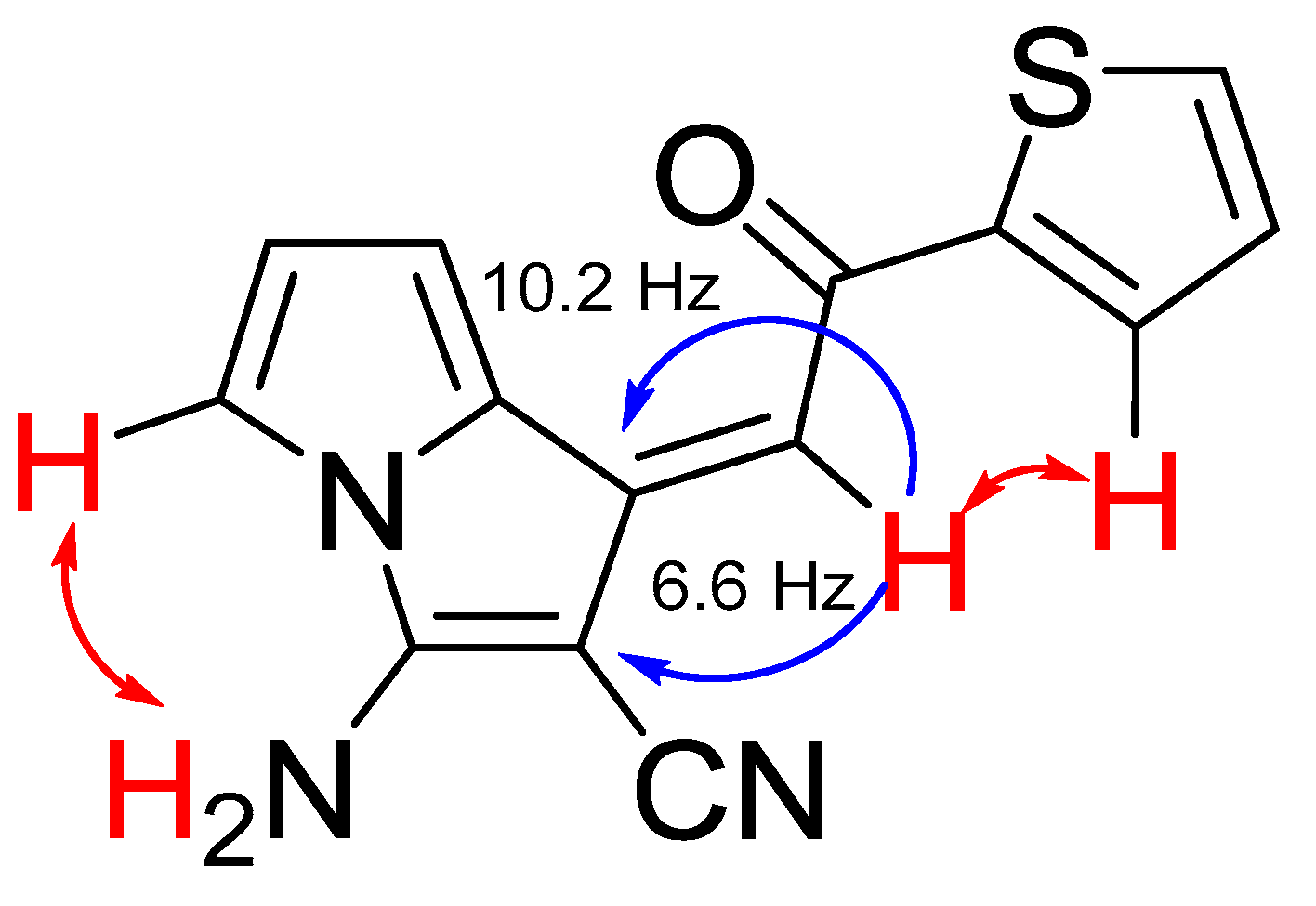
The formation of aminopyrrolizines obviously °C curs as intramolecular addition of the NH pyrrole moiety to the cyano group followed by prototropic isomerization of the imino-group to NH2 substituent (Scheme 3). The formation of aminopyrrolizines is strictly stereoselective: the Z-isomers are formed exclusively with cis-position of the proton at the double bond and the ortho-proton of aryl group, which follows from the main NOESY ( ) and HMBC (
) and HMBC ( ) correlations in 2D NMR spectra (Figure 2). 2M NMR spectra of aminopyrrolizine 6c confirm its the Z-form.
) correlations in 2D NMR spectra (Figure 2). 2M NMR spectra of aminopyrrolizine 6c confirm its the Z-form.
 ) and HMBC (
) and HMBC ( ) correlations in 2D NMR spectra (Figure 2). 2M NMR spectra of aminopyrrolizine 6c confirm its the Z-form.
) correlations in 2D NMR spectra (Figure 2). 2M NMR spectra of aminopyrrolizine 6c confirm its the Z-form.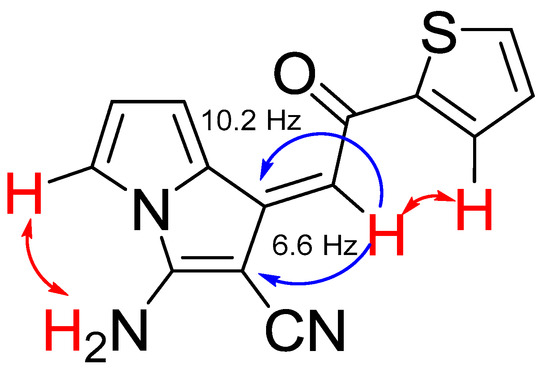
Figure 2.
Main NOESY ( ) and HMBC (
) and HMBC ( ) correlations for pyrolizine 6c.
) correlations for pyrolizine 6c.
 ) and HMBC (
) and HMBC ( ) correlations for pyrolizine 6c.
) correlations for pyrolizine 6c.
Thus, the substituent-dependent divergent synthesis of three different products has been achieved: in the case of acylethynylpyrroles with substituents in the pyrrole ring, 2-(3-amino-2,4-dicyanophenyl)pyrroles 3 are formed, while for substrate with unsubstituted pyrrole ring, pyrrolyldienols 4 and their keto-tautomers 5 are produced, which latter can be cyclized to aminopyrrolizines 6.
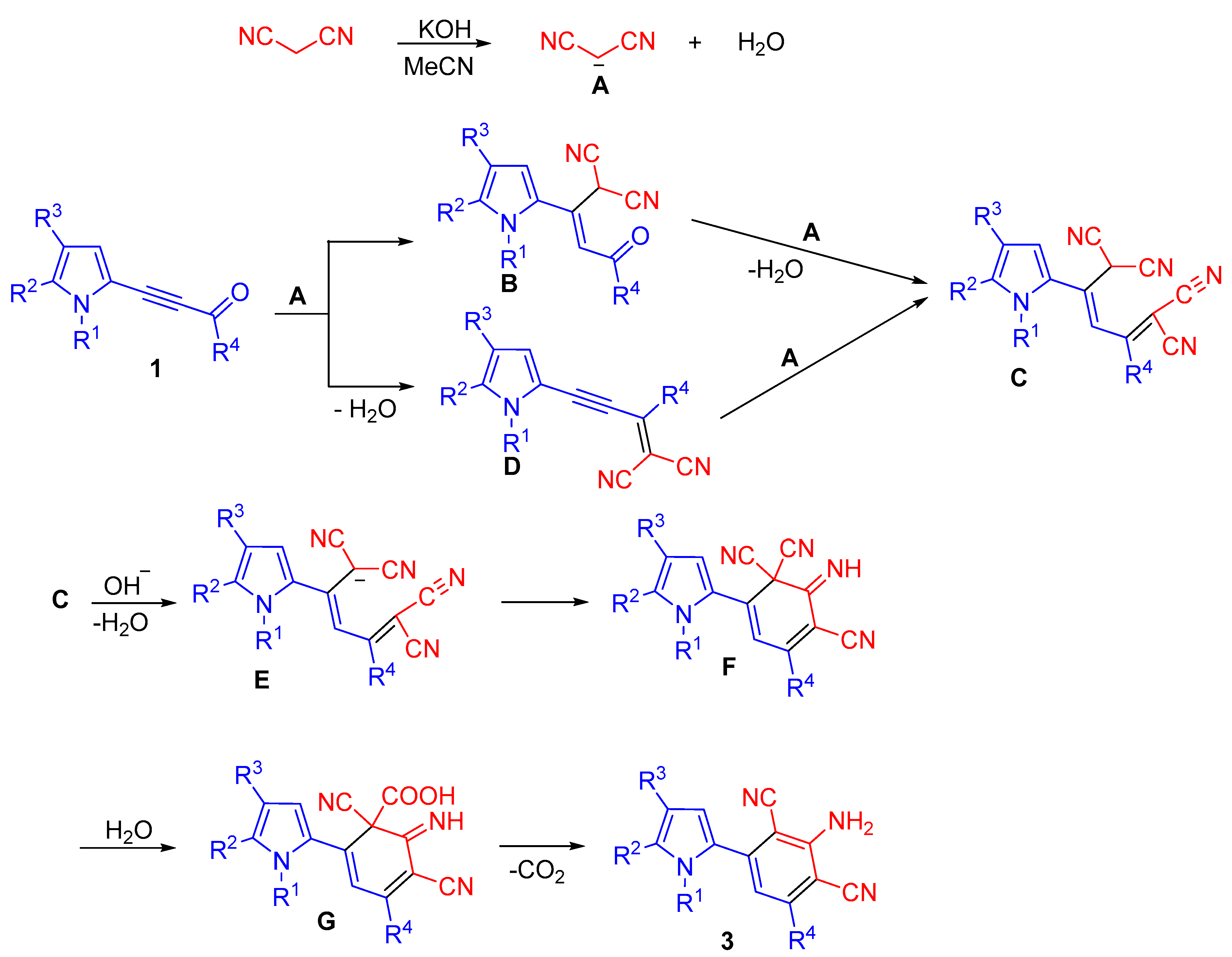
Apparently, the formation of 2-(3-amino-2,4-dicyanophenyl)pyrroles 3 starts with proton abstraction from the CH2-group of malononitrile. The carbanion A, thus generated, adds to the triple bond of acylethynylpyrroles 1, followed by Knoevenagel condensation of the adduct B, thus formed, with second molecule of malononitrile. Otherwise, malononitrile first reacts with the carbonyl group, and its second molecule adds to the triple bond of the intermediate pyrrolylenyne D. Next, diadduct C undergoes intramolecular cyclization with the participation of carbanion E to give cyclic imine F. Hydrolysis of one of the nitrile groups followed by decarboxylation and aromatization of the intermediate G finishes the process (Scheme 5).
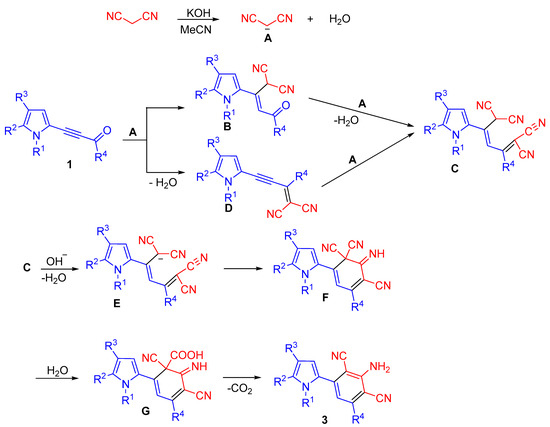
Scheme 5.
Possible scheme of formation of 2-(3-amino-2,4-dicyanophenyl)pyrroles 3 from 2-acylethynylpyrroles 1 and malononitrile 2.
Our assumption that one of the stages in the assembly of the aniline ring is the addition of acetonitrile to the carbonyl group, as we recently showed on the example of the formation of pyridines from acylethynylpyrroles [73], was not confirmed: when the reaction of acylethynylpyrrole 1d with malononitrile was carried out without acetonitrile in THF, the yield of pyrrole 3d was 38%.
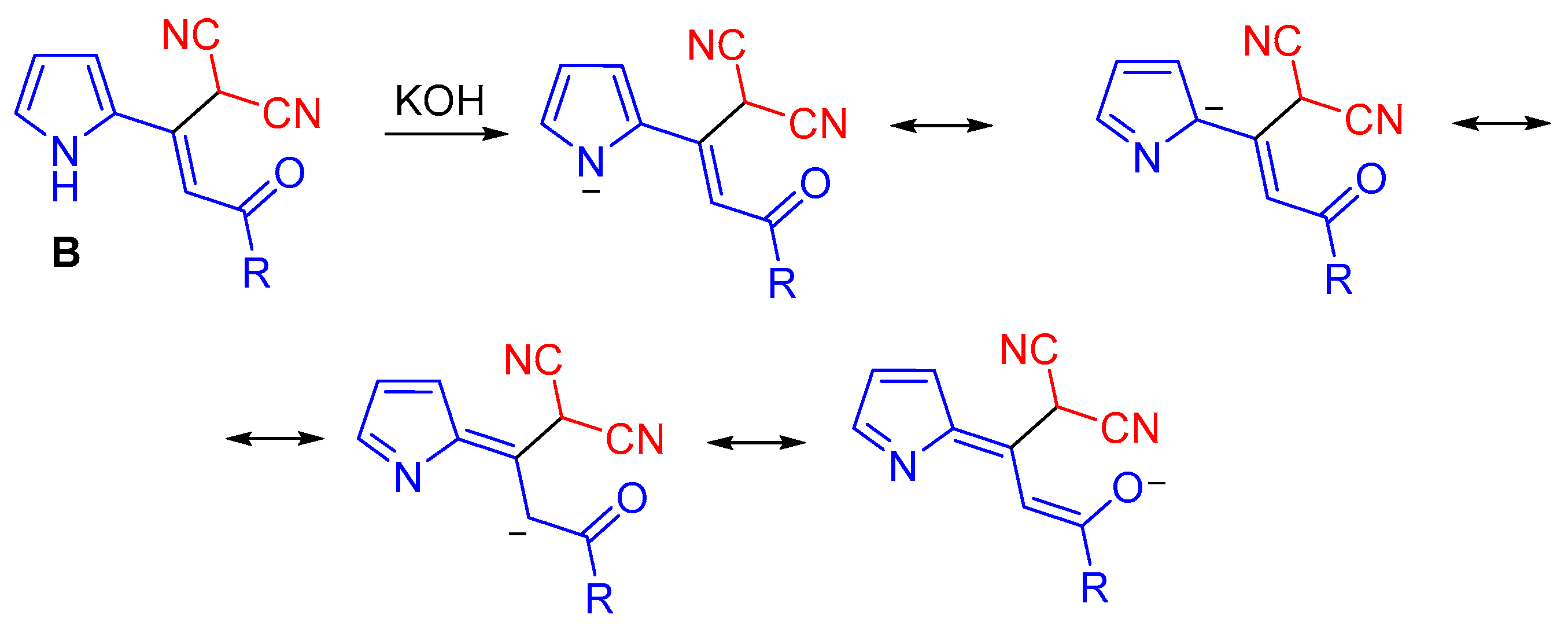
In the framework of the above mechanism, the substituent-dependent divergence of the synthesis can be explained as follows. In the presence of KOH, monoadducts of malononitrle to acylethynylpyrroles 1n–p should exist mainly as pyrrolate-anions with the negative charge distributed over the whole molecules along the conjugated chain up to the carbonyl group, which accepts a part of the negative charge, as shown by the resonance forms in (Scheme 6).
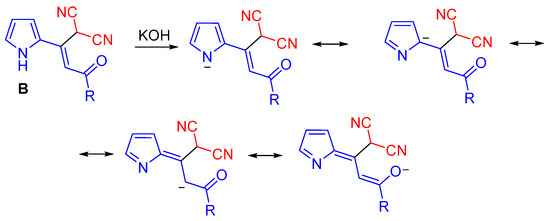
Scheme 6.
The resonance forms of the deprotonated monoadducts.
Such a charge transfer should be mostly expressed for the substrates with unsubstituted pyrrole counterpart that results in the decreasing of electrophilicity of the carbonyl group, which loses the ability to add the second molecule of malononitrile. Alkyl substituents in the pyrrole ring disfavor the above charge transfer from the pyrrolate-anions due to the reduction of the pyrrole moiety acidity. In the case of 5-arylsubstituted pyrrole structures, the negative charges of the pyrrolates are partially distributed over the aromatic substituents. N-substituted substrates do not form pyrrolate-anions at all. Thus, monoadducts with unsubstituted pyrrole counterparts 1n–p appear to be incapable of being attacked by the second molecule of malononitrile and hence of affording 2-(3-amino-2,4-dicyanophenyl)pyrroles 3. Instead, they isomerize to dienols 4a–c, which in favorable conformation undergo the ring closure to pyrrolizines 6a–c. On the other hand, monoadducts having any substituents in the pyrrole ring are readily attacked by the second carbanion of malononitrile at their more electrophilic carbonyl group to construct dicyanoaniline structures.
3. Materials and Methods
3.1. General Information
IR spectra were obtained with a Bruker Vertex 70 spectrometer (400–4000 cm−1, KBr). 1H (400.13 MHz), 13C (100.6 MHz) spectra were recorded on a Bruker DPX-400 spectrometer at ambient temperature in CDCl3 solutions and referenced to CDCl3 (residual protons of solvent in 1H NMR δ = 7.27 ppm; 13C NMR δ = 77.1 ppm) and DMSO-d6 (residual protons of solvent in 1H NMR δ = 2.50 ppm; 13C NMR δ = 39.5 ppm). The assignment of signals in the 1H NMR spectra was made using COSY and NOESY experiments. Resonance signals of carbon atoms were assigned based on 1H- 13C HSQC and 1H-13C HMBC experiments. The C, H, N, S microanalyses were performed on a Flash EA 1112 CHNS-O/MAS analyzer. Clorine was determined by mercurimetric titration. Sulfur was determined by complexometric titration with Chlorasenazo III. Melting point (uncorrected) was determined on a Kofler micro hot-stage apparatus.
Malononitrile 2, KOH and MeCN are commercial products. Acylethynylpyrroles were prepared according to the procedure reported [66].
3.2. Synthesis of 2-(3-amino-2,4-dicyanophenyl)pyrroles (3a–m), pyrrolyldienols (4a–c) and pyrrolizines (6b,c) (General Procedure)
The suspension of malononitrile 2 (132 mg, 2 mmol) and KOH·0.5H2O (130 mg, 2 mmol) in acetonitrile (15 mL) was stirred at 20–25 °C for 30 min. Then the reaction mixture was cooled to 0 °C, the 2-acylethynylpyrrole 1 (1 mmol) in acetonitrile (5 mL) was added dropwise to a reaction mixture within 10 min. Reaction mixture was stirred at 0 °C for 2 h and then was diluted with water (40 mL), extracted with diethyl ether (4 × 10 mL). Extracts were washed with water and dried over Na2SO4. The residue, after removing solvent, was fractionated by column chromatography (Al2O3, n-hexane: diethyl ether, 1 : 1) to afford the pyrroles 3a–m.
Under analogous conditions, pyrrolyldienols 4a–c are formed from 2-acylethynylpyrroles 1n–p and malononitrile 2. They are isolated by filtering the formed precipitates by diluting the reaction mixtures with water (1:3). Pyrrolyldienols 4b,c are formed with additive of the keto form. Products 4b,c were transformed in the corresponding pyrrolizines 6b,c in the course of isolation and drying.
3.3. Characterization Data of Products 3, 4, 6b,c
3-Amino-5-(1-methyl-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3a). Yield 233 mg (78%); yellow solid, m.p. 213–215 °C. Rf = 0.68. 1H NMR (400.13 MHz, DMSO-d6): δ 7.62–7.61 (m, 2H, o-Ph), 7.54–7.51 (m, 3H, m,p-Ph), 7.01–6.98 (m, 1H, H-5, pyrrole), 6.75 (s, 1H, CH, aniline), 6.74 (br. s, 2H, NH2), 6.46–6.45 (m, 1H, H-4, pyrrole), 6.17–6.15 (m, 1H, H-3, pyrrole), 3.65 (s, 3H, CH3). 13C NMR (100.6 MHz, DMSO-d6): δ 154.1, 149.3, 140.9, 137.4, 129.3, 128.9, 128.6 (2C), 128.5 (2C), 126.6, 118.5, 116.2, 116.1, 112.2, 107.9, 94.4, 93.2, 34.9. IR (KBr, cm−1): 3471, 3356, 3240, 2927, 2853, 2214, 2161, 1640, 1585, 1572, 1550, 1498, 1463, 1419, 1380, 1289, 1143, 1076, 1032, 959, 909, 733, 700, 633, 496. Elemental analysis calculated (%) for C19H14N4: C, 76.49; H, 4.73; N, 18.78; found: C, 76.6; H, 4.86; N, 18.96.
3-Amino-5-(4-ethyl-5-propyl-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3b). Yield 266 mg (75%); yellow solid, m.p. 118–120 °C. Rf = 0.66. 1H NMR (400.13 MHz, CDCl3): δ 8.91 (br. s, 1H, NH), 7.56–7.53 (m, 2H, o-Ph), 7.49–7.47 (m, 3H, m,p-Ph), 6.92 (s, 1H, CH, aniline), 6.85 (d, J = 2.8 Hz, 1H, H-3, pyrrole), 5.27 (br. s, 2H, NH2), 2.63–2.57 (m, 2H, CH2), 2.45 (q, J = 7.6 Hz, 2H, CH2), 1.71–1.62 (m, 4H, CH2), 1.19 (t, J = 7.5 Hz, 3H, CH3), 0.99 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (100.6 MHz, CDCl3): δ 154.0, 149.7, 139.3, 138.0, 134.6, 129.6, 128.9 (2C), 128.4 (2C), 125.2, 125.0, 118.2, 116.7, 115.5, 113.7, 91.3, 88.0, 28.2, 23.0, 18.9, 15.6, 14.0. IR (KBr, cm−1): 3473, 3357, 3239, 2962, 2928, 2871, 2208, 2156, 1959, 1633, 1587, 1571, 1541, 1490, 1459, 1329, 1274, 1208, 1176, 1139, 1075, 1041, 1018, 908, 858, 820, 772, 731, 700, 649, 503. Elemental analysis calculated (%) for C23H22N4: C, 77.94; H, 6.26; N, 15.81; found: C, 77.75; H, 6.40; N, 15.91.
3-Amino-5-(4,5,6,7-tetrahydro-1H-indol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3c). Yield 244 mg (72%); yellow solid, m.p. 175–177 °C. Rf = 0.67. 1H NMR (400.13 MHz, DMSO-d6): δ 11.21 (br. s, 1H, NH), 7.63–7.60 (m, 2H, o-Ph), 7.54–7.51 (m, 3H, m,p-Ph), 7.01 (s, 1H, CH, aniline), 6.90 (s, 1H, H-3, pyrrole), 6.47 (br. s, 2H, NH2), 2.61–2.57 (m, 2H, CH2-7), 2.50–2.46 (m, 2H, CH2-4), 1.78–1.74 (m, 2H, CH2-5), 1.71–1.64 (m, 2H, CH2-6). 13C NMR (100.6 MHz, DMSO-d6): δ 154.8, 149.1, 139.5, 138.0, 133.0, 129.2, 128.5 (2C), 128.4 (2C), 124.9, 119.0, 117.4, 116.6, 114.0, 111.2, 90.0, 87.9, 23.2, 22.7, 22.4, 22.4. IR (KBr, cm−1): 3468, 3348, 3241, 2924, 2852, 2361, 2209, 1637, 1570, 1544, 1489, 1460, 1362, 1270, 1205, 1144, 1126, 1012, 931, 897, 851, 805, 771, 699, 637, 505. Elemental analysis calculated (%) for C22H18N4: C, 78.08; H, 5.36; N, 16.56; found: C, 77.86; H, 5.55; N, 16.38.
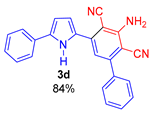
3-Amino-5-(5-phenyl-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3d). Yield 303 mg (84%); yellow solid, m.p. 82–84 °C. Rf = 0.66. 1H NMR (400.13 MHz, CDCl3): δ 9.49 (br. s, 1H, NH), 7.59–7.40 (m, 10H, Ph), 7.04–7.03 (m, 1H, H-3, pyrrole), 7.02 (s, 1H, CH, aniline), 6.66–6.64 (m, 1H, H-4, pyrrole), 5.31 (br. s, 2H, NH2). 13C NMR (100.6 MHz, CDCl3): δ 153.9, 150.0, 138.9, 137.8, 136.9, 131.3, 129.8, 129.3 (2C), 129.0 (2C), 128.4 (2C), 128.3, 127.8, 124.5 (2C), 118.2, 116.4, 116.1, 115.1, 108.8, 92.4, 88.7. IR (KBr, cm−1): 3456, 3357, 3242, 3061, 2923, 2852, 2361, 2253, 2211, 1634, 1577, 1546, 1476, 1460, 1424, 1385, 1298, 1262, 1216, 1077, 1058, 856, 758, 700, 497. Elemental analysis calculated (%) for C24H16N4: C, 79.98; H, 4.47; N, 15.55; found: C, 79.71; H, 4.32; N, 15.63.
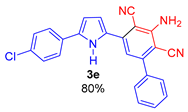
3-Amino-5-(5-(4-chlorophenyl)-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3e). Yield 316 mg (80%); yellow solid, m.p. 236–238 °C. Rf = 0.68. 1H NMR (400.13 MHz, DMSO-d6): δ 11.64 (br. s, 1H, NH), 7.82–7.80 (m, 2H, Ph), 7.67–7.65 (m, 2H, Ph), 7.58–7.53 (m, 3H, Ph), 7.47–7.44 (m, 2H, Ph), 7.23 (s, 1H, CH, aniline), 7.11–7.10 (m, 1H, H-3, pyrrole), 6.78–6.77 (m, 1H, H-4, pyrrole), 6.63 (br. s, 2H, NH2). 13C NMR (100.6 MHz, DMSO-d6): δ 154.6, 149.4, 139.1, 137.8, 134.7, 131.3, 130.4, 129.3, 128.9, 128.6 (2C), 128.6 (2C), 128.5 (2C), 126.4 (2C), 117.0, 116.3, 115.6, 113.7, 108.9, 91.8, 89.7. IR (KBr, cm−1): 3464, 3446, 3353, 3240, 2214, 1645, 1584, 1574, 1541, 1472, 1450, 1368, 1309, 1293, 1256, 1245, 1216, 1098, 1077, 1054, 1011, 933, 819, 767, 751, 696, 639, 607, 529. Elemental analysis calculated (%) for C24H15ClN4: C, 73.00; H, 3.83; Cl, 8.98; N, 14.19; found: C, 72.63; H, 3.64; N, 14.00.
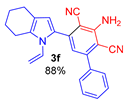
3-Amino-5-(1-vinyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3f). Yield 321 mg (88%), yellow solid, m.p. 182–184 °C. Rf = 0.65. 1H NMR (400.13 MHz, CDCl3): δ 7.55–7.52 (m, 2H, Ph), 7.50–7.45 (m, 3H, Ph), 6.82 (s, 1H, CH, aniline), 6.73 (dd, J = 15.6, 8.9 Hz, 1H, Hx), 6.51 (s, 1H, H-3, pyrrole), 5.31 (br. s, 2H, NH2), 4.95 (d, J = 8.9 Hz, 1H, Hb), 4.88 (d, J =15.6 Hz, 1H, 1Ha), 2.68–2.65 (m, 2H, CH2-7), 2.56–2.53 (m, 2H, CH2-4), 1.88–1.83 (m, 2H, CH2-5), 1.79–1.73 (m, 2H, CH2-6). 13C NMR (100.6 MHz, CDCl3) δ 153.5, 149.1, 141.2, 137.7, 133.7, 130.9, 129.7, 129.0 (2C), 128.5 (2C), 128.4, 120.5, 120.4, 116.6, 116.4, 114.8, 107.1, 94.2, 93.3, 23.9, 23.3, 23.2, 23.1. IR (KBr, cm−1): 3471, 3356, 3240, 2927, 2853, 2214, 2161, 1640, 1585, 1572, 1550, 1498, 1463, 1419, 1380, 1289, 1143, 1076, 1032, 959, 909, 733, 700, 633, 496. Elemental analysis calculated (%) for C24H20N4: C, 79.10; H, 5.53; N, 15.37; found: C, 79.21; H, 5.71; N, 15.55.
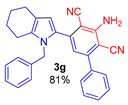
3-Amino-5-(1-benzyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3g). Yield 347 mg (81%), yellow solid, m.p. 137–139 °C. Rf = 0.67. 1H NMR (400.13 MHz, CDCl3): δ 7.43–7.28 (m, 7H, Ph), 7.23–7.19 (m, 1H, Ph), 6.87–6.85 (m, 2H, o-H, CH2Ph), 6.62 (s, 1H, CH, aniline), 6.51 (s, 1H, H-3, pyrrole), 5.29 (br. s, 2H, NH2), 5.11 (s, 2H, CH2-Ph), 2.61–2.58 (m, 2H, CH2-7), 2.45–2.43 (m, 2H, CH2-4), 1.83–1.73 (m, 4H, CH2-5,6). 13C NMR (100.6 MHz, CDCl3): δ 153.6, 149.1, 141.3, 138.5, 137.4, 134.2, 129.6, 129.0 (2C), 128.8 (2C), 128.6, 128.3 (2C), 127.3, 125.6 (2C), 119.6, 119.5, 116.6, 116.4, 112.8, 94.6, 93.1, 48.0, 23.5, 23.2, 23.1, 22.5. IR (KBr, cm−1): 3467, 3354, 3239, 3061, 3031, 2928, 2850, 2214, 1632, 1568, 1552, 1496, 1457, 1382, 1287, 1118, 1029, 910, 805, 731, 700, 649, 495. Elemental analysis calculated (%) for C29H24N4: C, 81.28; H, 5.65; N, 13.07; found: C, 81.01; H, 5.41; N, 13.24.
2-Amino-4-(1-benzyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-6-(furan-2-yl)isophthalonitrile (3h). Yield 352 mg (84%), yellow solid, m.p. 215–217 °C. Rf = 0.66. 1H NMR (400.13 MHz, CDCl3): δ 7.41–7.40 (m, 1H, H-5, furan), 7.33–7.29 (m, 2H, m-H, CH2Ph), 7.25–7.23 (m, 1H, H-3, furan), 7.02–7.01 (m, 1H, p-H, CH2Ph), 7.00 (s, 1H, CH, aniline), 6.90–6.88 (m, 2H, o-H, CH2Ph), 6.53 (s, 1H, H-3, pyrrole), 6.47 (dd, J = 3.5, 1.7 Hz, 1H, H-4, furan), 5.26 (br. s, 2H, NH2), 5.12 (s, 2H, CH2-Ph), 2.60 (m, 2H, CH2-7), 2.46 (m, 2H, CH2-4), 1.73 (m, 4H, CH2-5,6). 13C NMR (100.6 MHz, CDCl3) δ 153.8, 149.3, 144.4, 141.2, 138.6, 135.9, 134.3, 129.0 (2C), 128.6, 127.3, 125.7 (2C), 119.6, 116.7 (2C), 115.1, 112.8, 112.6, 112.5, 93.7, 88.2, 48.0, 23.6, 23.2, 23.2, 22.5. IR (KBr, cm−1): 3356, 2923, 2852, 2212, 1723, 1634, 1587, 1558, 1493, 1380, 1294, 1029, 731. Elemental analysis calculated (%) for C27H22N4O: C, 77.49; H, 5.30; N, 13.39; found: C, 77.26; H, 5.45; N, 13.58.
2-Amino-4-(1-benzyl-4,5,6,7-tetrahydro-1H-indol-2-yl)-6-(thiophen-2-yl)isophthalonitrile (3i). Yield 343 mg (79%), yellow solid, m.p. 197–199 °C. Rf = 0.66. 1H NMR (400.13 MHz, CDCl3): δ 7.51–7.45 (m, 1H, H-5, thiophene), 7.38–7.37 (m, 1H, H-3, thiophene), 7.32–7.21 (m, 3H, Ph, H-4 thiophene), 7.08–7.05 (m, 1H, Ph), 6.90–6.88 (m, 2H, Ph), 6.77 (s, 1H, CH, aniline), 6.53 (s, 1H, H-3, pyrrole), 5.29 (br. s, 2H, NH2), 5.11 (s, 2H, CH2-Ph), 2.61–2.58 (m, 2H, CH2-7), 2.45–2.42 (m, 2H, CH2-4), 1.83–1.73 (m, 4H, CH2-5,6). 13C NMR (100.6 MHz, CDCl3): δ 154.0, 141.2, 140.8, 139.0, 138.4, 134.3, 129.1 (2C), 128.6, 128.5, 128.4, 128.2, 127.4, 125.6 (2C), 119.7, 118.3, 116.7, 116.6, 112.9, 94.2, 91.21 48.0, 23.5, 23.2, 23.1, 22.5. IR (KBr, cm−1): 3467, 3354, 3239, 2927, 2851, 2211, 2157, 1632, 1566, 1494, 1463, 1437, 1381, 1357, 1289, 1144, 1112, 909, 844, 806, 728. Elemental analysis calculated (%) for C27H22N4S: C, 74.63; H, 5.10; N, 12.89; S, 7.38; found: C, 74.29; H, 5.27; N, 13.03; S, 7.27.
3-Amino-5-(1-methyl-5-phenyl-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3j). Yield 326 mg (87%), yellow solid, m.p. 228–230 °C. Rf = 0.68. 1H NMR (400.13 MHz, CDCl3): δ 7.60–7.58 (m, 2H, Ph), 7.54–7.42 (m, 7H, Ph), 7.38–7.34 (m, 1H, Ph), 6.88 (s, 1H, CH, aniline), 6.69 (d, J = 3.8 Hz, 1H, H-3, pyrrole), 6.38 (d, J = 3.8 Hz, 1H, H-4, pyrrole), 5.39 (br. s, 2H, NH2), 3.62 (s, 3H, CH3). 13C NMR (100.6 MHz, CDCl3): δ 153.5, 149.7, 141.1, 140.5, 137.6, 132.6, 131.9, 129.8, 129.2 (2C), 129.1 (2C), 128.7 (2C), 128.5 (2C), 127.8, 120.1, 116.6, 116.3, 113.6, 109.9, 94.8, 94.0, 34.9. IR (KBr, cm−1): 3357, 2923, 2854, 2214, 1634, 1576, 1549, 1460, 1285, 758, 728, 700. Elemental analysis calculated (%) for C25H18N4: C, 80.19; H, 4.85; N, 14.96. found: C, 80.03; H, 5.01; N, 15.08.
3-Amino-5-(1-benzyl-5-phenyl-1H-pyrrol-2-yl)-[1,1′-biphenyl]-2,4-dicarbonitrile (3k). Yield 369 mg (82%); yellow solid, m.p. 150–152 °C. Rf = 0.68. 1H NMR (400.13 MHz, CDCl3): δ 7.47–7.45 (m, 3H, Ph), 7.42–7.33 (m, 7H, Ph), 7.14–7.12 (m, 3H, Ph), 6.77 (s, 1H, CH, aniline), 6.67 (d, J = 3.7 Hz, 1H, H-3, pyrrole), 6.63–6.61 (m, 2H, Ph), 6.44 (d, J = 3.7 Hz, 1H, H-4, pyrrole), 5.30 (br. s, 2H, NH2), 5.24 (s, 2H, CH2-Ph). 13C NMR (100.6 MHz, CDCl3): δ 153.2, 149.5, 141.5, 140.5, 138.5, 137.4, 132.8, 131.7, 129.7, 129.3 (2C), 129.0 (2C), 128.9, 128.7 (2C), 128.6 (2C), 128.4 (2C), 127.9, 127.3, 126.0 (2C), 120.2, 116.2, 114.3, 110.7, 95.6, 94.1, 49.8. IR (KBr, cm−1): 3464, 3356, 3062, 3031, 2928, 2216, 1630, 1580, 1550, 1500, 1456, 1389, 1352, 1286, 1181, 1075, 1032, 910, 760, 732, 701. Elemental analysis calculated (%) for C31H22N4: C, 82.64; H, 4.92; N, 12.44; found: C, 82.32; H, 5.02; N, 12.57.
2-Amino-4-(1-benzyl-5-phenyl-1H-pyrrol-2-yl)-6-(furan-2-yl)isophthalonitrile (3l). Yield 374 mg (85%), yellow solid, m.p. 176–178 °C. Rf = 0.67. 1H NMR (400.13 MHz, CDCl3): δ 7.56–7.54 (m, 1H, H-5, furan), 7.44–7.32 (m, 5H, Ph), 7.27–7.26 (m, 1H, Ph), 7.21 (s, 1H, CH, aniline), 7.14–7.10 (m, 3H, Ph, H-3, furan), 6.70 (d, J = 3.7 Hz, 1H, H-3, pyrrole), 6.64–6.62 (m, 2H, Ph), 6.57 (dd, J = 3.4, 1.6 Hz, 1H, H-4, furan), 6.45 (d, J = 3.7 Hz, 1H, H-4, pyrrole), 5.30–5.23 (m, 4H, NH2, CH2-Ph). 13C NMR (100.6 MHz, CDCl3): δ 153.5, 149.2, 144.6, 141.4, 140.5, 138.5, 136.1, 132.9, 131.7, 129.4 (2C), 128.7 (2C), 128.6 (2C), 127.9, 127.3, 126.0 (2C), 116.6, 116.2, 115.7, 114.3, 112.9, 112.8, 110.8, 94.7, 89.0, 49.7. IR (KBr, cm−1): 3357, 2922, 2853, 2213, 1633, 1586, 1554, 1479, 1454, 1294, 1029, 910, 755, 732, 701. Elemental analysis calculated (%) for C29H20N4O: C, 79.07; H, 4.58; N, 12.72; found: C, 78.84; H, 4.35; N, 12.56.
2-Amino-4-(1-benzyl-5-phenyl-1H-pyrrol-2-yl)-6-(thiophen-2-yl)isophthalonitrile (3m). Yield 402 mg (88%), yellow solid, m.p. 203–205 °C. Rf = 0.66. 1H NMR (400.13 MHz, CDCl3): δ 7.63–7.58 (m, 1H, H-5, thiophene), 7.50–7.47 (m, 1H, H-3, thiophene), 7.41–7.32 (m, 5H, Ph), 7.18–7.12 (m, 4H, Ph, H-4, thiophene), 6.91 (s, 1H, CH, aniline), 6.67–6.65 (m, 1H, H-3, pyrrole), 6.62–6.61 (m, 2H, Ph), 6.43–6.41 (m, 1H, H-3, pyrrole), 5.28 (br. s, 2H, NH2), 5.24 (s, 2H, CH2-Ph). 13C NMR (100.6 MHz, CDCl3): δ 153.6, 141.5, 141.2, 140.6, 138.8, 138.4, 132.8, 131.8, 129.4 (2C), 128.7 (2C), 128.7 (4C), 128.5, 127.9, 127.4, 126.0 (2C), 119.3, 116.5, 116.1, 114.3, 110.6, 95.3, 92.2, 49.8. IR (KBr, cm−1): 3355, 2921, 2852, 2213, 1632, 1576, 1546, 1458, 1425, 1288, 1254, 910, 845, 759, 731, 702. Elemental analysis calculated (%) for C29H20N4S: C, 76.29; H, 4.42; N, 12.27; S, 7.02; found: C, 76.01; H, 4.32; N, 12.04; S, 6.84.
(Z)-2-(3-Hydroxy-3-phenyl-1-(1H-pyrrol-2-yl)allylidene)malononitrile (4a). Yield 196 mg (75%), yellow solid, m.p. 175–177 °C. Rf = 0.42. 1H NMR (400.13 MHz, DMSO-d6): δ 12.08 (br. s, 1H, OH), 8.36 (br. s, 1H, NH), 8.00–7.98 (m, 2H, Ph), 7.59–7.57 (m, 3H, Ph), 7.51–7.50 (m, 1H, H-5, pyrrole), 7.44–7.42 (m, 1H, H-4, pyrrole), 7.31 (s, 1H, = CH), 6.46–6.45 (m, 1H, H-3, pyrrole). 13C NMR (100.6 MHz, DMSO-d6): δ 158.4, 156.1, 144.8, 131.6, 130.6, 129.0 (2C), 126.4, 125.8 (2C), 125.0, 117.6, 116.2, 112.0, 97.6, 85.6. IR (KBr, cm−1): 3300, 2216, 1630, 1540, 1500, 1467, 1362, 1325, 1052, 994, 910, 780, 699, 642. Elemental analysis calculated (%) for C16H11N3O: C, 73.55; H, 4.24; N, 16.08; found: C, 73.21; H, 4.42; N, 15.88.
(Z)-2-(3-Hydroxy-3-(2-furyl)-1-(1H-pyrrol-2-yl)allylidene)malononitrile (4b). 1H NMR (400.13 MHz, DMSO-d6): δ 12.22 (br. s, 1H, OH), 8.78 (br. s, 1H, NH), 8.06–8.04 (m, 1H, H-5, furan), 7.52–7.51 (m, 1H, H-3, furan), 7.40–7.37 (m, 1H, H-3, pyrrole), 7.16–7.15 (m, 1H, H-4, furan), 7.13 (s, 1H, = CH), 6.81–6.80 (m, 1H, H-5, pyrrole), 6.49–6.46 (m, 1H, H-4, pyrrole).
(Z)-2-(3-Hydroxy-1-(1H-pyrrol-2-yl)-3-(thiophen-2-yl)allylidene)malononitrile (4c). 1H NMR (400.13 MHz, DMSO-d6): δ 12.14 (br. s, 1H, OH), 8.81 (br. s, 1H, NH), 7.96–7.95 (m, 1H, H-5, thiophene), 7.84–7.83 (m, 1H, H-3, thiophene), 7.52–7.51 (m, 1H, H-3, pyrrole), 7.47–7.45 (m, 1H, H-5, pyrrole), 7.32–7.30 (m, 1H, H-4, thiophene), 7.24 (s, 1H, = CH), 6.47–6.46 (m, 1H, H-4, pyrrole). 13C NMR (100.6 MHz, DMSO-d6): δ 157.0, 154.7, 145.3, 133.9, 132.1, 129.2, 129.0, 127.3, 124.9, 117.3, 116.9, 112.3, 97.0, 83.8.
(Z)-3-Amino-1-(2-(furan-2-yl)-2-oxoethylidene)-1H-pyrrolizine-2-carbonitrile (6b). Yield 161 mg (64%), orange solid, m.p. 206–208 °C. Rf = 0.36. 1H NMR (400.13 MHz, DMSO-d6): δ 9.15 (s, 2H, NH2), 7.97–7.94 (m, 1H, H-5, furan), 7.53–7.51 (m, 1H, H-5, pyrrole), 7.47–7.44 (m, 1H, H-3, furan), 6.33–6.31 (m, 1H, H-4, furan), 6.72–6.69 (m, 1H, H-3, pyrrole), 6.57 (s, 1H, = CH), 6.48–6.45 (m, 1H, H-4, pyrrole). 13C NMR (100.6 MHz, DMSO-d6): δ 175.2, 154.9, 154.2, 146.4, 142.0, 131.5, 118.3, 117.1, 116.7, 115.6, 115.5, 112.7, 104.1, 67.2. IR (KBr, cm−1): 3134, 2205, 1687, 1622, 1557, 1540, 1492, 1466, 1398, 1260, 1235, 1206, 1114, 1087, 1067, 1039, 1017, 968, 886, 811, 722, 669. Elemental analysis calculated (%) for C14H9N3O2: C, 66.93; H, 3.61; N, 16.73; found: C, 67.12; H, 3.80; N, 16.91.
(Z)-3-Amino-1-(2-oxo-2-(thiophen-2-yl)ethylidene)-1H-pyrrolizine-2-carbonitrile (6c). Yield 147 mg (55%), red solid, m.p. 283–285 °C. Rf = 0.36. 1H NMR (400.13 MHz, DMSO-d6): δ 9.15 (s, 2H, NH2), 7.91–7.90 (m, 1H, H-5, thiophene), 7.88–7.86 (m, 1H, H-3, thiophene), 7.53–7.51 (m, 1H, H-5, pyrrole), 7.45–7.44 (m, 1H, H-3, pyrrole), 7.25–7.22 (m, 1H, H-4, thiophene), 6.56 (s, 1H, = CH), 6.48–6.45 (m, 1H, H-4, pyrrole). 13C NMR (100.6 MHz, DMSO-d6): δ 179.1, 155.0, 146.9, 142.0, 133.3, 131.5, 130.4, 128.7, 118.2, 117.2, 116.7, 115.6, 104.3, 67.1. IR (KBr, cm−1): 3323, 3153, 2204, 2173, 1629, 1590, 1556, 1509, 1449, 1416, 1382, 1339, 1250, 1152, 1059, 998, 959, 877, 857, 813, 775, 738, 715, 600, 534. Elemental analysis calculated (%) for C14H9N3OS: C, 62.91; H, 3.39; N, 15.72; S, 11.99; found: C, 63.05; H, 3.55; N, 15.91; S, 11.86.
3.4. The synthesis of (Z)-3-amino-1-(2-oxo-2-phenylethylidene)-1H-pyrrolizine-2-carbonitrile (6a)
The triethylamine (101 mg, 1 mmol) was added to the solution of pyrrolyldienol 4a (261 mg, 1 mmol) in EtOH (40 mL) and refluxed for 30 min. After the reaction mixture was cooled to room temperature, the resulting crystalline precipitate 6a was filtered off on a Schott filter and dried under vacuum. Yield 209 mg (80%), brown solid, m.p. 158–160 °C. Rf = 0.35. 1H NMR (400.13 MHz, DMSO-d6): δ 9.15 (s, 2H, NH2), 7.97–7.95 (m, 2H, H-5, H-3, pyrrole), 7.61–7.53 (m, 5H, Ph), 6.68 (m, 1H, H-4, pyrrole), 6.46 (s, 1H, = CH). 13C NMR (100.6 MHz, DMSO-d6): δ 186.4, 154.9, 142.3, 139.5, 131.9, 131.6, 128.7 (2C), 127.5 (2C), 118.3, 117.1, 116.7, 115.7, 104.5, 67.4. IR (KBr, cm−1): 3314, 2206, 1638, 1591, 1512, 1443, 1398, 1366, 1282, 1180, 1147, 1057, 966, 872, 823, 757, 698, 608, 531. Elemental analysis calculated (%) for C16H11N3O: C, 73.55; H, 4.24; N, 16.08; found: C, 73.73; H, 4.38; N, 16.27.
4. Conclusions
In summary, an effective access to 2-(3-amino-2,4-dicyanophenyl)pyrroles, pyrrolyldienols and 3-amino-1-acylethylidene-2-cyanopyrrolizines, attractive objects for drug design, via substituent-dependent divergent reaction of acylethynylpyrroles with malononitrile, has been developed. The method has several synthetic advantages, such as the one-pot procedure, very mild conditions (0 °C), the use of readily available starting materials and good-to-excellent yields of the above compounds, and therefore can stimulate the interest of both synthetic and pharmaceutical communities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238528/s1. Synthesis of detailed procedures and physicochemical characterization of products: 2-(3-amino-2,4-dicyanophenyl)pyrroles 3a-m, pyrrolyldienols 4a-c and pyrrolizines 6b,c (S3–S8); synthesis of 3-amino-1-acylethylidene-2-cyanopyrrolizine 6a (S9); 1 H NMR and 13 C NMR spectra of compounds 3a-m, 4a-c and 6a-c (S10–S49); the 2D NMR spectra of (Z)-3-amino-1-(2-oxo-2-(thiophen-2-yl)ethylidene)-1H-pyrrolizine-2-carbonitrile 6c (S50–S58).
Author Contributions
Conceptualization, B.A.T.; investigation, M.D.G. and I.V.S.; writing—review and editing, L.N.S. and B.A.T.; writing—original draft preparation, L.N.S.; formal analysis, I.A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Acknowledgments
Experimental work was carried out using the equipment of the Baikal Analytical Centre for Collective Use of the Siberian Branch of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1,3,4,5 and 6 are available from the authors.
References
- O’Malley, D.P.; Li, K.; Maue, M.; Zografos, A.L.; Baran, P.S. Total Synthesis of Dimeric Pyrrole−Imidazole Alkaloids: Sceptrin, Ageliferin, Nagelamide E, Oxysceptrin, Nakamuric Acid, and the Axinellamine Carbon Skeleton. J. Am. Chem. Soc. 2007, 129, 4762–4775. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2007, 108, 264–287. [Google Scholar] [CrossRef]
- Hughes, C.C.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. The Marinopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Org. Lett. 2008, 10, 629–631. [Google Scholar] [CrossRef]
- Li, R. Marinopyrroles: Unique Drug Discoveries Based on Marine Natural Products. Med. Res. Rev. 2015, 36, 169–189. [Google Scholar] [CrossRef]
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Bianco, M.d.C.A.D.; Marinho, D.I.L.F.; Hoelz, L.V.B.; Bastos, M.M.; Boechat, N. Pyrroles as Privileged Scaffolds in the Search for New Potential HIV Inhibitors. Pharmaceuticals 2021, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Jeelan Basha, N.; Basavarajaiah, S.M.; Shyamsunder, K. Therapeutic potential of pyrrole and pyrrolidine analogs: An update. Mol. Divers. 2022, 26, 2915–2937. [Google Scholar] [CrossRef]
- Mir, R.H.; Mir, P.A.; Mohi-Ud-Din, R.; Sabreen, S.; Maqbool, M.; Shah, A.J.; Shenmar, K.; Raza, S.N.; Pottoo, F.H. A Comprehensive Review on Journey of Pyrrole Scaffold Against Multiple Therapeutic Targets. Anticancer Agents Med. Chem. 2022, 22, 3291–3303. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, V.; Abbot, V.; Kapoor, Y.; Konar, D.; Kumar, K. Recent synthetic and medicinal perspectives of pyrroles: An overview. J. Pharm. Chem. Sci. 2017, 1, 17–32. [Google Scholar]
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.; Alam, M.M.; Iqbal, M. Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [CrossRef] [PubMed]
- Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.-W.; Hwang, H.; Lee, C.-H. Synthesis of a Strapped Calix[4]pyrrole: Structure and Anion Binding Properties. Angew. Chem. Int. Ed. 2002, 41, 1757–1759. [Google Scholar] [CrossRef]
- Gale, P.A. Structural and Molecular Recognition Studies with Acyclic Anion Receptors. Acc. Chem. Res. 2006, 39, 465–475. [Google Scholar] [CrossRef]
- Plitt, P.; Gross, D.E.; Lynch, V.M.; Sessler, J.L. Dipyrrolyl-Functionalized Bipyridine-Based Anion Receptors for Emission-Based Selective Detection of Dihydrogen Phosphate. Chem. Eur. J. 2007, 13, 1374–1381. [Google Scholar] [CrossRef]
- Piliego, C.; Holcombe, T.W.; Douglas, J.D.; Woo, C.H.; Beaujuge, P.M.; Fréchet, J.M.J. Synthetic Control of Structural Order in N-Alkylthieno[3,4-c]pyrrole-4,6-dione-Based Polymers for Efficient Solar Cells. J. Am. Chem. Soc. 2010, 132, 7595–7597. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, T.; Li, S.; Yu, T.; Zhang, X.; Hu, R.; Feng, J.; Wang, S.; Liang, T.; Chen, J.; et al. Ultrasensitive reversible chromophore reaction of BODIPY functions as high ratio double turn on probe. Nat. Commun. 2018, 9, 362. [Google Scholar] [CrossRef]
- Biava, M.; Porretta, G.C.; Poce, G.; Supino, S.; Deidda, D.; Pompei, R.; Molicotti, P.; Manetti, F.; Botta, M. Antimycobacterial Agents. Novel Diarylpyrrole Derivatives of BM212 Endowed with High Activity toward Mycobacterium tuberculosis and Low Cytotoxicity. J. Med. Chem. 2006, 49, 4946–4952. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Krim, O.; Bouachrine, M.; Hammouti, B.; Elidrissi, A.; Hamidi, M. 2,5-Difuryl-N-Methylpyrrole as Corrosion Inhibitor for Steel in 1 M HCl. Port. Electrochim. Acta 2007, 26, 283–289. [Google Scholar] [CrossRef]
- Onnis, V.; De Logu, A.; Cocco, M.T.; Fadda, R.; Meleddu, R.; Congiu, C. 2-Acylhydrazino-5-arylpyrrole derivatives: Synthesis and antifungal activity evaluation. Eur. J. Med. Chem. 2009, 44, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-L.; Ko, C.-C.; Lam, W.H.; Zhu, N.; Yam, V.W.-W. Design and Synthesis of a New Class of Photochromic Diarylethene-Containing Dithieno[3,2-b:2′,3′-d]pyrroles and Their Switchable Luminescence Properties. Chem. Eur. J. 2009, 15, 10005–10009. [Google Scholar] [CrossRef]
- Gomha, S.M.; Eldebss, T.M.A.; Abdulla, M.M.; Mayhoub, A.S. Diphenylpyrroles: Novel p53 activators. Eur. J. Med. Chem. 2014, 82, 472–479. [Google Scholar] [CrossRef]
- Cheon, K.H.; Cho, J.; Kim, Y.-H.; Chung, D.S. Thin Film Transistor Gas Sensors Incorporating High-Mobility Diketopyrrolopyrole-Based Polymeric Semiconductor Doped with Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 14004–14010. [Google Scholar] [CrossRef]
- Jung, E.-K.; Leung, E.; Barker, D. Synthesis and biological activity of pyrrole analogues of combretastatin A-4. Bioorg. Med. Chem. Lett. 2016, 26, 3001–3005. [Google Scholar] [CrossRef]
- Mishra, S.J.; Ghosh, S.; Stothert, A.R.; Dickey, C.A.; Blagg, B.S.J. Transformation of the Non-Selective Aminocyclohexanol-Based Hsp90 Inhibitor into a Grp94-Seletive Scaffold. ACS Chem. Biol. 2017, 12, 244–253. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Sun, Y.; Zhang, M.; Guo, C.; Mirza, I.A.; Li, Y.-Q. Vonoprazan: A Novel and Potent Alternative in the Treatment of Acid-Related Diseases. Dig. Dis. Sci. 2017, 63, 302–311. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Miao, Z.-H. Marine-derived angiogenesis inhibitors for cancer therapy. Mar. Drugs 2013, 11, 903–933. [Google Scholar] [CrossRef]
- Alešković, M.; Basarić, N.; Halasz, I.; Liang, X.; Qin, W.; Mlinarić-Majerski, K. Aryl substituted adamantane–dipyrromethanes: Chromogenic and fluorescent anion sensors. Tetrahedron 2013, 69, 1725–1734. [Google Scholar] [CrossRef]
- Cai, Z.; Lei, Y.; Dong, Y. Synthesis of Multi-phenyl-substituted Pyrrole (MPP)-based AIE Materials and Their Applications. Handb. Aggreg. -Induc. Emiss. 2022, 195–220. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Sobenina, L.N.; Vasil’tsov, A.M.; Petrova, O.V.; Petrushenko, K.B.; Ushakov, I.A.; Clavier, G.; Meallet-Renault, R.; Mikhaleva, A.I.; Trofimov, B.A. General Route to Symmetric and Asymmetric meso-CF3-3(5)-Aryl(hetaryl)- and 3,5-Diaryl(dihetaryl)-BODIPY Dyes. Org. Lett. 2011, 13, 2524–2527. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Qi, F.; Ma, H.; Wang, X.; Pan, Y.; Chen, R.; Shen, Z.; Liu, Z.; Huang, L.; Huang, W. Domino-like multi-emissions across red and near infrared from solid-state 2-/2,6-aryl substituted BODIPY dyes. Nat. Commun. 2018, 9, 2688. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, S.; She, M.; Chen, J.; Wang, Z.; Liu, P.; Zhang, S.; Li, J. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Marfin, Y.; Merkushev, D.; Khalabudin, D. Fast Synthesis of Tetra-Aryl-Substituted Aza-BODIPYs. J. Phys. Conf. Ser. 2021, 1822, 012004. [Google Scholar] [CrossRef]
- Gadomska, A.V.; Nevidimov, A.V.; Tovstun, S.A.; Petrova, O.V.; Sobenina, L.N.; Trofimov, B.A.; Razumov, V.F. Fluorescence from 3,5-diphenyl-8-CF3-BODIPYs with amino substituents on the phenyl rings: Quenching by aromatic molecules. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119632. [Google Scholar] [CrossRef]
- Harman, W.H.; Chang, C.J. N2O Activation and Oxidation Reactivity from a Non-Heme Iron Pyrrole Platform. J. Am. Chem. Soc. 2007, 129, 15128–15129. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, F.; Zhang, S.F. Synthesis of 5-Aryl-1,2-dihydro-1-pyrrolizinones. Chin. Chem. Lett. 2003, 14, 565–568. [Google Scholar]
- Belal, A.; El-Gendy, B.E.-D.M. Pyrrolizines: Promising scaffolds for anticancer drugs. Biorg. Med. Chem. 2014, 22, 46–53. [Google Scholar] [CrossRef]
- Wang, X.; Lane, B.S.; Sames, D. Direct C-Arylation of Free (NH)-Indoles and Pyrroles Catalyzed by Ar−Rh(III) Complexes Assembled In Situ. J. Am. Chem. Soc. 2005, 127, 4996–4997. [Google Scholar] [CrossRef] [PubMed]
- Swartz, D.L.; Odom, A.L. Synthesis, Structure, and Hydroamination Kinetics of (2,2‘-Diaryldipyrrolylmethane)- and Bis(2-arylpyrrolyl)titanium Complexes. Organometallics 2006, 25, 6125–6133. [Google Scholar] [CrossRef]
- Lee, P.H. Synthesis of 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene DyesBearing New Aryl Substituents at C3- and C5-Positions. Bull. Korean Chem. Soc. 2008, 29, 261–264. [Google Scholar] [CrossRef][Green Version]
- Wen, J.; Qin, S.; Ma, L.-F.; Dong, L.; Zhang, J.; Liu, S.-S.; Duan, Y.-S.; Chen, S.-Y.; Hu, C.-W.; Yu, X.-Q. Iron-Mediated Direct Suzuki−Miyaura Reaction: A New Method for the ortho-Arylation of Pyrrole and Pyridine. Org. Lett. 2010, 12, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.Y.; Wong, K.L.; Zhang, M.W.; Kwok, T.Y.; To, C.T.; Chan, K.S. Catalytic C–H arylation of unactivated heteroaromatics with aryl halides by cobalt porphyrin. Tetrahedron Lett. 2012, 53, 1571–1575. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, R.-Y.; Chen, S.-Y.; Zhang, J.; Yu, X.-Q. Direct Arylation of Arene and N-Heteroarenes with Diaryliodonium Salts without the Use of Transition Metal Catalyst. J. Org. Chem. 2011, 77, 766–771. [Google Scholar] [CrossRef]
- Cui, K.; Gao, M.; Zhao, H.; Zhang, D.; Yan, H.; Huang, H. An Efficient Synthesis of Aryl-Substituted Pyrroles by the Suzuki⁻Miyaura Coupling Reaction of SEM-Protected Pyrroles. Molecules 2019, 24, 1594. [Google Scholar] [CrossRef]
- Prati, F.; Spaggiari, A.; Vaccari, D.; Davoli, P. The Triphenyl Phosphite-Chlorine Reagent in the Synthesis of Pyrroles from N-Allylamides. Synthesis 2006, 2006, 995–998. [Google Scholar] [CrossRef]
- Du, W.; Zhao, M.-N.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Copper-catalyzed 5-endo-trig cyclization of ketoxime carboxylates: A facile synthesis of 2-arylpyrroles. Chem. Commun. 2014, 50, 7437. [Google Scholar] [CrossRef]
- Kaewchangwat, N.; Sukato, R.; Vchirawongkwin, V.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. Direct synthesis of aryl substituted pyrroles from calcium carbide: An underestimated chemical feedstock. Green Chem. 2015, 17, 460–465. [Google Scholar] [CrossRef]
- Karimi, S.; Ma, S.; Liu, Y.; Ramig, K.; Greer, E.M.; Kwon, K.; Berkowitz, W.F.; Subramaniam, G. Substituted pyrrole synthesis from nitrodienes. Tetrahedron Lett. 2017, 58, 2223–2227. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Mikhaleva, A.b.I.; Schmidt, E.Y.; Sobenina, L.N. Chemistry of Pyrroles; CRC Press Inc.: Boca-Raton, FL, USA, 2014. [Google Scholar]
- Singh, F.V.; Parihar, A.; Chaurasia, S.; Singh, A.B.; Singh, S.P.; Tamrakar, A.K.; Srivastava, A.K.; Goel, A. 5,6-Diarylanthranilo-1,3-dinitriles as a new class of antihyperglycemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Kanwal; Khan, K.M.; Chigurupati, S.; Andriani, Y.; Solangi, M.; Hameed, S.; Abdel Monem Abdel Hafez, A.; Begum, F.; Arif Lodhi, M.; et al. Dicyanoanilines as potential and dual inhibitors of α-amylase and α-glucosidase enzymes: Synthesis, characterization, in vitro, in silico, and kinetics studies. Arab. J. Chem. 2022, 15, 103651. [Google Scholar] [CrossRef]
- Borate, H.B.; Kudale, A.S.; Agalave, S.G. Synthesis of Substituted 2,6-Dicyanoanilines and Related Compounds. A Review. Org. Prep. Proced. Int. 2012, 44, 467–521. [Google Scholar] [CrossRef]
- Yi, C.; Blum, C.; Liu, S.-X.; Frei, G.; Neels, A.; Stoeckli-Evans, H.; Leutwyler, S.; Decurtins, S. An efficient one-pot synthesis of strongly fluorescent (hetero)arenes polysubstituted with amino and cyano groups. Tetrahedron 2008, 64, 9437–9441. [Google Scholar] [CrossRef]
- Zonouzi, A.; Izakian, Z.; Abdi, K.; Ng, S.W. Synthesis of Fluorescent 2,6-Dicyano-3,5-Disubstituted Anilines Using Cellulose Sulfuric Acid in Aqueous Media. Helv. Chim. Acta 2016, 99, 355–360. [Google Scholar] [CrossRef]
- Kulkarni, R.C.; Samundeeswari, S.; Shaikh, F.; Naik, N.S.; Madar, J.M.; Shastri, L.A.; Sunagar, V.A. Synthesis of Naked-eye Detectable Fluorescent 2H-chromen-2-One 2, 6-Dicyanoanilines: Effect of Substituents and pH on Its Luminous Behavior. J. Fluoresc. 2017, 27, 1613–1619. [Google Scholar] [CrossRef]
- Kudale, A.S.; Kamble, S.B.; Gore, A.H.; Pisal, M.M.; Salokhe, A.T.; Kolekar, G.B.; Helavi, V.B. One-pot three-component synthesis and photophysical properties of highly fluorescent novel 4-alkyl-3-aryl-2,6-dicyanoanilines by using tris(hydroxymethyl)aminomethane as a catalyst. Chem. Data Collect. 2019, 19, 100172. [Google Scholar] [CrossRef]
- Cardozo, T.M.; Nascimento, M.A.C. New class of molecules predicted to exhibit non-linear optical properties. J. Mater. Sci. 2005, 40, 3549–3551. [Google Scholar] [CrossRef]
- Plass, F.; Bönisch, S.; Held, F.; Ullrich, T.; Fischer, F.E.J.; Guryev, A.; Görling, A.; Kahnt, A.; Tsogoeva, S.B. Controlling and Fine-Tuning Charge-Transfer Emission in 2,6-Dicyanoaniline Multichromophores Prepared through Domino Reactions: Entry to a Potentially New Class of OLEDs. J. Org. Chem. 2021, 86, 6111–6125. [Google Scholar] [CrossRef]
- Pisal, M.M.; Annadate, R.A.; Athalye, M.C.; Kumar, D.; Chavan, S.P.; Sarkar, D.; Borate, H.B. Synthesis and cell imaging applications of fluorescent mono/di/tri-heterocyclyl-2,6-dicyanoanilines. Bioorg. Med. Chem. Lett. 2017, 27, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, A.; Vernon, J.M.; Dransfield, T.A.; Fouli, F.A.; Youssef, A.S.A. Reactions of malononitrile with acetylenic esters and ketones. J. Chem. Res. Synop. 1990, 9, 276–277. [Google Scholar]
- Yi, C.; Blum, C.; Liu, S.-X.; Frei, G.; Neels, A.; Renaud, P.; Leutwyler, S.; Decurtins, S. An Efficient and Facile Synthesis of Highly Substituted 2,6-Dicyanoanilines. J. Org. Chem. 2008, 73, 3596–3599. [Google Scholar] [CrossRef]
- He, J.; Li, Z. Synthesis of 3,5-Diaryl-2,6-dicyanoanilines from Tandem Reactions of Ynones with Malononitrile. ChemistrySelect 2019, 4, 5732–5734. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Stepanova, Z.V.; Sobenina, L.N.; Mikhaleva, A.b.I.; Ushakov, I.A. Ethynylation of pyrroles with 1-acyl-2-bromoacetylenes on alumina: A formal ‘inverse Sonogashira coupling’. Tetrahedron Lett. 2004, 45, 6513–6516. [Google Scholar] [CrossRef]
- Trofimov, B.; Sobenina, L.; Stepanova, Z.; Ushakov, I.; Petrova, O.; Tarasova, O.; Volkova, K.; Mikhaleva, A. Regioselective Cross-Coupling of 1-Vinylpyrroles with Acylbromoacetylenes on Al2O3: Synthesis of 2-(2-Acylethynyl)-1-vinylpyrroles. Synthesis 2007, 2007, 447–451. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sobenina, L.N. Ethynylation of pyrrole nucleus with haloacetylenes on active surfaces. In Targets in Heterocyclic Chemistry; Attanasi, O.A., Spinelli, D., Eds.; Societa Chimica Italiana: Rome, Italy, 2009; pp. 92–119. [Google Scholar]
- Sobenina, L.N.; Tomilin, D.N.; Petrova, O.V.; Gulia, N.; Osowska, K.; Szafert, S.; Mikhaleva, A.I.; Trofimov, B.A. Cross-coupling of 4,5,6,7-tetrahydroindole with functionalized haloacetylenes on active surfaces of metal oxides and salts. Russ. J. Org. Chem. 2010, 46, 1373–1377. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Petrova, O.V.; Tomilin, D.N.; Gotsko, M.D.; Ushakov, I.A.; Klyba, L.V.; Mikhaleva, A.I.; Trofimov, B.A. Ethynylation of 2-(furan-2-yl)- and 2-(thiophen-2-yl)pyrroles with acylbromoacetylenes in the Al2O3 medium: Relative reactivity of heterocycles. Tetrahedron 2014, 70, 9506–9511. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Tomilin, D.N.; Trofimov, B.A. C-Ethynylpyrroles: Synthesis and reactivity. Russ. Chem. Rev. 2014, 83, 475–501. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Trofimov, B.A. Recent Strides in the Transition Metal-Free Cross-Coupling of Haloacetylenes with Electron-Rich Heterocycles in Solid Media. Molecules 2020, 25, 2490. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Sobenina, L.N.; Saliy, I.V.; Ushakov, I.A.; Belogolova, A.M.; Trofimov, B.A. Substituted pyrrolyl-cyanopyridines on the platform of acylethynylpyrroles their 1 : 2 annulation with acetonitrile under the action of lithium metal. New J. Chem. 2022, 46, 13149–13155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).