Discovery of Hyrtinadine A and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents
Abstract
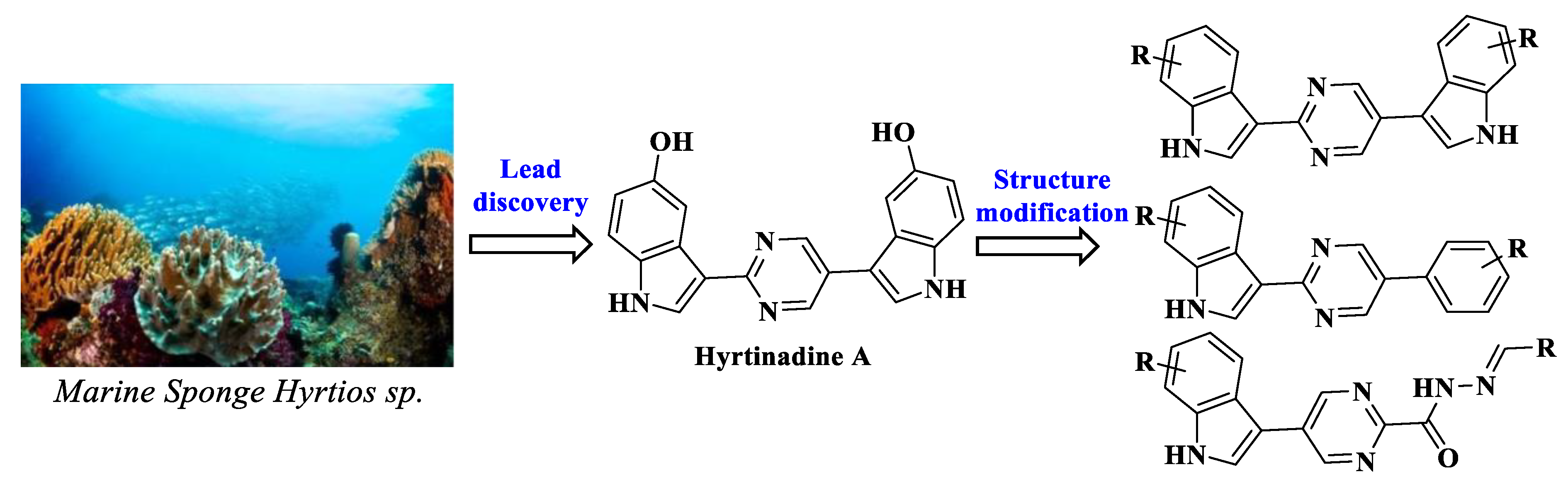
1. Introduction
2. Results and Discussion
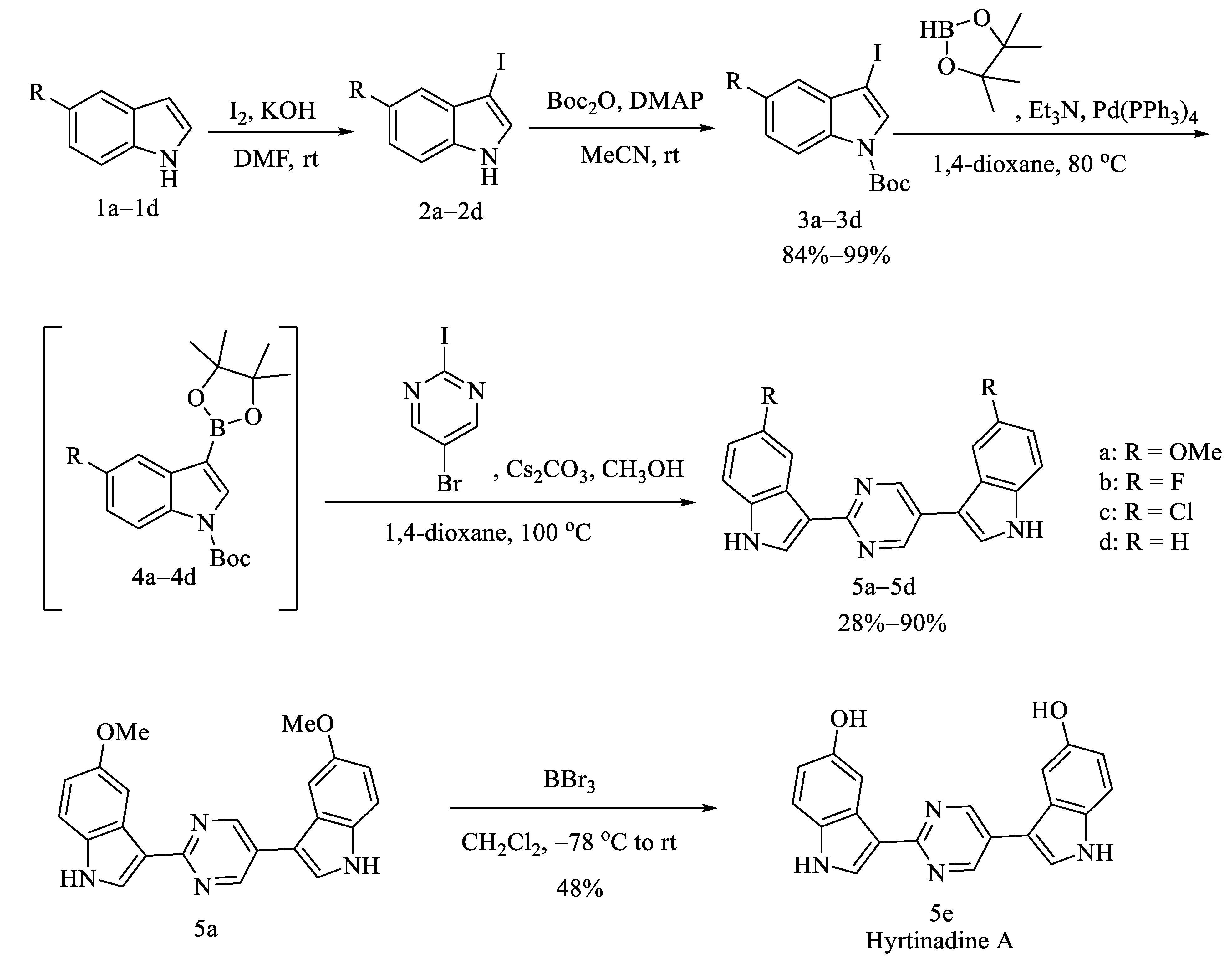
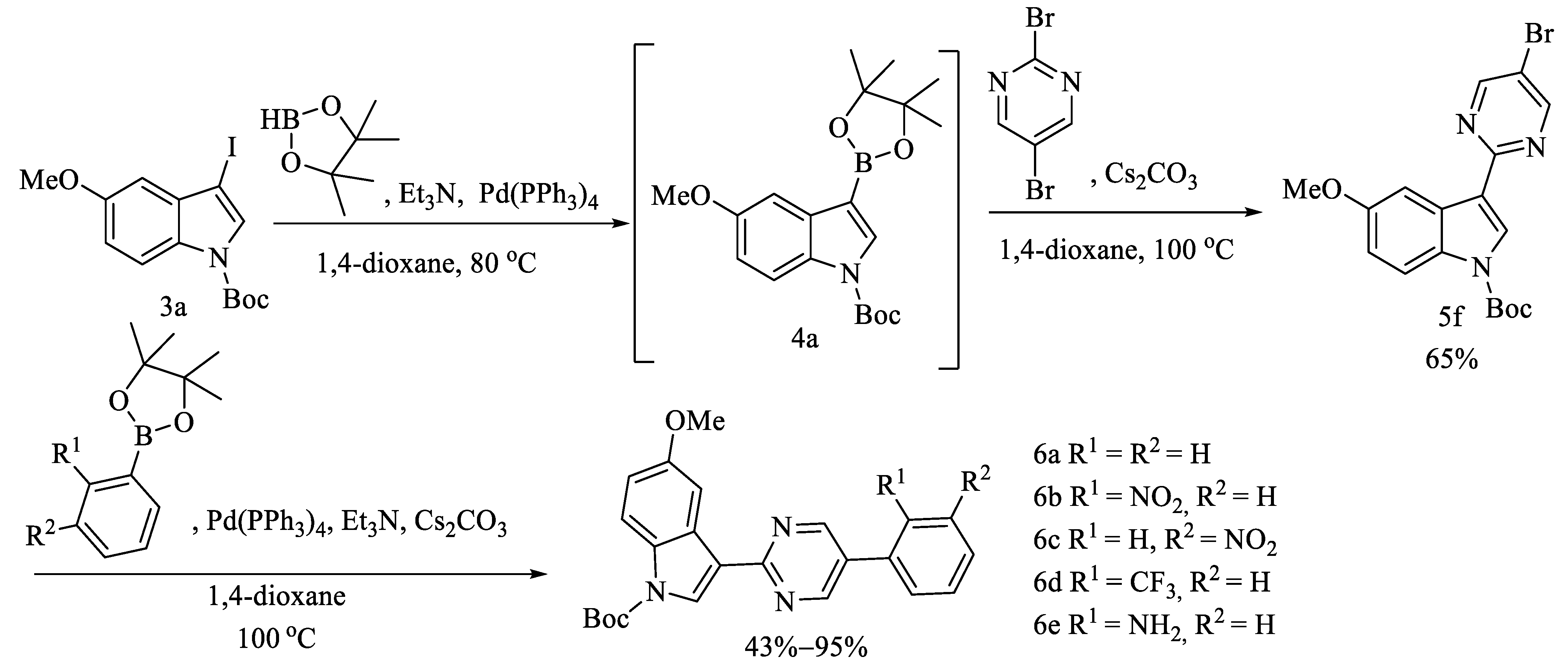
2.1. Chemistry
2.2. Phytotoxic Activity
2.3. Antiviral Activity
2.3.1. In Vivo Anti-TMV Activity
2.3.2. Structure-Activity Relationship (SAR)
2.4. Fungicidal Activity
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. Chemicals
3.1.2. Instruments
3.1.3. General Procedures for the Preparation of 3a–3d
3.1.4. General Procedures for the Preparation of 5a–5d
3.1.5. Procedures for the Preparation of 3,3′-(Pyrimidine-2,5-diyl)bis(1H-indol-5-ol) (Hyrtinadine A, 5e)
3.1.6. Procedures for the Preparation 5f and 5g
3.1.7. General Procedures for the Preparation of 6a–6e
3.1.8. General Procedures for the Preparation of 7a–7f
3.1.9. General Procedures for the Preparation of Compounds 8a and 8b
3.1.10. Procedures for the Preparation of Compound 5-(5-Methoxy-1H-indol-3-yl)pyrimidine-2-carbohydrazide (8c)
3.1.11. General Procedures for the Preparation of 9a–9g
3.2. Biological Assay
3.2.1. Antiviral Biological Assay
3.2.2. Antifungal Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, S.Z.; Hu, X.J.; Wang, J.H.; Tang, L.; Li, P.; Zheng, S.G.; Zheng, L.; Huang, L.S.; Xin, Z.H. Advanced application of raman spectroscopy and surface-enhanced raman spectroscopy in plant disease diagnostics: A review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Path. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Liu, L.R. The Control of Disease and Pests of Tobacco; Science Press Beijing: Beijing, China, 1998; p. 31. [Google Scholar]
- Song, B.A.; Yang, S.; Jin, L.H.; Bhadury, P.S. Environment Friendly Anti-Plant Viral Agents; Chemical Industry Press: Beijing, China; Springer: Berlin, Germany, 2009; pp. 1–305. [Google Scholar]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdis. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a case study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Metal nanoparticles against fungicide resistance: Alternatives or partners? Pest Manag. Sci. 2022, 78, 3953–3956. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xie, J.C.; Zhang, J.X.; Yan, H.; Xiong, Y.M.; Liu, W.; Min, S.G. A global model for the determination of prohibited addition in pesticide formulations by near infrared spectroscopy. Infrared Phys. Techn. 2020, 105, 103191. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from marine sources: An assessment of the current "state of play". Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R.; et al. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, Q. Clinical efficacy of combination of vidarabine monophosphate with compound glycyrrhizin in treatment of herpes zoster. Food Drug 2009, 11, 48–49. [Google Scholar]
- Liu, M.Y.; Zhang, X.W.; Li, G.Q. Structural and biological insights into the hot-spot marine natural products reported from 2012 to 2021. Chin. J. Chem. 2022, 40, 1867–1889. [Google Scholar] [CrossRef]
- Endo, T.; Tsuda, M.; Fromont, J.; Kobayashi, J. Hyrtinadine A, a bis-indole alkaloid from a marine sponge. J. Nat. Prod. 2007, 70, 423–424. [Google Scholar] [CrossRef]
- Boris, O.A.; Tasch, E.M.; Müller, T.J.J. One-pot synthesis of diazine-bridged bisindoles and concise synthesis of the marine alkaloid hyrtinadine A. Eur. J. Org. Chem. 2011, 24, 4532–4535. [Google Scholar]
- Ansari, N.H.; Söderberg, B.C.G. Short syntheses of the indole alkaloids alocasin A, scalaridine A, and hyrtinadines A-B. Tetrahedron 2016, 72, 4214–4221. [Google Scholar] [CrossRef]
- Ji, X.F.; Guo, J.C.; Liu, Y.X.; Lu, A.D.; Wang, Z.W.; Li, Y.Q.; Yang, S.X.; Wang, Q.M. Marine-natural-product development: First discovery of nortopsentin alkaloids as novel antiviral, anti-phytopathogenic-fungus, and insecticidal agents. J. Agric. Food Chem. 2018, 66, 4062–4072. [Google Scholar] [CrossRef]
- Hao, Y.N.; Wang, K.H.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Wang, Q.M. Luotonin A and its derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef]
- Zhang, M.J.; Ding, X.; Kang, J.; Gao, Y.Y.; Wang, Z.W.; Wang, Q.M. Marine natural product for pesticide candidate: Pulmonarin alkaloids as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 11350–11357. [Google Scholar] [CrossRef]
- Mosquera, A.; Riveiros, R.; Sestelo, J.P.; Sarandeses, L.A. Cross-coupling reactions of indium organometallics with 2,5-dihalopyrimidines: Synthesis of hyrtinadine A. Org. Lett. 2008, 10, 3745–3748. [Google Scholar] [CrossRef]
- Lamberth, C. Agrochemical lead optimization by scaffold hopping. Pest Manag. Sci. 2018, 74, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, R.; Li, Y.N.; Li, S.Y.; Yu, J.; Zhao, B.F.; Liao, A.C.; Wang, Y.; Wang, Z.W.; Lu, A.D.; et al. Discovery of pimprinine alkaloids as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.N.; Guo, J.C.; Wang, Z.W.; Liu, Y.X.; Li, Y.Q.; Ma, D.J.; Wang, Q.M. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wei, P.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Li, S.Z., Ed.; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]
- Leberman, R. Isolation of plant viruses by means of simple coacervates. Virol 1966, 30, 341–347. [Google Scholar] [CrossRef]
- Fraenkel Conrat, H.; Williams, R.C. Reconstitution of active tobacco mosaic virus fromits inactive protein and nucleic acid components. Proc. Natl. Acad. Sci. USA 1955, 41, 690–698. [Google Scholar] [CrossRef]
- Zhao, H.P.; Liu, Y.X.; Cui, Z.P.; Beattie, D.; Gu, Y.C.; Wang, Q.M. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef]


| Compound | Conc. (µg/mL) | Inhibition Rate (%) a | ||
|---|---|---|---|---|
| Inactivation Effect | Curative Effect | Protection Effect | ||
| 5a | 500 | 53 ± 1 | 48 ± 2 | 55 ± 1 |
| 100 | 21 ± 2 | 14 ± 1 | 20 ± 2 | |
| 5b | 500 | 25 ± 1 | / | / |
| 5c | 500 | 51 ± 2 | 54 ± 4 | 47 ± 2 |
| 100 | 18 ± 1 | 22 ± 2 | 15 ± 1 | |
| 5d | 500 | 43 ± 4 | 47 ± 4 | 51 ± 3 |
| 100 | 10 ± 1 | 9 ± 2 | 15 ± 1 | |
| 5e | 500 | 11 ± 1 | / | / |
| 5f | 500 | 34 ± 2 | / | / |
| 5g | 500 | 43 ± 3 | 47 ± 4 | 48 ± 3 |
| 100 | 14 ± 1 | 12 ± 1 | 18 ± 2 | |
| 6a | 500 | 18 ± 1 | / | / |
| 6b | 500 | 28 ± 3 | / | / |
| 6c | 500 | 16 ± 1 | / | / |
| 6e | 500 | 40 ± 3 | 45 ± 1 | 36 ± 4 |
| 100 | 9 ± 2 | 15 ± 1 | 8 ± 1 | |
| 7a | 500 | 36 ± 1 | / | / |
| 7b | 500 | 46 ± 1 | 38 ± 2 | 40 ± 1 |
| 100 | 8 ± 4 | 13 ± 1 | 16 ± 1 | |
| 7c | 500 | 44 ± 1 | 48 ± 4 | 39 ± 4 |
| 100 | 12 ± 2 | 17 ± 2 | 7 ± 1 | |
| 7d | 500 | 43 ± 2 | 46 ± 4 | 38 ± 4 |
| 100 | 13 ± 1 | 9 ± 1 | 15 ± 1 | |
| 7e | 500 | 33 ± 2 | / | / |
| 7f | 500 | 40 ± 1 | 35 ± 4 | 47 ± 3 |
| 100 | 15 ± 1 | 6 ± 2 | 12 ± 1 | |
| 8a | 500 | 55 ± 3 | 52 ± 3 | 57 ± 2 |
| 100 | 19 ± 1 | 22 ± 1 | 26 ± 1 | |
| 8b | 500 | 39 ± 1 | / | / |
| 9a | 500 | 42 ± 3 | 43 ± 2 | 37 ± 4 |
| 100 | 16 ± 1 | 10 ± 2 | 12 ± 1 | |
| 9b | 500 | 45 ± 2 | 39 ± 3 | 47 ± 4 |
| 100 | 18 ± 1 | 10 ± 2 | 16 ± 1 | |
| 9c | 500 | 3 ± 1 | / | / |
| 9d | 500 | 42 ± 1 | 33 ± 5 | 37 ± 2 |
| 100 | 9 ± 1 | 13 ± 1 | 10 ± 3 | |
| 9e | 500 | 51 ± 1 | 45 ± 4 | 54 ± 3 |
| 100 | 24 ± 2 | 18 ± 1 | 23 ± 1 | |
| 9f | 500 | 55 ± 2 | 51 ± 1 | 55 ± 3 |
| 100 | 22 ± 1 | 18 ± 2 | 19 ± 1 | |
| 9g | 500 | 21 ± 2 | / | / |
| Ningnanmycin | 500 | 58 ± 1 | 55 ± 2 | 57 ± 1 |
| 100 | 26 ± 1 | 24 ± 1 | 28 ± 1 | |
| Ribavirin | 500 | 39 ± 1 | 38 ± 2 | 40 ± 1 |
| 100 | 12 ± 1 | 13 ± 1 | 14 ± 2 | |
| Compound | Fungicidal Activity %/50 μg/mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.C | C.H | P.P | R.C | B.M | W.A | F.M | A.S | F.G | P.I | P.C | S.S | R.S | B.C | |
| 5a | 32 ± 1 | 38 ± 3 | 63 ± 2 | 56 ± 2 | 26 ± 1 | 24 ± 1 | 42 ± 2 | 29 ± 1 | 21 ± 1 | 6 ± 1 | 19 ± 2 | 47 ± 3 | 27 ± 2 | 33 ± 1 |
| 5b | 24 ± 2 | 29 ± 2 | 61 ± 1 | 49 ± 1 | 29 ± 2 | 30 ± 3 | 33 ± 1 | 50 ± 1 | 57 ± 2 | 11 ± 1 | 48 ± 2 | 60 ± 2 | 18 ± 1 | 59 ± 2 |
| 5c | 32 ± 3 | 33 ± 2 | 56 ± 2 | 70 ± 1 | 23 ± 1 | 36 ± 2 | 25 ± 1 | 50 ± 2 | 21 ± 1 | 17 ± 2 | 62 ± 1 | 47 ± 1 | 18 ± 2 | 7 ± 1 |
| 5d | 29 ± 1 | 25 ± 1 | 49 ± 1 | 51 ± 2 | 29 ± 2 | 24 ± 1 | 42 ± 3 | 21 ± 1 | 7 ± 2 | 11 ± 1 | 48 ± 2 | 47 ± 3 | 46 ± 1 | 46 ± 3 |
| 5e | 29 ± 2 | 29 ± 2 | 66 ± 2 | 56 ± 1 | 34 ± 1 | 30 ± 2 | 33 ± 1 | 36 ± 2 | 21 ± 1 | 11 ± 2 | 33 ± 1 | 27 ± 1 | 27 ± 1 | 20 ± 1 |
| 5f | 32 ± 1 | 25 ± 1 | 56 ± 1 | 60 ± 3 | 26 ± 2 | 30 ± 1 | 33 ± 3 | 36 ± 3 | 21 ± 1 | 22 ± 1 | 48 ± 3 | 20 ± 1 | 27 ± 2 | 13 ± 1 |
| 5g | 42 ± 3 | 46 ± 2 | 75 ± 2 | 71 ± 3 | 49 ± 1 | 52 ± 3 | 42 ± 1 | 36 ± 2 | 14 ± 2 | 11 ± 1 | 48 ± 1 | 20 ± 2 | 27 ± 1 | 26 ± 2 |
| 6a | 32 ± 1 | 54 ± 2 | 69 ± 1 | 70 ± 1 | 34 ± 2 | 42 ± 1 | 54 ± 3 | 14 ± 1 | 14 ± 3 | 6 ± 1 | 10 ± 1 | 60 ± 1 | 64 ± 3 | 7 ± 1 |
| 6b | 32 ± 2 | 33 ± 1 | 64 ± 2 | 60 ± 2 | 23 ± 1 | 36 ± 2 | 33 ± 1 | 36 ± 2 | 57 ± 1 | 11 ± 1 | 33 ± 2 | 27 ± 2 | 27 ± 1 | 13 ± 2 |
| 6c | 24 ± 1 | 29 ± 2 | 49 ± 1 | 49 ± 1 | 20 ± 2 | 21 ± 1 | 33 ± 2 | 29 ± 1 | 64 ± 2 | 22 ± 1 | 29 ± 1 | 27 ± 1 | 18 ± 2 | 3 ± 1 |
| 6d | 21 ± 2 | 25 ± 1 | 54 ± 2 | 41 ± 2 | 23 ± 1 | 24 ± 2 | 29 ± 1 | 14 ± 1 | 7 ± 1 | 11 ± 1 | 14 ± 2 | 47 ± 2 | 27 ± 1 | 7 ± 1 |
| 6e | 45 ± 1 | 50 ± 1 | 79 ± 1 | 77 ± 1 | 46 ± 2 | 46 ± 1 | 46 ± 2 | 14 ± 1 | 14 ± 1 | 6 ± 1 | 19 ± 2 | 13 ± 1 | 18 ± 2 | 13 ± 1 |
| 7a | 18 ± 1 | 29 ± 2 | 49 ± 2 | 56 ± 2 | 23 ± 1 | 18 ± 1 | 29 ± 1 | 36 ± 2 | 7 ± 1 | 11 ± 1 | 48 ± 2 | 13 ± 2 | 46 ± 1 | 13 ± 1 |
| 7b | 37 ± 1 | 42 ± 1 | 66 ± 1 | 54 ± 1 | 34 ± 1 | 42 ± 2 | 46 ± 2 | 29 ± 1 | 14 ± 2 | 11 ± 1 | 38 ± 1 | 20 ± 1 | 18 ± 2 | 40 ± 2 |
| 7c | 21 ± 2 | 21 ± 2 | 49 ± 3 | 37 ± 3 | 20 ± 2 | 21 ± 1 | 29 ± 1 | 21 ± 2 | 7 ± 1 | 6 ± 1 | 24 ± 2 | 13 ± 1 | 46 ± 1 | 20 ± 1 |
| 7d | 26 ± 1 | 38 ± 1 | 49 ± 1 | 60 ± 1 | 23 ± 1 | 27 ± 2 | 33 ± 2 | 29 ± 1 | 29 ± 2 | 17 ± 1 | 33 ± 1 | 40 ± 2 | 36 ± 3 | 40 ± 2 |
| 7e | 21 ± 1 | 33 ± 3 | 48 ± 2 | 56 ± 2 | 26 ± 1 | 18 ± 1 | 25 ± 1 | 21 ± 2 | 43 ± 1 | 6 ± 1 | 24 ± 2 | 13 ± 1 | 27 ± 1 | 13 ± 1 |
| 7f | 24 ± 2 | 25 ± 1 | 46 ± 3 | 27 ± 1 | 20 ± 2 | 21 ± 2 | 33 ± 2 | 14 ± 1 | 7 ± 1 | 6 ± 1 | 24 ± 2 | 7 ± 1 | 27 ± 2 | 46 ± 3 |
| 8a | 32 ± 1 | 42 ± 2 | 61 ± 1 | 66 ± 3 | 34 ± 1 | 27 ± 1 | 38 ± 1 | 21 ± 2 | 14 ± 2 | 11 ± 2 | 33 ± 1 | 13 ± 1 | 18 ± 1 | 20 ± 1 |
| 8b | 32 ± 2 | 13 ± 1 | 64 ± 3 | 33 ± 1 | 26 ± 2 | 21 ± 2 | 21 ± 1 | 21 ± 2 | 21 ± 1 | 6 ± 1 | 38 ± 2 | 13 ± 1 | 46 ± 3 | 26 ± 2 |
| 9a | 18 ± 1 | 29 ± 2 | 41 ± 1 | 34 ± 2 | 23 ± 1 | 27 ± 1 | 25 ± 2 | 43 ± 1 | 57 ± 2 | 33 ± 1 | 33 ± 1 | 27 ± 2 | 46 ± 1 | 53 ± 2 |
| 9b | 37 ± 2 | 33 ± 1 | 51 ± 2 | 34 ± 1 | 11 ± 1 | 30 ± 1 | 33 ± 3 | 21 ± 2 | 7 ± 1 | 6 ± 1 | 24 ± 1 | 13 ± 2 | 9 ± 1 | 26 ± 1 |
| 9c | 21 ± 1 | 46 ± 2 | 57 ± 3 | 49 ± 2 | 23 ± 3 | 18 ± 1 | 42 ± 2 | 29 ± 1 | 43 ± 2 | 17 ± 2 | 38 ± 3 | 27 ± 1 | 9 ± 2 | 46 ± 2 |
| 9d | 24 ± 1 | 21 ± 1 | 53 ± 1 | 34 ± 1 | 20 ± 1 | 24 ± 2 | 33 ± 1 | 43 ± 3 | 14 ± 1 | 17 ± 1 | 24 ± 1 | 7 ± 1 | 18 ± 1 | 53 ± 2 |
| 9e | 21 ± 2 | 54 ± 2 | 57 ± 3 | 63 ± 2 | 14 ± 1 | 21 ± 1 | 42 ± 2 | 36 ± 1 | 7 ± 1 | 22 ± 2 | 10 ± 1 | 7 ± 1 | 18 ± 2 | 46 ± 2 |
| 9f | 32 ± 2 | 13 ± 1 | 57 ± 2 | 34 ± 1 | 40 ± 2 | 30 ± 2 | 25 ± 2 | 21 ± 2 | 7 ± 1 | 6 ± 1 | 14 ± 1 | 13 ± 1 | 18 ± 2 | 26 ± 1 |
| 9g | 32 ± 1 | 13 ± 2 | 59 ± 1 | 39 ± 2 | 29 ± 1 | 42 ± 2 | 29 ± 1 | 7 ± 1 | 7 ± 1 | 6 ± 1 | 10 ± 2 | 7 ± 2 | 9 ± 1 | 40 ± 3 |
| Carbendazim b | 97 ± 1 | 29 ± 2 | 97 ± 1 | 99 ± 1 | 97 ± 1 | 97 ± 1 | 88 ± 2 | 21 ± 1 | 100 | 100 | 29 ± 1 | 93 ± 2 | 9 ± 1 | 100 |
| Chlorothalonil b | 76 ± 2 | 54 ± 1 | 82 ± 1 | 66 ± 2 | 69 ± 1 | 67 ± 3 | 79 ± 1 | 43 ± 1 | 57 ± 2 | 83 ± 2 | 95 ± 1 | 80 ± 1 | 73 ± 2 | 88 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Ma, H.; Wang, B.; Yang, S.; Wang, Z.; Li, Y.; Liu, Y.; Wang, Q. Discovery of Hyrtinadine A and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents. Molecules 2022, 27, 8439. https://doi.org/10.3390/molecules27238439
Dong J, Ma H, Wang B, Yang S, Wang Z, Li Y, Liu Y, Wang Q. Discovery of Hyrtinadine A and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents. Molecules. 2022; 27(23):8439. https://doi.org/10.3390/molecules27238439
Chicago/Turabian StyleDong, Ji, Henan Ma, Beibei Wang, Shaoxiang Yang, Ziwen Wang, Yongqiang Li, Yuxiu Liu, and Qingmin Wang. 2022. "Discovery of Hyrtinadine A and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents" Molecules 27, no. 23: 8439. https://doi.org/10.3390/molecules27238439
APA StyleDong, J., Ma, H., Wang, B., Yang, S., Wang, Z., Li, Y., Liu, Y., & Wang, Q. (2022). Discovery of Hyrtinadine A and Its Derivatives as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents. Molecules, 27(23), 8439. https://doi.org/10.3390/molecules27238439





