Abstract
Obstructive sleep apnea (OSA) is a common syndrome that features a complex etiology and set of mechanisms. Here we summarized the molecular pathogenesis of OSA, especially the prospective mechanism of upper? airway dilator fatigue and the current breakthroughs. Additionally, we also introduced the molecular mechanism of OSA in terms of related studies on the main signaling pathways and epigenetics alterations, such as microRNA, long non-coding RNA, and DNA methylation. We also reviewed small molecular compounds, which are potential targets for gene regulations in the future, that are involved in the regulation of OSA. This review will be beneficial to point the way for OSA research within the next decade.
1. Introduction
Obstructive sleep apnea (OSA) is a clinical condition characterized by sleep-related recurrent upper airway obstruction, hypopnea and apnea, resulting in chronic intermittent hypoxemia (CIH) and sleep disorders [1]. It estimated that 936 million adults aged 30–69 years (men and women) have mild to severe obstructive sleep apnoea and 425 million adults aged 30–69 years have moderate to severe obstructive sleep apnoea globally. The number of affected individuals was highest in China, followed by the USA, Brazil, and India [2]. It is a highly prevalent disorder which has rapidly evolved into a major global public health burden, independently linked with the development and control of numerous cardiovascular and metabolic conditions including hypertension, coronary artery disease, stroke, heart failure, type 2 diabetes or on-alcoholic fatty liver disease [3]. Polysomnography, the gold standard for the diagnosis of OSA, is utilized to monitor the frequency of obstructive respiratory events (apneas and hypopneas) during sleep. The severity of OSA is defined by apnea– hypopnea index(AHI), persons with an AHI of 5 to 15, 16 to 30, or more than 30 events per hour are considered to have mild, moderate, or severe obstructive sleep apnea, respectively [2]. There is a wide range of treatment options for OSA, including surgical interventions, lifestyle modifications, drug control, continuous positive airway pressure (CPAP), oral appliances (OAs) and hypoglossal nerve stimulation (HGNS) [4]. However, although there were some improvements in some aspects of OSA, no revolutionary changes have emerged in the progress of diagnosis and clinical treatment. Therefore, we reviewed current knowledge about pathogenesis, molecular mechanism of OSA, and exploration of some new breakthroughs, thus develop novel ideas for OSA.
2. The Pathogenesis of OSA and Pharyngeal Muscle Fatigue
The pathogenesis of OSA can be attributed to anatomical stenosis and pharyngeal dilator dysfunction (Figure 1). Anatomical stenosis includes upper airway anatomical structure stenosis, negative airway pressure and an increase in external tissue in pharyngeal space such as fatty tissue [5,6]. However, the pharyngeal collapse of OSA is partly due to stenosis of the upper airway anatomy [7]. The dysfunction of pharyngeal dilator might also play a key role in the pathophysiology of OSA [8,9]. Obesity can lead to soft tissue enlargement of the upper airway and craniofacial abnormalities, which are also important factors for the anatomical risk of OSA [10].
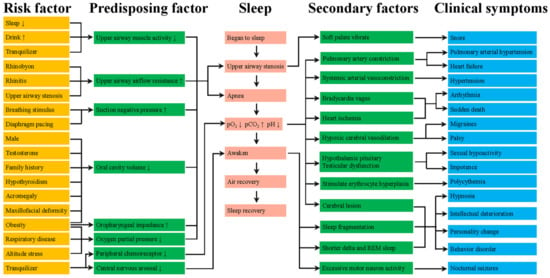
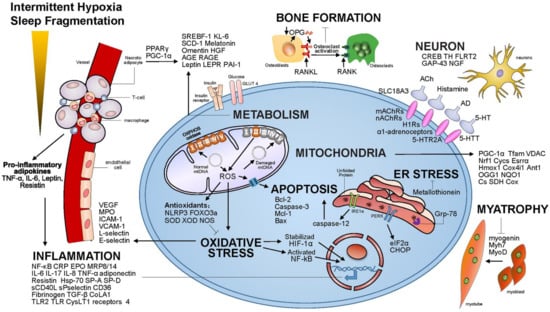
Figure 1.
The pathogenesis of OSA.
More details about the pathological process in OSA could be found in Figure 1. Among these, we are most interested in the pathogenesis of upper airway dilator neurological impairment. The basic mechanism of neuropathology in obstructive sleep apnea syndrome was controversial and single mechanism was unlikely to explain all the changes. The most reasonable explanation is that these changes reflect the effects of repeated exposure to hypoxia, vibration, abnormal movement, which may lead to local trauma caused by inflammation, impair nerve function by axonal injury, resulting in the vulnerability of motor nerve endings [11,12]. Therefore, these effects might induce upper airway muscle remodeling, alter contraction frequency and fatigue resistance of upper airway muscle [12,13]. Chronically, these changes make the airway narrower and easier to collapse. We think that active remodeling may help maintain muscle functions.
4. MicroRNA (miRNA) in OSA
MicroRNA (miRNA) is a kind of non-coding RNA, which is widely used in organ development, inflammation, tumor development and other aspects because of its inhibitory effect on target genes. As OSA is a systemic disease, miRNA is bound to play an indispensable role in its occurrence and development (Table 2). Researchers indicate that the presence of endothelial dysfunction, atherosclerosis, and hypertension in OSA may be associated with up-regulations or down-regulations of some miRNAs [116,117,118,119]. Recent studies found that several miRNAs could influence IH process and affect hypoxia-induced cell apoptosis [120]. Some miRNAs up-regulated or down-regulated by hypoxia are direct targets of HIF-1α, HIF-2α, NF-κB, or their responsive genes, or some inflammatory signalings [121,122,123,124]. Therefore, it is suggested to identifying differentially expressed miRNAs and their potential spots in order to understand mechanism of OSA with targeted therapies. At present, although there have been some reports on the functional studies of miRNA in the OSA patients or animal models, systematic and in-depth studies on epigenetics still remain to be seen.

Table 2.
miRNAs in OSA.
5. Long Noncoding RNAs (lncRNAs) in OSA
Long noncoding RNAs (lncRNAs), a novel class of non-coding RNAs, which function in regulating gene expression [136,137], affect numerous cellular processes [82] and are implicated in multiple diseases such as liver disease, cancer, and psychiatric disease [136,138,139]. Regarding lncRNAs in OSA, researchers are now at the initial and tentative launching stages. A well-established CIH rat model was used to conduct lncRNA microarray experiments on the heart samples of rats with CIH and under normoxia control. A total of 157 lncRNAs were upregulated and 132 lncRNAs were downregulated in a rat model of CIH compared with a sham control [140]. More details could be found in Table 3.

Table 3.
The lncRNAs involved in OSA.
6. DNA Methylation in OSA
Very few studies have so far focused on the role of DNA methylation in OSA, which might bridge the gap in the molecular mechanisms underlying the pathophysiology of OSA. Studies to explore the potential association of DNA methylation patterns with the disease severity in the adult population with OSA are starting to emerge [144,145,146]. More details are found in Table 4. Further studies are required to elucidate the role of DNA methylation as a potential biomarker in the context of OSA.

Table 4.
DNA methylation in OSA.
7. Chemical Compounds for OSA Treatment
Because of their many unique natural advantages, small molecular compounds are of great significance in regulating OSA and mechanism research. Most of these small-molecule compounds are important gene inhibitors or activators of OSA-correlated signaling pathways (Table 5). These chemical compounds are mainly targeted with signaling pathways that include oxidative stress, apoptosis, mitochondria, inflammation, metabolism, and neuro-muscular connection [154,155,156,157,158,159,160,161,162,163,164,165,166]. Some clinical trials were aimed at evaluating the potential benefits of melatonin, which is a hormone that regulates sleep patterns; these benefits include being a potent antioxidant, reducing chemoreflex sensitivity, stabilizing ventilatory control, and reducing OSA severity. This clinical trial is registered with www.clinicaltrials.gov (accessed on 2 October 2022) (NCT02484300, NCT05309681). Other trials were aimed at exploring the benefits of Venlafaxine, which is an agent that increases the respiratory arousal threshold (neural drive) based on the hypothesis that OSA patients with a low arousal threshold may wake up too early before upper airway muscles can be activated to achieve stable ventilation. This clinical trial was registered with www.clinicaltrials.gov (accessed on 2 October 2022) (NCT02714400, NCT00084669). There are also some clinical trials targeted toward orexin and investigating the effects of ACT-541468, which is an orexin receptor antagonist against nighttime respiratory function in patients with mild-to-moderate obstructive sleep apnea. This clinical trial was registered with www.clinicaltrials.gov (accessed on 2 October 2022) (NCT03765294, NCT02841709).

Table 5.
The chemical compounds involved in OSA.
8. Conclusions and Perspectives
1. The research on the signaling pathway and the popularization of rapid clinical diagnosis suggest that new small-molecule targeted drugs will be developed and applied rapidly in the next decade. Although the clinical diagnosis of OSA was recently standardized and the clinical treatment of OSA has been progressing rapidly, the relevant small-molecule targeted drugs have not made important progress due to our insufficient understanding of the signaling pathways involved in this disease, including the epigenetic pathways.
2. The field of epigenetics has attracted much attention in the past few years as a potential mechanism for the etiology and phenotypic variation of multiple diseases. Recent studies on the epigenetics of OSA phenotype expression further attest to the complexity of OSA and provide inspiring prospects for controlling OSA and its consequences with more individualized diagnosis and treatment methods. For example, if OSA is the cause of epigenetic changes in a gene, such a change might reverse after treatment of OSA, and may require incremental therapies that specifically target the epigenetic modification. Future research should focus on genome-wide association methods to identify epigenomic characteristics associated with certain phenotypes, which will help to provide new diagnostic biomarkers and targeted therapy for genetically susceptible individuals.
3. For the establishment of an OSA model, we need to simulate the pathogenesis of OSA in a manner that is as close to reality as possible. The electrophysiological states of the upper airway dilator muscle are diverse in waking and the different stages of sleeping and are also associated with sleep-related genes. As such, how can we get closer to the real OSA model? As far as we know, the OSA model of non-human primates has been seldom reported, except for earlier studies. We believe that the OSA model in non-human primates is of great significance to the study of the relevant pathogenesis, targeted drug screening, and therapeutic device development.
4. Although upper airway stenosis can be expanded by surgery, the relevant soft tissue research is still in the early stage. Targeted drug therapy and functional rehabilitation of the genioglossus muscle are likely to be an important direction regarding OSA in the future. We can expect to place these drugs in these oral appliances and treat OSA with a slow-release gel, which can additionally improve the function of an upper airway dilator.
5. We believe that among the related genes, it is more important to study those involved in nerve and muscle regulation. The study of these genes will make it easier to find a breakthrough in the treatment of OSA. For example, genes related to mitochondrial function include Hmox1, Cs, Cox4i1, Ant1, 8-OGG1, and NQO1.
6. Summary: OSA, as a representative of human systemic diseases whose hypoxia mechanism can be attributed to anatomical stenosis and pharyngeal dilator dysfunction, has the above characteristics of systemic diseases and is enough to trigger (or influence) various diseases. Therefore, we should pay more attention to the main molecular mechanisms of OSA pathogenesis when referring to the treatment, and thus, to effect a cure or prevent the occurrence of OSA. Preventive and therapeutic drugs targeting the relevant molecular targets are expected. We remain optimistic about the treatment of OSA in light of the current progress and OSA will be alleviated within decades.
Author Contributions
L.S., M.Z., S.L. and Y.L. (Yun Lu): performed the literature searches and wrote the draft manuscript.; X.H., L.Y., W.Z. and Y.L. (Yuehua Liu): discussion and interpretation; S.L. and Y.L. (Yuehua Liu): final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (81771109, 21703031, 81600897, 81901031), National Science Foundation of Shanghai (19411961900), Shanghai Health Commission Funds for Young Scientists (20164Y0031), Shanghai Municipal Commission of Health and Family Planning Priority Projects (201640023), Three-Year Action Plan for Promoting Clinical Skills and Innovation in Municipal Hospital (16CR2044B), Shanghai Talent Development Funding, Shanghai Wumengchao Medical Science Foundation (JJHXM-2019018), and Shanghai Stomatological Hospital Talent Project (SSDC-2019-RC01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ryan, S.; Cummins, E.P.; Farre, R.; Gileles-Hillel, A.; Jun, J.; Oster, H.; Pepin, J.-L.; Ray, D.W.; Reutrakul, S.; Sanchez-De-La-Torre, M.; et al. Understanding the pathophysiological mechanisms of cardiometabolic complications in obstructive sleep apnoea: Towards personalised treatment approaches. Eur. Respir. J. 2020, 56, 1902295. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Markt, S.C.; Stampfer, M.J.; Laden, F.; Hu, F.B.; Tworoger, S.S.; Redline, S. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur. Respir. J. 2018, 51, 1702421. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Gold, A.R.; Schubert, N.; Stryzak, A.; Wise, R.A.; Permutt, S.; Smith, P.L. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 1991, 144 Pt 1, 494–498. [Google Scholar] [CrossRef]
- Ng, S.S.S.; Tam, W.W.S.; Lee, R.W.W.; Chan, T.O.; Yiu, K.; Yuen, B.T.Y.; Wong, K.T.; Woo, J.; Ma, R.C.W.; Chan, K.K.P.; et al. Effect of Weight Loss and Continuous Positive Airway Pressure on Obstructive Sleep Apnea and Metabolic Profile Stratified by Craniofacial Phenotype: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 711–720. [Google Scholar] [CrossRef]
- Strohl, K.P. Con: Sleep apnea is not an anatomic disorder. Am. J. Respir. Crit. Care Med. 2003, 168, 271–272;discussion 272–273. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- White, D.P. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 1363–1370. [Google Scholar] [CrossRef]
- Xu, L.; Keenan, B.T.; Wiemken, A.S.; Chi, L.; Staley, B.; Wang, Z.; Wang, J.; Benedikstdottir, B.; Juliusson, S.; Pack, A.I.; et al. Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep 2020, 43, zsz273. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Lundborg, G. Vibration-induced hand problems: Role of the peripheral nerves in the pathophysiology. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 225–232. [Google Scholar] [CrossRef]
- Zhu, L.; Chamberlin, N.L.; Arrigoni, E. Muscarinic Inhibition of Hypoglossal Motoneurons: Possible Implications for Upper Airway Muscle Hypotonia during REM Sleep. J. Neurosci. 2019, 39, 7910–7919. [Google Scholar] [CrossRef]
- Cori, J.M.; Nicholas, C.L.; Avraam, J.; Lee, V.V.; Schembri, R.; Jackson, M.L.; Jordan, A.S. The Effects of Experimental Sleep Fragmentation and Sleep Deprivation on the Response of the Genioglossus Muscle to Inspiratory Resistive Loads. J. Clin. Sleep Med. 2018, 14, 715–724. [Google Scholar] [CrossRef]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Liu, H.M.; Chiang, I.J.; Kuo, K.N.; Liou, C.M.; Chen, C. The effect of acetazolamide on sleep apnea at high altitude: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2017, 11, 20–29. [Google Scholar] [CrossRef]
- Hao, T.; Liu, Y.H.; Li, Y.Y.; Lu, Y.; Xu, H.Y. Transcriptomic Analysis of Physiological Significance of Hypoxia-inducible Factor-1α in Myogenesis and Carbohydrate Metabolism of Genioglossus in Mice. Chin. Med. J. 2017, 130, 1570–1577. [Google Scholar] [CrossRef]
- Schulz, R.; Hummel, C.; Heinemann, S.; Seeger, W.; Grimminger, F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am. J. Respir. Crit. Care Med. 2002, 165, 67–70. [Google Scholar] [CrossRef]
- Ryan, S.; McNicholas, W.T. Intermittent hypoxia and activation of inflammatory molecular pathways in OSAS. Arch. Physiol. Biochem. 2008, 114, 261–266. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Mador, M.J.; Sikka, P.; Dhillon, R.S.; Amsterdam, D.; Grant, B.J. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest 2002, 121, 1541–1547. [Google Scholar] [CrossRef]
- Aydin, Ş.; Özdemir, C.; Küçükali, C.I.; Sökücü, S.N.; Giriş, M.; Akcan, U.; Tüzün, E. Reduced Peripheral Blood Mononuclear Cell ROCK1 and ROCK2 Levels in Obstructive Sleep Apnea Syndrome. In Vivo 2018, 32, 319–325. [Google Scholar]
- Ugur, K.S.; Acar, M.; Ozol, D.; Dagli, E.; Oznur, M.; Kosus, A.; Gunduz, M. Gene Expression Profiles of Tumor Necrosis Factor-α and Endothelin-1 in Obstructive Sleep Apnea. ORL-J. Oto-Rhino-Laryngol. Head Neck Surg. 2019, 81, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Serrano, F.; Fenik, P.; Hsu, R.; Kong, L.; Pratico, D.; Klann, E.; Veasey, S.C. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Ip, M.S.; Lam, B.; Chan, L.Y.; Zheng, L.; Tsang, K.W.; Fung, P.C.; Lam, W.K. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 2000, 162, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Priou, P.; Gagnadoux, F.; Tesse, A.; Mastronardi, M.L.; Agouni, A.; Meslier, N.; Racineux, J.L.; Martinez, M.C.; Trzepizur, W.; Andriantsitohaina, R. Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am. J. Pathol. 2010, 177, 974–983. [Google Scholar] [CrossRef]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef]
- Wu, X.; Chang, S.C.; Jin, J.; Gu, W.; Li, S. NLRP3 inflammasome mediates chronic intermittent hypoxia-induced renal injury implication of the microRNA-155/FOXO3a signaling pathway. J. Cell. Physiol. 2018, 233, 9404–9415. [Google Scholar] [CrossRef]
- Xu, L.H.; Xie, H.; Shi, Z.H.; Du, L.D.; Wing, Y.K.; Li, A.M.; Ke, Y.; Yung, W.H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Cohen-Kaplan, V.; Lavie, P.; Lavie, L. Bax/Mcl-1 balance affects neutrophil survival in intermittent hypoxia and obstructive sleep apnea: Effects of p38MAPK and ERK1/2 signaling. J. Transl. Med. 2012, 10, 211. [Google Scholar] [CrossRef]
- Shan, X.; Chi, L.; Ke, Y.; Luo, C.; Qian, S.; Gozal, D.; Liu, R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol. Dis. 2007, 28, 206–215. [Google Scholar] [CrossRef]
- Seo, Y.J.; Ju, H.M.; Lee, S.H.; Kwak, S.H.; Kang, M.J.; Yoon, J.H.; Kim, C.H.; Cho, H.J. Damage of Inner Ear Sensory Hair Cells via Mitochondrial Loss in a Murine Model of Sleep Apnea With Chronic Intermittent Hypoxia. Sleep 2017, 40, zsx106. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Dong, Y.; Zhang, X.; Ding, N.; Liu, J.; Hutchinson, S.Z.; Lu, G.; Zhang, X. Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS ONE 2014, 9, e109284. [Google Scholar] [CrossRef][Green Version]
- Stål, P.S.; Johansson, B. Abnormal mitochondria organization and oxidative activity in the palate muscles of long-term snorers with obstructive sleep apnea. Respiration 2012, 83, 407–417. [Google Scholar] [CrossRef]
- Zhu, Y.; Fenik, P.; Zhan, G.; Sanfillipo-Cohn, B.; Naidoo, N.; Veasey, S.C. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J. Neurosci. 2008, 28, 2168–2178. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, X.; Zheng, Y.; Miao, X.; Feng, W.; Cai, J.; Cai, L. Metallothionein prevents intermittent hypoxia-induced cardiac endoplasmic reticulum stress and cell death likely via activation of Akt signaling pathway in mice. Toxicol. Lett. 2014, 227, 113–123. [Google Scholar] [CrossRef]
- Jelic, S.; Lederer, D.J.; Adams, T.; Padeletti, M.; Colombo, P.C.; Factor, P.H.; Le Jemtel, T.H. Vascular inflammation in obesity and sleep apnea. Circulation 2010, 121, 1014–1021. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. Is C-reactive protein a marker of obstructive sleep apnea?: A meta-analysis. Medicine 2017, 96, e6850. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Toujani, S.; Kaabachi, W.; Mjid, M.; Hamzaoui, K.; Cherif, J.; Beji, M. Vitamin D deficiency and interleukin-17 relationship in severe obstructive sleep apnea-hypopnea syndrome. Ann. Thorac. Med. 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Yokoe, T.; Minoguchi, K.; Matsuo, H.; Oda, N.; Minoguchi, H.; Yoshino, G.; Hirano, T.; Adachi, M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003, 107, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.E.; Holty, J.C.; Bogan, R.K.; Hwang, D.; Ferouz-Colborn, A.S.; Budhiraja, R.; Redline, S.; Mensah-Osman, E.; Osman, N.I.; Li, Q.; et al. Use of blood biomarkers to screen for obstructive sleep apnea. Nat. Sci. Sleep 2018, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, N.; Heizhati, M.; Yao, X.; Abdireim, A.; Wang, Y.; Abulikemu, Z.; Zhang, D.; Chang, G.; Kong, J.; et al. What do changes in concentrations of serum surfactant proteins A and D in OSA mean? Sleep Breath. 2015, 19, 955–962. [Google Scholar] [CrossRef]
- Akinnusi, M.; Jaoude, P.; Kufel, T.; El-Solh, A.A. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013, 17, 1009–1016. [Google Scholar] [CrossRef]
- Cherneva, R.V.; Cherneva, Z.V.; Georgiev, O.B.; Petrova, D.S.; Petrova, J.I. 8-isoprostanes and resistin as markers of vascular damage in non-hypersomnolent obstructive sleep apnoea patients. Clin. Physiol. Funct. Imaging 2017, 37, 695–702. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Bäck, M.; Arnaud, C.; Belaïdi, E.; Tamisier, R.; Lévy, P.; Arnol, N.; Perrin, M.; Pépin, J.L.; Stanke-Labesque, F. Cysteinyl-leukotriene pathway as a new therapeutic target for the treatment of atherosclerosis related to obstructive sleep apnea syndrome. Pharmacol. Res. 2018, 134, 311–319. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Snow, A.B.; Capdevila, O.S.; Kheirandish-Gozal, L.; Gozal, D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur. Respir. J. 2010, 35, 843–850. [Google Scholar] [CrossRef]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Oda, N.; Tanaka, A.; Yamamoto, M.; Ohta, S.; O’Donnell, C.P.; Adachi, M. Silent brain infarction and platelet activation in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2007, 175, 612–617. [Google Scholar] [CrossRef]
- Cortese, R.; Gileles-Hillel, A.; Khalyfa, A.; Almendros, I.; Akbarpour, M.; Khalyfa, A.A.; Qiao, Z.; Garcia, T.; Andrade, J.; Gozal, D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci. Rep. 2017, 7, 43648. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.; Amin, R.S.; Calvin, A.D.; Davison, D.; Somers, V.K. Severity of obstructive sleep apnea is associated with elevated plasma fibrinogen in otherwise healthy patients. Sleep Breath. 2014, 18, 761–766. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujimoto, K.; Urushibata, K.; Takamizawa, A.; Kinoshita, O.; Kubo, K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006, 11, 24–31. [Google Scholar] [CrossRef]
- Steffanina, A.; Proietti, L.; Antonaglia, C.; Palange, P.; Angelici, E.; Canipari, R. The Plasminogen System and Transforming Growth Factor-β in Subjects With Obstructive Sleep Apnea Syndrome: Effects of CPAP Treatment. Respir. Care 2015, 60, 1643–1651. [Google Scholar] [CrossRef]
- Ding, X.; Yu, C.; Liu, Y.; Yan, S.; Li, W.; Wang, D.; Sun, L.; Han, Y.; Li, M.; Zhang, S.; et al. Chronic obstructive sleep apnea accelerates pulmonary remodeling via TGF-β/miR-185/CoLA1 signaling in a canine model. Oncotarget 2016, 7, 57545–57555. [Google Scholar] [CrossRef]
- Lederer, D.J.; Jelic, S.; Basner, R.C.; Ishizaka, A.; Bhattacharya, J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnoea. Eur. Respir. J. 2009, 33, 793–796. [Google Scholar] [CrossRef]
- Aihara, K.; Oga, T.; Harada, Y.; Chihara, Y.; Handa, T.; Tanizawa, K.; Watanabe, K.; Tsuboi, T.; Hitomi, T.; Mishima, M.; et al. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Respir. Med. 2011, 105, 939–945. [Google Scholar] [CrossRef]
- Li, J.; Thorne, L.N.; Punjabi, N.M.; Sun, C.K.; Schwartz, A.R.; Smith, P.L.; Marino, R.L.; Rodriguez, A.; Hubbard, W.C.; O’Donnell, C.P.; et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005, 97, 698–706. [Google Scholar] [CrossRef]
- Li, J.; Grigoryev, D.N.; Ye, S.Q.; Thorne, L.; Schwartz, A.R.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005, 99, 1643–1648. [Google Scholar] [CrossRef]
- Savransky, V.; Jun, J.; Li, J.; Nanayakkara, A.; Fonti, S.; Moser, A.B.; Steele, K.E.; Schweitzer, M.A.; Patil, S.P.; Bhanot, S.; et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ. Res. 2008, 103, 1173–1180. [Google Scholar] [CrossRef]
- Zirlik, S.; Hildner, K.M.; Targosz, A.; Neurath, M.F.; Fuchs, F.S.; Brzozowski, T.; Konturek, P.C. Melatonin and omentin: Influence factors in the obstructive sleep apnoea syndrome? J. Physiol. Pharmacol. 2013, 64, 353–360. [Google Scholar]
- Kurt, O.K.; Tosun, M.; Alcelik, A.; Yilmaz, B.; Talay, F. Serum omentin levels in patients with obstructive sleep apnea. Sleep Breath. 2014, 18, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Meng, B.; Ding, J.H. The expression of serum hepatocyte growth factor in OSAHS. J. Clin. Otorhinolaryngol. Head Neck Surg. 2017, 31, 690–693. [Google Scholar]
- Jeon, B.; Luyster, F.S.; Sereika, S.M.; DiNardo, M.M.; Callan, J.A.; Chasens, E.R. Comorbid obstructive sleep apnea and insomnia and its associations with mood and diabetes-related distress in type 2 diabetes mellitus. J. Clin. Sleep Med. 2022, 18, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lesser, D.J.; Bhatia, R.; Tran, W.H.; Oliveira, F.; Ortega, R.; Keens, T.G.; Mittelman, S.D.; Khoo, M.C.; Davidson Ward, S.L. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatric Res. 2012, 72, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Cai, W.; Sun, J.F.; Liao, W.J.; Liu, Y.; Xiao, J.R.; Zhu, L.Y.; Liu, J.Y.; Zhang, W. Serum advanced glycation end products are associated with insulin resistance in male nondiabetic patients with obstructive sleep apnea. Sleep Breath. 2015, 19, 827–833. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Tang, T.; Wu, J.; Liu, F.; Gu, L. 5-HTR2A and IL-6 polymorphisms and obstructive sleep apnea-hypopnea syndrome. Biomed. Rep. 2016, 4, 203–208. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, L.; Cao, Y.; Chen, P.; Chen, Y.; Zong, D.; Ouyang, R. Relation between serum leptin levels, lipid profiles and neurocognitive deficits in Chinese OSAHS patients. Int. J. Neurosci. 2017, 127, 981–987. [Google Scholar] [CrossRef]
- Imayama, I.; Prasad, B. Role of Leptin in Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621. [Google Scholar] [CrossRef]
- Schiza, S.E.; Mermigkis, C.; Bouloukaki, I. Leptin and leptin receptor gene polymorphisms and obstructive sleep apnea syndrome: Is there an association? Sleep Breath. 2015, 19, 1079–1080. [Google Scholar] [CrossRef][Green Version]
- Gharib, S.A.; Hayes, A.L.; Rosen, M.J.; Patel, S.R. A pathway-based analysis on the effects of obstructive sleep apnea in modulating visceral fat transcriptome. Sleep 2013, 36, 23–30. [Google Scholar] [CrossRef]
- Badran, M.; Gozal, D. PAI-1: A Major Player in the Vascular Dysfunction in Obstructive Sleep Apnea? Int. J. Mol. Sci. 2022, 23, 5516. [Google Scholar] [CrossRef]
- Chen, H.H.; Lu, J.; Guan, Y.F.; Li, S.J.; Hu, T.T.; Xie, Z.S.; Wang, F.; Peng, X.H.; Liu, X.; Xu, X.; et al. Estrogen/ERR-α signaling axis is associated with fiber-type conversion of upper airway muscles in patients with obstructive sleep apnea hypopnea syndrome. Sci. Rep. 2016, 6, 27088. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y. Effects of genistein and estrogen on the genioglossus in rats exposed to chronic intermittent hypoxia may be HIF-1α dependent. Oral Dis. 2013, 19, 702–711. [Google Scholar] [CrossRef]
- Ma, X.R.; Wang, Y.; Sun, Y.C. Imbalance of osteoprotegerin/receptor activator of nuclear factor-κB ligand and oxidative stress in patients with obstructive sleep apnea-hypopnea syndrome. Chin. Med. J. 2019, 132, 25–29. [Google Scholar] [CrossRef]
- Ragia, G.; Archontogeorgis, K.; Simmaco, M.; Gentile, G.; Borro, M.; Zissimopoulos, A.; Froudarakis, M.; Manolopoulos, V.G.; Steiropoulos, P. Genetics of Obstructive Sleep Apnea: Vitamin D Receptor Gene Variation Affects Both Vitamin D Serum Concentration and Disease Susceptibility. Omics-A J. Integr. Biol. 2019, 23, 45–53. [Google Scholar] [CrossRef]
- Qin, B.; Sun, Z.; Liang, Y.; Yang, Z.; Zhong, R. The association of 5-HT2A, 5-HTT, and LEPR polymorphisms with obstructive sleep apnea syndrome: A systematic review and meta-analysis. PLoS ONE 2014, 9, e95856. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zeng, Y.M.; Chen, X.Y.; Zhang, Y.X.; Ding, J.Z.; Xue, C. Decreased expression of hepatic cytochrome P450 1A2 (CYP1A2) in a chronic intermittent hypoxia mouse model. J. Thorac. Dis. 2018, 10, 825–834. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wu, X.; Luo, Y.J.; Wang, L.; Qu, W.M.; Li, S.Q.; Huang, Z.L. Signaling mechanism underlying the histamine-modulated action of hypoglossal motoneurons. J. Neurochem. 2016, 137, 277–286. [Google Scholar] [CrossRef]
- Grace, K.P.; Hughes, S.W.; Shahabi, S.; Horner, R.L. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir. Physiol. Neurobiol. 2013, 188, 277–288. [Google Scholar] [CrossRef]
- Chamberlin, N.L.; Bocchiaro, C.M.; Greene, R.W.; Feldman, J.L. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience 2002, 115, 861–870. [Google Scholar] [CrossRef]
- Kubin, L. Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia. Prog. Brain Res. 2014, 209, 255–274. [Google Scholar] [PubMed]
- Ling, J.; Yu, Q.; Li, Y.; Yuan, X.; Wang, X.; Liu, W.; Guo, T.; Duan, Y.; Li, L. Edaravone Improves Intermittent Hypoxia-Induced Cognitive Impairment and Hippocampal Damage in Rats. Biol. Pharm. Bulletin 2020, 43, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Liaw, S.F.; Chiu, C.H.; Lin, M.W. Effects of continuous positive airway pressure on exhaled transforming growth factor-β and vascular endothelial growth factor in patients with obstructive sleep apnea. J. Thorac. Disease 2020, 12, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Tsai, C.N.; Lee, Y.S.; Chu, S.F.; Chen, N.H. Gene expression profiles in peripheral blood mononuclear cells of Asian obstructive sleep apnea patients. Biomed. J. 2014, 37, 60–70. [Google Scholar]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nature reviews. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Chang, H.R.; Lien, C.F.; Jeng, J.R.; Hsieh, J.C.; Chang, C.W.; Lin, J.H.; Yang, K.T. Intermittent Hypoxia Inhibits Na+-H+ Exchange-Mediated Acid Extrusion Via Intracellular Na+ Accumulation in Cardiomyocytes. Cell. Physiol. Biochem. 2018, 46, 1252–1262. [Google Scholar] [CrossRef]
- Veasey, S.C.; Davis, C.W.; Fenik, P.; Zhan, G.; Hsu, Y.J.; Pratico, D.; Gow, A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004, 27, 194–201. [Google Scholar] [CrossRef]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pépin, J.L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef]
- Lam, S.Y.; Liu, Y.; Ng, K.M.; Lau, C.F.; Liong, E.C.; Tipoe, G.L.; Fung, M.L. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem. Cell Biol. 2012, 137, 303–317. [Google Scholar] [CrossRef]
- Grieve, D.J.; Shah, A.M. Oxidative stress in heart failure. More than just damage. Eur. Heart J. 2003, 24, 2161–2163. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Molecular mechanisms of cardiovascular disease in OSAHS: The oxidative stress link. Eur. Respir. J. 2009, 33, 1467–1484. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Pilkauskaite, G.; Miliauskas, S.; Sakalauskas, R. Reactive oxygen species production in peripheral blood neutrophils of obstructive sleep apnea patients. Sci. World J. 2013, 2013, 421763. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. CrossTalk opposing view: Most cardiovascular diseases in sleep apnoea are not caused by sympathetic activation. J. Physiol.-Lond. 2012, 590, 2817–2819;discussion 2821. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, G.; Huang, J.; Chen, L.; Liu, Y.; Huang, J.; Zhang, S.; Lin, Q. Inhibition of miR-193a-3p protects human umbilical vein endothelial cells against intermittent hypoxia-induced endothelial injury by targeting FAIM2. Aging 2020, 12, 1899–1909. [Google Scholar] [CrossRef]
- Periasamy, S.; Hsu, D.Z.; Fu, Y.H.; Liu, M.Y. Sleep deprivation, oxidative stress and inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwak, J.W.; Lee, K.E.; Cho, H.S.; Lim, S.J.; Kim, K.S.; Yang, H.S.; Kim, H.J. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid. Redox Signaling 2014, 21, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cheresh, P.; Jablonski, R.P.; Williams, D.B.; Kamp, D.W. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. Int. J. Mol. Sci. 2015, 16, 21486–24519. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, J.H.; Stewart, J.B. Mitochondrial DNA: Radically free of free-radical driven mutations. Biochim. Et Biophys. Acta 2015, 1847, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Groenendyk, J.; Agellon, L.B.; Michalak, M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 2013, 75, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Sack, U.; König, B.; Serebrovska, Z.; Egorov, E. Effects of Intermittent Hypoxia in Training Regimes and in Obstructive Sleep Apnea on Aging Biomarkers and Age-Related Diseases: A Systematic Review. Front. Aging Neurosci. 2022, 14, 878278. [Google Scholar] [CrossRef]
- Kong, W.; Zheng, Y.; Xu, W.; Gu, H.; Wu, J. Biomarkers of Alzheimer’s disease in severe obstructive sleep apnea-hypopnea syndrome in the Chinese population. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 865–872. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Li, Z.; Li, X.; Liu, Y.; Li, N.; Zhang, X.; Gao, Z.; Zhang, X.; Liu, Y.; et al. Genome-Wide Association Study of Obstructive Sleep Apnea and Objective Sleep-Related Traits Identifies Novel Risk Loci in Han Chinese Individuals. Am. J. Respir. Crit. Care Med. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Strausz, S.; Ruotsalainen, S.; Ollila, H.M.; Karjalainen, J.; Kiiskinen, T.; Reeve, M.; Kurki, M.; Mars, N.; Havulinna, A.S.; Luonsi, E.; et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur. Respir. J. 2021, 57, 2003091. [Google Scholar] [CrossRef]
- Li, J.; Lv, Q.; Sun, H.; Yang, Y.; Jiao, X.; Yang, S.; Yu, H.; Qin, Y. Combined Association Between ADIPOQ, PPARG, and TNF Genes Variants and Obstructive Sleep Apnea in Chinese Han Population. Nat. Sci. Sleep 2022, 14, 363–372. [Google Scholar] [CrossRef]
- Tanizawa, K.; Chin, K. Genetic factors in sleep-disordered breathing. Respir. Investig. 2018, 56, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Cade, B.E.; Sofer, T.; Sands, S.A.; Chen, H.; Browning, S.R.; Stilp, A.M.; Louie, T.L.; Thornton, T.A.; Johnson, W.C.; et al. Admixture mapping identifies novel loci for obstructive sleep apnea in Hispanic/Latino Americans. Hum. Mol. Genet. 2019, 28, 675–687. [Google Scholar] [CrossRef]
- Kalra, M.; Pal, P.; Kaushal, R.; Amin, R.S.; Dolan, L.M.; Fitz, K.; Kumar, S.; Sheng, X.; Guha, S.; Mallik, J.; et al. Association of ApoE genetic variants with obstructive sleep apnea in children. Sleep Med. 2008, 9, 260–265. [Google Scholar] [CrossRef]
- Gozal, D.; Khalyfa, A.; Capdevila, O.S.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Kim, J. Cognitive function in prepubertal children with obstructive sleep apnea: A modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid. Redox Signal. 2012, 16, 171–177. [Google Scholar] [CrossRef]
- Cade, B.E.; Chen, H.; Stilp, A.M.; Gleason, K.J.; Sofer, T.; Ancoli-Israel, S.; Arens, R.; Bell, G.I.; Below, J.E.; Bjonnes, A.C.; et al. Genetic Associations with Obstructive Sleep Apnea Traits in Hispanic/Latino Americans. Am. J. Respir. Crit. Care Med. 2016, 194, 886–897. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Khalyfa, A.; Sánchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-García, M.Á.; Barceló, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef]
- Goodchild, T.T.; Lefer, D.J. Obstructive Sleep Apnea: The Not-So-Silent Killer. Circ. Res. 2020, 126, 229–231. [Google Scholar] [CrossRef]
- Pinilla, L.; Barbé, F.; de Gonzalo-Calvo, D. MicroRNAs to guide medical decision-making in obstructive sleep apnea: A review. Sleep Med. Rev. 2021, 59, 101458. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in obstructive sleep apnea patients. Medicine 2017, 96, e7917. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Yang, G.; Zou, L.; Huang, X.; Liu, S. The Role of miRNAs during Endoplasmic Reticulum Stress Induced Apoptosis in Digestive Cancer. J. Cancer 2021, 12, 6787–6795. [Google Scholar] [CrossRef]
- Liu, K.X.; Chen, Q.; Chen, G.P.; Huang, J.C.; Huang, J.F.; He, X.R.; Lin, T.; Lin, Q.C. Inhibition of microRNA-218 reduces HIF-1α by targeting on Robo1 in mice aortic endothelial cells under intermittent hypoxia. Oncotarget 2017, 8, 104359–104366. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Han, Z.; Huang, S.; Bai, R.; Ge, X.; Chen, F.; Lei, P. Intermittent hypoxia caused cognitive dysfunction relate to miRNAs dysregulation in hippocampus. Behav. Brain Res. 2017, 335, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, W.; Li, G.C.; Jin, M.; You, Z.X.; Liu, H.G.; Hu, Y. Atorvastatin Attenuates Myocardial Hypertrophy Induced by Chronic Intermittent Hypoxia In Vitro Partly through miR-31/PKCε Pathway. Curr. Med. Sci. 2018, 38, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Li, X.; Ma, Z.; Zhang, Y.; Yuan, M.; Suo, Y.; Liang, X.; Tse, G.; Goudis, C.A.; et al. Doxycycline attenuates chronic intermittent hypoxia-induced atrial fibrosis in rats. Cardiovasc. Ther. 2018, 36, e12321. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Qin, Y.; Wei, Y. MiR-664a-3p expression in patients with obstructive sleep apnea: A potential marker of atherosclerosis. Medicine 2018, 97, e9813. [Google Scholar] [CrossRef]
- An, Z.; Wang, D.; Yang, G.; Zhang, W.Q.; Ren, J.; Fu, J.L. Role of microRNA-130a in the pathogeneses of obstructive sleep apnea hypopnea syndrome-associated pulmonary hypertension by targeting the GAX gene. Medicine 2017, 96, e6746. [Google Scholar] [CrossRef]
- Hao, S.; Jiang, L.; Fu, C.; Wu, X.; Liu, Z.; Song, J.; Lu, H.; Wu, X.; Li, S. 2-Methoxyestradiol attenuates chronic-intermittent-hypoxia-induced pulmonary hypertension through regulating microRNA-223. J. Cell. Physiol. 2019, 234, 6324–6335. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Sun, L.; Wang, D.; Wang, Y.; Zhao, S.; Dai, H.; Zhao, J.; Zhang, S.; Li, M.; et al. Chronic obstructive sleep apnea promotes aortic remodeling in canines through miR-145/Smad3 signaling pathway. Oncotarget 2017, 8, 37705–37716. [Google Scholar] [CrossRef][Green Version]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Álvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Terán-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef]
- Liu, K.X.; Chen, G.P.; Lin, P.L.; Huang, J.C.; Lin, X.; Qi, J.C.; Lin, Q.C. Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 2018, 193, 194–199. [Google Scholar] [CrossRef]
- Bi, R.; Dai, Y.; Ma, Z.; Zhang, S.; Wang, L.; Lin, Q. Endothelial cell autophagy in chronic intermittent hypoxia is impaired by miRNA-30a-mediated translational control of Beclin-1. J. Cell. Biochem. 2019, 120, 4214–4224. [Google Scholar] [CrossRef]
- Lv, X.; Wang, K.; Tang, W.; Yu, L.; Cao, H.; Chi, W.; Wang, B. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J. Cell. Biochem. 2019, 120, 18871–18882. [Google Scholar] [CrossRef]
- Lin, G.; Huang, J.; Chen, Q.; Chen, L.; Feng, D.; Zhang, S.; Huang, X.; Huang, Y.; Lin, Q. miR-146a-5p Mediates Intermittent Hypoxia-Induced Injury in H9c2 Cells by Targeting XIAP. Oxidative Med. Cell. Longev. 2019, 2019, 6581217. [Google Scholar] [CrossRef]
- Yang, X.; Niu, X.; Xiao, Y.; Lin, K.; Chen, X. MiRNA expression profiles in healthy OSAHS and OSAHS with arterial hypertension: Potential diagnostic and early warning markers. Respir. Res. 2018, 19, 194. [Google Scholar] [CrossRef]
- Gu, W.; Gong, L.; Wu, X.; Yao, X. Hypoxic TAM-derived exosomal miR-155-5p promotes RCC progression through HuR-dependent IGF1R/AKT/PI3K pathway. Cell Death Discov. 2021, 7, 147. [Google Scholar] [CrossRef]
- Yuan, K.; Lan, J.; Xu, L.; Feng, X.; Liao, H.; Xie, K.; Wu, H.; Zeng, Y. Long noncoding RNA TLNC1 promotes the growth and metastasis of liver cancer via inhibition of p53 signaling. Mol. Cancer 2022, 21, 105. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef]
- Johnsson, P.; Ziegenhain, C.; Hartmanis, L.; Hendriks, G.J.; Hagemann-Jensen, M.; Reinius, B.; Sandberg, R. Transcriptional kinetics and molecular functions of long noncoding RNAs. Nat. Genet. 2022, 54, 306–317. [Google Scholar] [CrossRef]
- DiStefano, J.K.; Gerhard, G.S. Long Noncoding RNAs and Human Liver Disease. Annu. Rev. Pathol. -Mech. Disease 2022, 17, 1–21. [Google Scholar] [CrossRef]
- Mentis, A.A.; Dardiotis, E.; Katsouni, E.; Chrousos, G.P. From warrior genes to translational solutions: Novel insights into monoamine oxidases (MAOs) and aggression. Transl. Psychiatry 2021, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Wang, Y.; Wei, L.; Chen, H. Overexpressed long noncoding RNA CPS1-IT alleviates pulmonary arterial hypertension in obstructive sleep apnea by reducing interleukin-1β expression via HIF1 transcriptional activity. J. Cell. Physiol. 2019, 234, 19715–19727. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Liu, J.; Liu, F.; Sun, Y.; Yang, R. Long non-coding RNA ROR mitigates cobalt chloride-induced hypoxia injury through regulation of miR-145. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, G.; Huang, J.; Chen, G.; Huang, X.; Lin, Q. Expression profile of long non-coding RNAs in rat models of OSA-induced cardiovascular disease: New insight into pathogenesis. Sleep Breath. 2019, 23, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Chen, Y.C.; Tseng, C.C.; Chang, H.C.; Su, M.C.; Wang, T.Y.; Lin, Y.Y.; Zheng, Y.X.; Chang, J.C.; Chin, C.H.; et al. Aberrant DNA methylation of the toll-like receptors 2 and 6 genes in patients with obstructive sleep apnea. PLoS ONE 2020, 15, e0228958. [Google Scholar] [CrossRef] [PubMed]
- Perikleous, E.; Steiropoulos, P.; Tzouvelekis, A.; Nena, E.; Koffa, M.; Paraskakis, E. DNA Methylation in Pediatric Obstructive Sleep Apnea: An Overview of Preliminary Findings. Front. Pediatrics 2018, 6, 154. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, K.T.; Su, M.C.; Hsu, P.Y.; Chin, C.H.; Lin, I.C.; Liou, C.W.; Wang, T.Y.; Lin, Y.Y.; Hsiao, C.C.; et al. Aberrant DNA methylation levels of the formyl peptide receptor 1/2/3 genes are associated with obstructive sleep apnea and its clinical phenotypes. Am. J. Transl. Res. 2020, 12, 2521–2537. [Google Scholar]
- Chen, W.; Ye, J.; Han, D.; Yin, G.; Wang, B.; Zhang, Y. Association of prepro-orexin polymorphism with obstructive sleep apnea/hypopnea syndrome. Am. J. Otolaryngol. 2012, 33, 31–36. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.W.; Su, M.C.; Chen, C.J.; Chen, K.D.; Liou, C.W.; Tang, P.; Wang, T.Y.; Chang, J.C.; Wang, C.C.; et al. Whole Genome DNA Methylation Analysis of Obstructive Sleep Apnea: IL1R2, NPR2, AR, SP140 Methylation and Clinical Phenotype. Sleep 2016, 39, 743–755. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Khalyfa, A.; Kheirandish-Gozal, L.; Capdevila, O.S.; Wang, Y.; Gozal, D. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 330–338. [Google Scholar] [CrossRef]
- L Kheirandish-Gozal, L.; Khalyfa, A.; Gozal, D.; Bhattacharjee, R.; Wang, Y. Endothelial dysfunction in children with obstructive sleep apnea is associated with epigenetic changes in the eNOS gene. Chest 2013, 143, 971–977. [Google Scholar] [CrossRef]
- Nanduri, J.; Peng, Y.J.; Wang, N.; Khan, S.A.; Semenza, G.L.; Kumar, G.K.; Prabhakar, N.R. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J. Physiol.-Lond. 2017, 595, 63–77. [Google Scholar] [CrossRef]
- Cortese, R.; Almendros, I.; Wang, Y.; Gozal, D. Tumor circulating DNA profiling in xenografted mice exposed to intermittent hypoxia. Oncotarget 2015, 6, 556–569. [Google Scholar] [CrossRef]
- Chu, A.; Gozal, D.; Cortese, R.; Wang, Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatric Res. 2015, 77, 425–433. [Google Scholar] [CrossRef][Green Version]
- Sanz-Rubio, D.; Sanz, A.; Varona, L.; Bolea, R.; Forner, M.; Gil, A.V.; Cubero, P.; Marin-Oto, M.; Martin-Burriel, I.; Marin, J.M.; et al. Forkhead Box P3 Methylation and Expression in Men with Obstructive Sleep Apnea. Int. J. Mol. Sci. 2020, 21, 2233. [Google Scholar] [CrossRef]
- Lambert, A.A.; Parker, A.M.; Moon, K.K. High-dose N-acetylcysteine in chronic obstructive pulmonary disease, prone positioning in acute respiratory distress syndrome, and continuous positive airway pressure and exhaled nitric oxide in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 223–224. [Google Scholar] [CrossRef]
- Lu, W.; Kang, J.; Hu, K.; Tang, S.; Zhou, X.; Xu, L.; Li, Y.; Yu, S. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017, 21, 667–677. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Zhang, J.; Wang, S.; Ge, W.; Li, X.; Mou, W.; Wang, X.; Chai, W.; Zhao, J.; et al. Tim-3 is a potential regulator that inhibits monocyte inflammation in response to intermittent hypoxia in children with obstructive sleep apnea syndrome. Clin. Immunol. 2021, 222, 108641. [Google Scholar] [CrossRef]
- Harki, O.; Tamisier, R.; Pépin, J.L.; Bailly, S.; Mahmani, A.; Gonthier, B.; Salomon, A.; Vilgrain, I.; Faury, G.; Briançon-Marjollet, A. VE-cadherin cleavage in sleep apnoea: New insights into intermittent hypoxia-related endothelial permeability. Eur. Respir. J. 2021, 58, 2004518. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, C.L.; Yu, C.C. Trazodone improves obstructive sleep apnea after ischemic stroke: A randomized, double-blind, placebo-controlled, crossover pilot study. J. Neurol. 2021, 268, 2951–2960. [Google Scholar] [CrossRef]
- Moderie, C.; Carrier, J.; Dang-Vu, T.T. Sleep disorders in patients with a neurocognitive disorder. Enceph. -Rev. De Psychiatr. Clin. Biol. Et Ther. 2022, 48, 325–334. [Google Scholar]
- Jaffuel, D.; Mallet, J.P.; Dauvilliers, Y.; Bourdin, A. Is the Muscle the Only Potential Target of Desipramine in Obstructive Sleep Apnea Syndrome? Am. J. Respir. Crit. Care Med. 2017, 195, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.S.; Santos-Carvalho, A.; Santos, B.; Carvalhas-Almeida, C.; Barros-Viegas, A.T.; Oliveiros, B.; Donato, H.; Santos, C.; Moita, J.; Cavadas, C.; et al. Peripheral biomarkers to diagnose obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 64, 101659. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Fenik, P.; Panckeri, K.; Pack, A.I.; Hendricks, J.C. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am. J. Respir. Crit. Care Med. 1999, 160 Pt 1, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, J.H.; Wang, M.; Huang, M.; Wang, C.Y.; Xia, H.; Xu, J.G.; Li, M.X.; Wang, S. Injection of L-glutamate into the insular cortex produces sleep apnea and serotonin reduction in rats. Sleep Breath. 2012, 16, 845–853. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea. Sleep 2018, 41, zsx184. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Lu, Y.; Zhao, B. Inhibitory effects of 17β-estradiol or a resveratrol dimer on hypoxia-inducible factor-1α in genioglossus myoblasts: Involvement of ERα and its downstream p38 MAPK pathways. Int. J. Mol. Med. 2017, 40, 1347–1356. [Google Scholar] [CrossRef]
- Dasu, M.R.; Riosvelasco, A.C.; Jialal, I. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 2009, 202, 76–83. [Google Scholar] [CrossRef]
- Nakatsuka, R.; Nozaki, T.; Uemura, Y.; Matsuoka, Y.; Sasaki, Y.; Shinohara, M.; Ohura, K.; Sonoda, Y. 5-Aza-2’-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch. Oral Biol. 2010, 55, 350–357. [Google Scholar] [CrossRef]
- Ohike, Y.; Kozaki, K.; Iijima, K.; Eto, M.; Kojima, T.; Ohga, E.; Santa, T.; Imai, K.; Hashimoto, M.; Yoshizumi, M.; et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure--possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ. J. 2005, 69, 221–226. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Guilleminault, C.; Poyares, D.; Cintra, F.; Kauati, A.; Tufik, S. A double-blind, placebo-controlled, crossover study of sildenafil in obstructive sleep apnea. Arch. Intern. Med. 2006, 166, 1763–1767. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity. A Randomized, Placebo-controlled, Double-Blind Crossover Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Edwards, B.A.; Sands, S.A.; Marques, M.; Eckert, D.J.; White, D.P.; Wellman, A. Desipramine Increases Genioglossus Activity and Reduces Upper Airway Collapsibility during Non-REM Sleep in Healthy Subjects. Am. J. Respir. Crit. Care Med. 2016, 194, 878–885. [Google Scholar] [CrossRef]
- Zhao, F.; Meng, Y.; Wang, Y.; Fan, S.; Liu, Y.; Zhang, X.; Ran, C.; Wang, H.; Lu, M. Protective effect of Astragaloside IV on chronic intermittent hypoxia-induced vascular endothelial dysfunction through the calpain-1/SIRT1/AMPK signaling pathway. Front. Pharmacol. 2022, 13, 920977. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, H.; Cui, Z.; Tai, X.; Chu, Y.; Guo, X. Tauroursodeoxycholic acid attenuates endoplasmic reticulum stress and protects the liver from chronic intermittent hypoxia induced injury. Exp. Ther. Med. 2017, 14, 2461–2468. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Li, X.; Du, Y.; Li, L.; Hu, C.; Zhang, J.; Qin, Y.; Wei, Y.; Zhang, H. Extracellular Vesicles Derived from Intermittent Hypoxia-Treated Red Blood Cells Impair Endothelial Function Through Regulating eNOS Phosphorylation and ET-1 Expression. Cardiovasc. Drugs Therapy. 2021, 35, 901–913. [Google Scholar] [CrossRef]
- Sukys-Claudino, L.; Moraes, W.; Guilleminault, C.; Tufik, S.; Poyares, D. Beneficial effect of donepezil on obstructive sleep apnea: A double-blind, placebo-controlled clinical trial. Sleep Med. 2012, 13, 290–296. [Google Scholar] [CrossRef]
- Hedner, J.; Kraiczi, H.; Peker, Y.; Murphy, P. Reduction of sleep-disordered breathing after physostigmine. Am. J. Respir. Crit. Care Med. 2003, 168, 1246–1251. [Google Scholar] [CrossRef]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef]
- Aoki, C.R.; Liu, H.; Downey, G.P.; Mitchell, J.; Horner, R.L. Cyclic nucleotides modulate genioglossus and hypoglossal responses to excitatory inputs in rats. Am. J. Respir. Crit. Care Med. 2006, 173, 555–565. [Google Scholar] [CrossRef]
- Berry, R.B.; Yamaura, E.M.; Gill, K.; Reist, C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 1999, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).