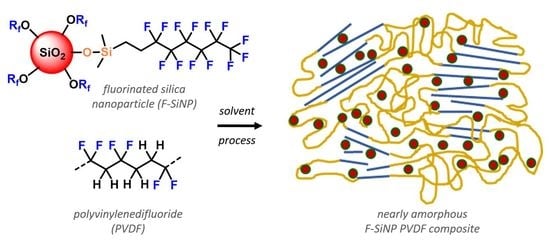
Influencing the Crystalline Domains of Poly(vinylidenedifluoride) Composites Using Fluorinated Silica Nanoparticles as Drop-In Modifiers
Abstract
1. Introduction
2. Results and Discussion
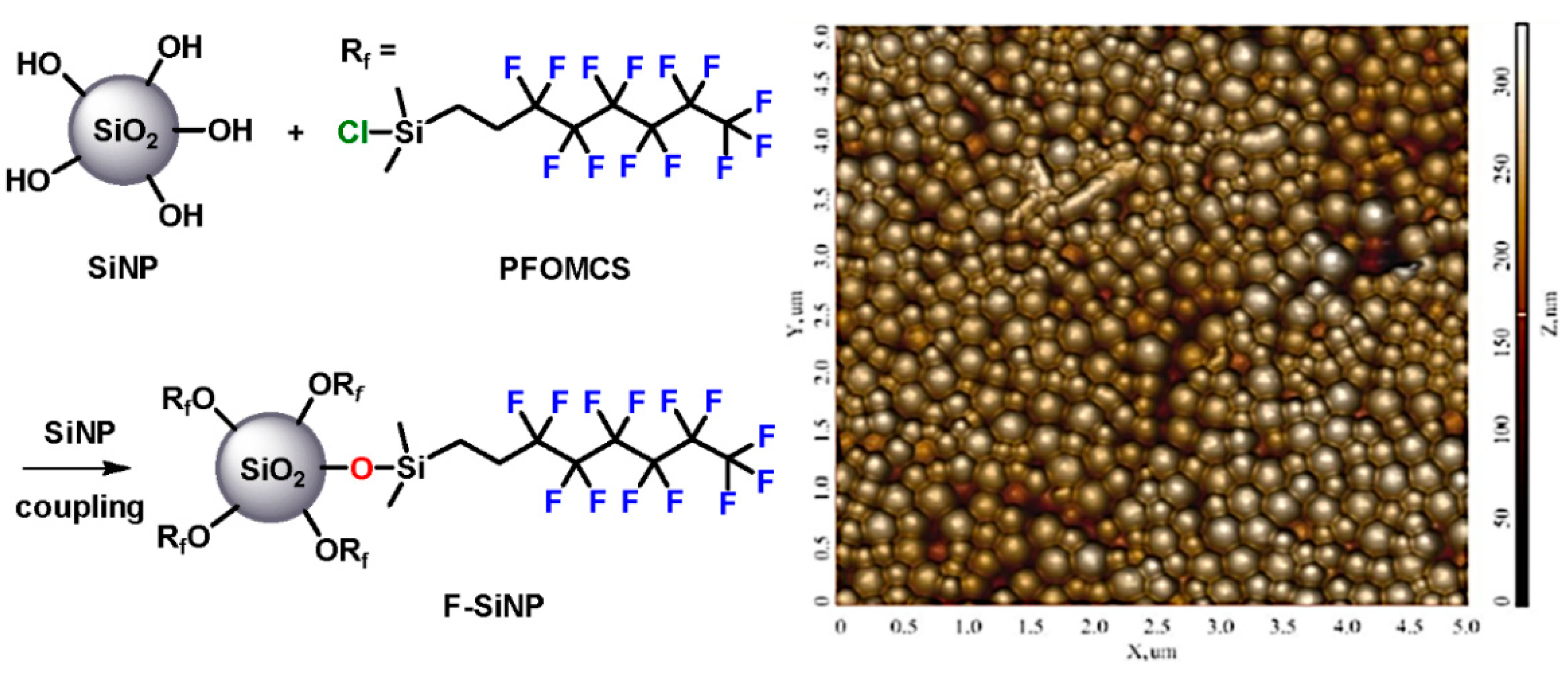
2.1. Preparation of Silica Nanoparticles (SiNPs) and Thier Surface Functionalization to Fluorinated Silica Nanoparticles (F-SiNPs)
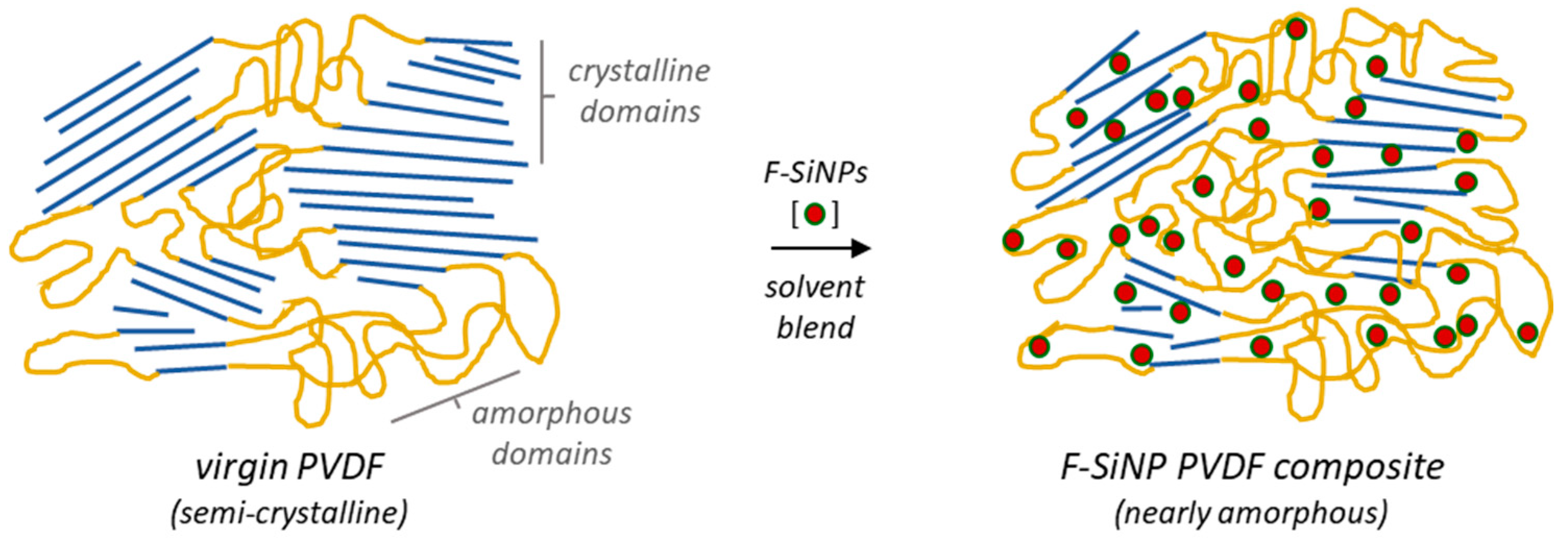
2.2. Formulation and Thermal Analysis of SiNPs and F-SiNPs Solvent-Blended PVDF Composites
3. Materials and Methods
3.1. Materials
3.2. Instrumental Methods
3.3. General Procedure for the Prepartion of Silica Nanoparticles (SiNPs) and Fluorinated Silica Nanoparticles (F-SiNPs)
3.4. General Procedure for Preparation of Solvent-Blended SiNP PVDF Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ameduri, B. The Promising Future of Fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573–1900587. [Google Scholar] [CrossRef]
- Henry, B.J.; Carlin, J.P.; Hammerschmidt, J.A.; Buck, R.C.; Buxton, L.W.; Fiedler, H.; Seed, J.; Hernandez, O. A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr. Environ. Assess. Manag. 2018, 14, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R.; Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lindstrom, A.B.; Miller, M.F.; Ng, C.A.; Patton, S.; et al. Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS? Environ. Sci. Technol. 2020, 54, 12820–12828. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S. Expanded PTFE Applications Handbook: Technology, Manufacturing and Applications; William Andrew: Oxford, UK, 2016. [Google Scholar]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(Vinylidene fluoride) (PVDF). Adv. Compos. Hybrid. Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Gebrekrstos, A.; Muzata, T.S.; Ray, S.S. Nanoparticle-Enhanced β-Phase Formation in Electroactive PVDF Composites: A Review of Systems for Applications in Energy Harvesting, EMI Shielding, and Membrane Technology. ACS Appl. Nano Mater. 2022, 5, 7632–7651. [Google Scholar] [CrossRef]
- Campos, R.; Guenthner, A.J.; Haddad, T.S.; Mabry, J.M. Fluoroalkyl-Functionalized Silica Particles: Synthesis, Characterization, and Wetting Characteristics. Langmuir 2011, 27, 10206–10215. [Google Scholar] [CrossRef]
- Iacono, S.T.; Jennings, A.R. Recent studies on fluorinated silica nanometer-sized particles. Nanomaterials 2019, 9, 684. [Google Scholar] [CrossRef]
- Ribeiro, S.; Meira, R.M.; Correia, D.M.; Tubio, C.R.; Ribeiro, C.; Baleizão, C.; Lanceros-Méndez, S. Silica nanoparticles surface charge modulation of the electroactive phase content and physical-chemical properties of poly(Vinylidene fluoride) nanocomposites. Compos. Part B Eng. 2020, 185, 107786. [Google Scholar] [CrossRef]
- Miyoshi, H.; Yumoto, A.; Shono, M.; Ueki, T.; Itsuki, Y. Visualization of PVDF nanofibers coated on filter paper using fluorescein silica nanoparticles. J. Appl. Polym. Sci. 2017, 134, 45125. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Boutevin, B.; Silly, G.; Améduri, B. Grafting from Polymerization of Vinylidene Fluoride (VDF) from Silica to Achieve Original Silica–PVDF Core–Shells. Macromolecules 2011, 44, 8487–8493. [Google Scholar] [CrossRef]
- Jennings, A.R.; Iacono, S.T.; Mabry, J.M. Polyhedral Silsesquioxanes. In Handbook of Sol-Gel Science and Technology, 2nd ed.; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3153–3176. [Google Scholar] [CrossRef]
- Campos, R.; Guenthner, A.J.; Meuler, A.J.; Tuteja, A.; Cohen, R.E.; McKinley, G.H.; Haddad, T.S.; Mabry, J.M. Superoleophobic Surfaces through Control of Sprayed-on Stochastic Topography. Langmuir 2012, 28, 9834–9841. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gazmin, E.; Glover, J.; Weeks, N.; Khan, F.; Iacono, S.T.; Corti, G. Improved electrical signal of non-poled 3D printed zinc oxide polyvinylidene fluoride nanocomposites. Polymers 2022, 14, 4312. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Gailhanou, H.; Raison, L.; Panizza, P.; Ushiki, H.; Sellier, E.; Delville, J.P.; Delville, M.H. Smart Control of Monodisperse Stöber Silica Particles: Effect of Reactant Addition Rate on Growth Process. Langmuir 2005, 21, 1516–1523. [Google Scholar] [CrossRef] [PubMed]

| SiNP Loading (wt %) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Xc (%) | Td (°C) | Char Yield in Ar at 900 °C (%) |
|---|---|---|---|---|---|---|---|
| 0 PVDF | 172 | 39.0 | 139 | 50.1 | 37 | 425 | 1 |
| 1 SiNP | 168, 172 | 30.4 | 143 | 44.7 | 29 | - | - |
| 2 SiNP | 168, 172 | 24.0 | 143 | 33.0 | 23 | - | - |
| 5 SiNP | 168, 172 | 20.9 | 143 | 32.6 | 20 | - | - |
| 10 SiNP | 167, 172 | 16.7 | 143 | 22.5 | 16 | 327 | 35 |
| 1 F-SiNP | 168, 171 | 44.2 | 143 | 51.8 | 42 | 407 | 2 |
| 2 F-SiNP | 168, 172 | 37.4 | 141 | 49.1 | 36 | 403 | 2 |
| 5 F-SiNP | 168, 172 | 32.7 | 144 | 45.9 | 31 | 430 | 3 |
| 10 F-SiNP | 167, 172 | 10.6 | 143 | 19.4 | 10 | 429 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weeks, N.J.; Phelps, C.R.; Gazmin, E.T.; Iacono, S.T. Influencing the Crystalline Domains of Poly(vinylidenedifluoride) Composites Using Fluorinated Silica Nanoparticles as Drop-In Modifiers. Molecules 2022, 27, 8398. https://doi.org/10.3390/molecules27238398
Weeks NJ, Phelps CR, Gazmin ET, Iacono ST. Influencing the Crystalline Domains of Poly(vinylidenedifluoride) Composites Using Fluorinated Silica Nanoparticles as Drop-In Modifiers. Molecules. 2022; 27(23):8398. https://doi.org/10.3390/molecules27238398
Chicago/Turabian StyleWeeks, Nathan J., Cole R. Phelps, Enrique T. Gazmin, and Scott T. Iacono. 2022. "Influencing the Crystalline Domains of Poly(vinylidenedifluoride) Composites Using Fluorinated Silica Nanoparticles as Drop-In Modifiers" Molecules 27, no. 23: 8398. https://doi.org/10.3390/molecules27238398
APA StyleWeeks, N. J., Phelps, C. R., Gazmin, E. T., & Iacono, S. T. (2022). Influencing the Crystalline Domains of Poly(vinylidenedifluoride) Composites Using Fluorinated Silica Nanoparticles as Drop-In Modifiers. Molecules, 27(23), 8398. https://doi.org/10.3390/molecules27238398