Abstract
In recent decades, there has been an increase in environmental problems caused by cosmetic products derived from toxic substances. Based on this issue, researchers and developers of new beauty cosmetics are looking for new natural alternatives that work well for the consumer and have biodegradable characteristics. This systematic review highlights the major publications of bacterial cellulose used strictly for cosmetics in the last 10 years. Bacterial cellulose is a natural product with great cosmetic properties and low cost that has shown excellent results. This study aimed at collecting rigorous information on bacterial cellulose in the cosmetic field in the last decade to produce a systematized review. A comprehensive search was conducted with selected descriptors involving the topic of “bacterial cellulose”, “cosmetics”, “clean beauty”, and “skin mask”. Seventy studies were found, which went through exclusion criteria that selected only those related to the topic that was searched. In the 12 remaining studies that met the criteria, bacterial cellulose showed conditions for use as a mask-forming product for facial care. The increase in the number of publications concerning bacterial cellulose in cosmetics in the last ten years is a strong indicator that this is a growing area for both research and the industry.
1. Introduction
There has been an increase in the environmental contamination caused by cosmetic and personal care products produced from raw materials with toxic properties and also substances derived from petroleum [1]. The substances used in cosmetics and personal care product are safe for consumers, but most of the time they are harmful to the environment. They can create problems when they are incorrectly discarded [2], or after use, when they go into water that eventually returns to the environment through effluents, as is the case with synthetic microbeads [3].
Some formulations use petroleum-derived substances as raw material, which end up resulting in bioaccumulation and biomagnification in aquatic species, causing a significant imbalance in their environment [2]. The long persistence of organic pollutants is also responsible for diseases such as allergy, endocrine and microbiota disorders, and cancer. Parabens, phthalates, toluene, formaldehyde, and sulfates are examples of toxic substances that may lead to these kinds of diseases. These substances interfere in the hormonal system, generating toxicity in the organism, causing imbalances in human health and the environment [1,4].
In this context, the need to seek new technologies arises in order to meet the demand of both final consumers, with efficient dermatological properties [5], and the ecosystem, without presenting toxicity [2]. With this movement of cosmetic and personal care industries in the search for products that are safe and ecofriendly, the application of bacterial cellulose as a natural vehicle for dermatological alternatives is highly appreciated [6].
Bacterial cellulose (BC) is a polymer, considered an ecofriendly biomaterial since it does not cause ecological pollution [7], and it also has a biodegradable nature [8]. As a cosmetic, BC is particularly promising with several dermo-pharmacological applications due to its biological properties, such as: morphology, which has organized arrangement of nanofibers, facilitating the transport and delivery of substances, without interfering in the product function [7,9]; high mechanical strength; purity; high water absorption; no toxic characteristics; and most importantly, high biocompatibility. In addition, it can replace synthetic, petroleum-derived and toxic-ecological cosmetic ingredients [6].
The production of BC is carried out through the synthesis of extracellular polysaccharides produced by some non-pathogenic bacterial strains. This polysaccharide is formed by fibers and nanofibers that form around the bacterial cells [10].
Considering skin care cosmetics, BC as a natural biodegradable polymer has proven to be a very versatile product [9,11] once its characteristics are appropriate for this type of application. Moreover, BC has been studied as a potential substitute for synthetic polymers that are commonly used by the cosmetics industry, such as polyacrylamide, nylon, and polyethylene, compounds whose toxicities are widely known and studied for replacement [12].
Also in cosmetic application, BC has been noticed as an excellent system for delivering compounds to the skin due to its high adherence and water absorption [6]. In a study reported in 2020, vitamin B was incorporated into BC membranes and its distribution property was tested. The results proved that there was release of vitamin B, and the in vitro tests showed no cytotoxicity, indicating that this type of BC-based product is suitable and safe for skin care [11,12].
Recently, in 2021, BC was evaluated as a delivery system for Epilobium angustifolium extract, a natural antioxidant. The natural compound was loaded into BC membranes and tested in fibroblast cells and in ex vivo pig skin, via a cell permeator. The results indicated that the delivery of the antioxidant was accomplished, and the system showed no cytotoxicity. In that study, BC membranes were indicated as a promising solution for the use of topical antioxidants [10].
When the environmental positive impact is added to the promising characteristics of the BC uses in cosmetics, the increase in the value chain for this biopolymer is remarkable [6,12]. Therefore, this review aimed systematically to highlight studies that contextualize the evolution of the research and development of the bacterial cellul1ose-based cosmetics in the last decade.
2. Results
The survey in the PubMed and Science Direct platforms resulted in a total of 70 articles. In Figure 1, it is possible to observe that over ten years, there was a significant increase in publications related to bacterial cellulose and cosmetics. Between the years 2011 and 2015, six articles were published. In 2017, 2018, and 2019, it is possible to visualize an ascendancy in the studies related to the topic with a slight increase in publications. However, in the last five years, the number of publications grew significantly.
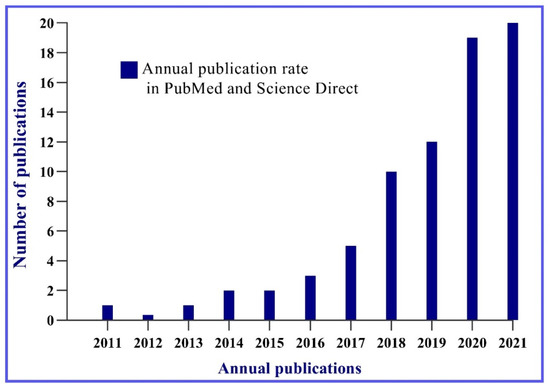
Figure 1.
Graphical representation of the perspective of publications on the use of bacterial cellulose in cosmetics in the last decade.
In the last two years—2020 and 2021—the greatest growth was observed, with an average of twelve to thirteen articles per year. This is still a relatively small average, but it allows to visualize the scientific prospect around bacterial cellulose in cosmetics, indicating an upward growth trend as illustrated in Figure 1.
Figure 2 shows that after the searches, the 70 articles went through the exclusion criteria method. After the first step of exclusion criteria for duplicate studies and reviews, 11 articles were excluded. The titles of the remaining 59 articles were read and evaluated. Then, another 42 articles were excluded because their titles did not reference what was being sought.
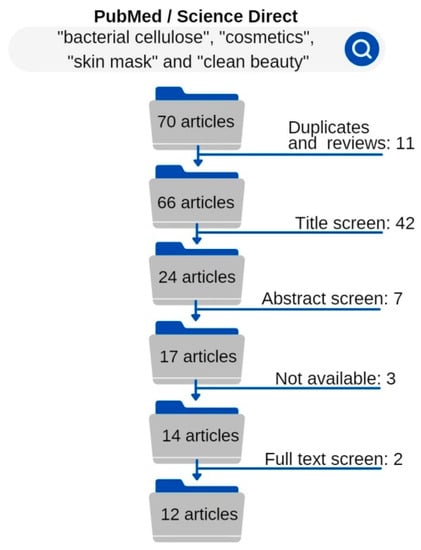
Figure 2.
Exclusion criteria delineation.
Subsequently, the abstracts of 24 articles were read and the abstracts that did not fit inclusion criteria of application of BC in cosmetics were excluded, leaving 17. From these 17, 3 articles were not available for reading. A full reading of 14 studies was then performed and 12 were within all the criteria for data collection.
Table 1 presents the main results. Analytical studies are here described as experimental ones, with direct measurements of chemical compounds. In vitro studies are those conducted outside a living organism, usually on cells kept alive and growing outside their original tissue. Ex vivo studies are performed on extracted tissues or organs. In vivo studies are performed on animals, usually rodents, also called pre-clinical tests. After the pre-clinical studies are performed, the clinical study is conducted, in which the substances are tested in humans.

Table 1.
Data demonstration collected in the studies on bacterial cellulose in cosmetics selected by the aforementioned exclusion criteria.
There is an emerging increase in studies related to BC when applied in cosmetics or in the development of new formulations. Although this review was limited to specific keywords and approaches during the searches, many studies of BC applications in various areas were found, also indicating its innovative and versatile potential.
3. Discussion
Among the studies found, another application of BC was described, by LI et al., 2015 [21], as a biomaterial of BC and hyaluronic acid with healing potential for severe skin injury. In this study, BC was tested in vitro on human fibroblast cells, showing no cytotoxicity, decreasing wound healing time, and improving tissue repair performance.
Recently, in 2021, Singh et al. [22], produced mucoadhesive BC for oral applications to protect tissue while healing. The mucoadhesive BC was tested ex vivo and indicated that the product withstood 7 days in a submerged aqueous environment under constant shear stress. The study demonstrated that the mucoadhesive BC has potential for new treatments for diseases and injuries in the oral cavity.
In the search for studies for this review of the application of BC in cosmetics, it was found that in 2011, Amnuaikit et al. [13], conducted a clinical study with 30 healthy Thai volunteers, aged between 21 and 40 years old. Among them, there were 21 women and 9 men. The masks used in this study had been produced from the fermentation of the bacteria Acetobacter xilinum in rice substrate. The masks were then steam-sterilized at 121 °C. In an attempt to test the moisturizing capacity of BC on the skin, the mask was applied to the volunteers for a period of 25 min, and within 5 min from the removal of the product, the levels of moisture and flaking, elasticity, and other parameters were evaluated. All volunteers participated until the end of the study because there was no skin irritation after 24 h of BC application. The results showed that BC significantly increased skin hydration after a single application for all volunteers. This can be considered one of the first scientific records of the potential of BC in human skin applications.
Almeida et al., 2013 [14], tested BC as a possible adhesive material on 15 healthy volunteers, 12 women and 3 men, aged 30–40 years old. The BC membranes in that study were produced using Gluconacetobacter sacchari in Hestrin and Shramm (HS) liquid medium, and subsequently washed, bleached, and oven dried at 40 °C for 16 h. After this process, 1% glycerin was incorporated into the BC membranes. Then, the product was applied to the forearm of the volunteers and two visual assessments of the degree of skin irritation were performed after the removal of the BC patches. No high levels of skin irritation were observed, proving the safety of BC as a cosmetic product.
In addition to positive results in its pure state as a potential moisturizer, BC was studied as a carrier and distributor of cosmetic pharmaceutical drugs by Numata et al. in 2015 [15]. In this study, BC gels were biosynthesized from the fermentation of Gluconacetobacter xylinus in liquid HS medium for 3 weeks under static conditions. Subsequently, the gels were purified in tap water for 2 days, deproteinized in 0.5% (w/w) NaOH solution, neutralized in 0.5% (w/w) acetic acid solution, and washed with distilled water. The incorporation process of the BC gels was performed by immersion in the retinol-loaded nanoparticle suspension for 24 h at room temperature. In in vitro release assays, BC also proved to be a great tool in the cosmetic industry.
In 2017, BC membranes produced from Gluconacetobacter xylinum bacteria in liquid medium (HS) were incorporated with ZnO and tested in 20 BALB/c mice to assess tissue regeneration capacity. For this assay, the produced BC membranes were processed by washing with distilled water followed by autoclaving in 3 M NaOH solution at 120 °C for 15 min to break up and dissolve remnants of organic matter. Next, the BC membranes were incorporated by dipping bacterial cellulose films into ZnO nanoparticle suspension and mixed in an incubator with stirring at 50 °C and 150 rpm for 24 h.
In this in vivo study, burn wounds were induced in animals. In one group, the wounds were treated with BC-ZnO nanocomposites and in the other one, only with BC. As a result, optimal performance of BC for potential tissue healing along with antimicrobial activity was obtained according to Khalid et al. [16]. These BC composites could be suggested as potential skin wound healing material after clinical evaluation.
In another study, the BC produced by Gluconacetobacter xylinum in liquid medium HS was incorporated with two formulations: one with an association of oat extract, rosemary, calendula, and hydroviton; and another one with propolis extract. The BCs were immersed in a tray and the cosmetic actives were homogeneously distributed on its surface. The trays were agitated for 30 min. The membranes were turned every 5 min to provide a good distribution of the actives. As a result, two types of mask were produced and tested on 20 women volunteers, between 20 and 40 years of age, to evaluate the moisturizing action of the extracts. The masks were applied for 20 min for 5 consecutive days. The volunteers did not use any type of cosmetic during the experimental period. The results obtained by Pacheco et al., 2017 [7], indicated that the BC mask was effective in delivering the substances; however, the increase in hydration was only observed in the BC mask with plant extracts.
In 2018, BC was studied by Jantarat et al. [17], as a composite biomaterial that allowed optimal ex vivo release time of piperine. Unlike the other studies, in this one, the membranes were purchased ready-made from a local market in Songkhla, Thailand. For the study, the membranes were ground and mixed with the piperine and curcumin solutions separately, then dried at 60 °C for 2 h in Petri dishes. Then, they were layered one on top of the other. The BC containing curcumin and piperine showed potential for the controlled release of curcumin transdermally, and was shown to facilitate the bioavailability of curcumin.
In 2018, Perugrini [18] tested BC masks purchased from two different suppliers to evaluate the effects of short- and long-term BC use. The study was conducted on 89 healthy women for one month with application three times a week. The short- and long-term effects of the BC masks were analyzed. It was concluded that the shelf life of BC is six months. No side effects were reported from use, again proving the safety of using BC in cosmetics.
And in 2019, Pergurini et al. [19] demonstrated the feasibility and efficacy of BC obtained by incubating Gluconacetobacter xylinus. Three different solutions were incorporated into BC. The first one was an anti-aging mascara with Adansonia digitata, Hibiscus sabdariffa flower, Arabic coffee seed, African Kigelia fruit and Crocus chrysanthus extracts as well as hydrolyzed hyaluronic acid and Senegal acacia gum.
The second one was a lifting mascara with hydrolyzed Quinoa seed extract and hydrolyzed hyaluronic acid. Lastly, the third solution was a purifying and regenerating mascara with Portulaca oleracea and Chlorella vulgaris extracts in addition to sodium hyaluronate, kaolin, and magnesium sulfate.
In the tests with volunteers, all three mascaras were applied for one month and the formulations showed excellent results indicating efficacy and safety. The extracts were chosen based on their natural biomedical properties such as antioxidant action, potential to increase skin hydration and elasticity, and activation of regeneration processes, among other benefits found in the literature regarding the chosen actives. Once again, the benefits of BC as an aid in the development of safe and natural cosmetics were proven.
Chantereau, et al., 2020 [12] studied BC membranes with the size of 2 × 2 cm produced by the Gluconacetobacter sacchari strain and incorporated in 1 mL of the ionic liquid loaded solution and vitamin B. The membranes were used as cosmetic adhesive material and showed high thermal stability and ability to replace the use of plasticizers. The incorporated BC also increased the bioavailability of vitamin B, aided skin hydration, and showed no cytotoxic activity.
Bacterial nanocellulose produced by Gluconacetobacter sacchari has also been studied as a delivery model for hyaluronic acid in patch form with regenerative moisturizing properties [8]. The model was tested in vitro on HaCaT cells to assess cytotoxicity and ex vivo on pig ear skin to assess its permeation ability. The results of the trials were successful and proved to be safe for the application of these adhesives.
In 2020, Malmir et al. [20], used BC produced by the bacterium Gluconacetobacter xylinus in HS medium to develop an antibacterial dressing with carbon quantum dioxide titanium nanoparticles (CQD-TiO2). To incorporate the nanoparticles into BC, the CQD-TiO2 solution was sprayed into wet BC at room temperature. Then, using the heat press machine, the membranes were dehydrated and dried for 3 days at 40 °C. This dressing model was tested in vitro on human L929 cells and CQD-TiO2 loaded into BC was non-cytotoxic and effective in skin wound healing. To evaluate the antibacterial ability, a minimum inhibitory concentration test was performed on Staphylococcus aureus, proving the antibacterial activity.
4. Experimental Articles
Figure 3 shows how the systematic electronic research was conducted on two search platforms (Science Direct and PubMed) throughout the month of January 2022. The literature search strategy combined the descriptors “bacterial cellulose”, “cosmetics”, “skin mask”, and “clean beauty”. The descriptors were chosen to be specific enough not to cover too much irrelevant literature. Furthermore, the descriptors were selected based on the “Medical Subject Headings (MeSH)”. The research was conducted over a ten-year period, between 2011 and 2021.
Figure 3.
Diagram used to define and limit the search for studies based on key words.
Searches were restricted by language (only English language). All original articles, related to development of products or new approaches, were considered for this review.
First, before the articles were evaluated, the year of each publication was collected to emphasize the prospect of growth of bacterial cellulose research in cosmetics. The software GraphPad Prism 8 was used to demonstrate this perspective.
After that, one reviewer independently accessed each article that was included in this review. Two other reviewers assisted in meticulous reading and confirmed the accuracy of the data that was included and excluded. Figure 4 shows there was a careful selection of articles based on the exclusion criteria, which defined the corresponding articles to be investigated.

Figure 4.
Exclusion criteria to define the studies that were used.
In the selection, duplicate studies and review articles were excluded, as well as studies that did not specifically address the subject, and studies that did not use bacterial cellulose as primary material. After this first selection, the titles were screened by abstracts. Studies that did not correspond to the criteria of the searched data were excluded.
For the complete review of the data sought, the full texts of the pre-selected articles were obtained, but not all of them were available to collect the necessary and pertaining data. Data were collected based on the application of BC in cosmetics, the type of microorganisms that produced the BC, the type of the study (in vitro, in vivo, and clinical studies), and also the purpose of the product or technology developed in the article. Data were grouped in a descriptive table.
5. Conclusions
Although limited in number, the studies on the application of bacterial cellulose in cosmetics used in this review indicate that this is a very promising area for researchers interested in natural cosmetics. In the presented studies, BC shows ideal pharmaco-cosmetic characteristics, such as moisturizing potential, oiliness control, controlled release of substances, biocompatibility with human tissues, and increased bioavailability of certain substances. In addition, it also proves to be safe for skin application because it is a bioproduct that does not harm the consumer.
We believe that cosmetics developed from the green technology of bacterial cellulose are committed to innovation, sustainable practices, circular economy and conscious beauty replacing unhealthy and dangerous substances. Bacterial cellulose may be a potential ally for the consumer and environment due to its natural origins.
Author Contributions
Conceptualization, T.J.O. and T.C.M.S.; Methodology, T.J.O. and T.C.M.S.; software, T.J.O.; Validation, A.F.J. and D.G.; Formal analysis, A.F.J. and D.G.; Investigation, T.J.O., T.C.M.S. and G.P.M.; Resources, A.F.J. and D.G.; Data curation, T.J.O.; Writing—original draft preparation, T.J.O., T.C.M.S. and G.P.M.; Writing—review and editing, A.F.J. and D.G.; supervision, A.F.J. and D.G. All authors have read and agreed to the published version of the manuscript.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), process number 16/05930-4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge financial support from Coordination for Higher Level Graduate Improvements (CAPES/Brazil, finance code 001), National Council for Scientific and Technological Development (CNPq/Brazil), and the State of São Paulo Research Foundation (FAPESP/Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Shaifuddin, S.N.; Mohamad, A.S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef]
- Anagnosti, L.; Varvaresou, A.; Pavlou, P.; Protopapa, E.; Carayanni, V. Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively? Mar. Pollut. Bull. 2021, 162, 1596–1605. [Google Scholar] [CrossRef]
- Alharbi, O.M.L.; Basheer, A.A.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Dent, M.P.; Vaillancourt, E.; Thomas, R.S.; Carmichael, P.L.; Ouedraogo, G.; Kojima, H.; Barroso, J.; Ansell, J.; Barton-Maclaren, T.S.; Bennekou, S.H.; et al. Paving the way for application of next generation risk assessment to safety decision-making for cosmetic ingredients. Regul. Toxicol. Pharmacol. 2021, 125, 105026. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Bacterial nanocellulose toward green cosmetics: Recent progresses and challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, G.; De Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosmet. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.F.S.; Vilela, C.; Pinto, R.J.B.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.; Freire, C. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111350. [Google Scholar] [CrossRef] [PubMed]
- Mbituyimana, B.; Liu, L.; Ye, W.; Ode Boni, B.O.; Zhang, K.; Chen, J.; Thomas, S.; Vasilievich, R.V.; Shi, Z.; Yang, G. Bacterial cellulose-based composites for biomedical and cosmetic applications: Research progress and existing products. Carbohydr. Polym. 2021, 273, 118565. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Ossowicz-Rupniewska, P.; Rakoczy, R.; Konopacki, M.; Perużyńska, M.; Droździk, M.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Wenelska, K.; et al. Bacterial cellulose membrane containing epilobium angustifolium l. Extract as a promising material for the topical delivery of antioxidants to the skin. Int. J. Mol. Sci. 2021, 22, 6269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Chusuit, T.; Raknam, P.; Boonme, P. Effects of a cellulose mask synthesized by a bacterium on facial skin characteristics and user satisfaction. Med. Devices Evid. Res. 2011, 4, 77–81. [Google Scholar]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.; Freire, C.S.; Sousa Lobo, J.M.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2013, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Mazzarino, L.; Borsali, R. A slow-release system of bacterial cellulose gel and nanoparticles for hydrophobic active ingredients. Int. J. Pharm. 2015, 486, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Boonmee, S.; Meechoodin, W.; Wangpittaya, H. Effect of piperine on skin permeation of curcumin from a bacterially derived cellulose-composite double-layer membrane for transdermal curcumin delivery. Sci. Pharm. 2018, 86, 39. [Google Scholar] [CrossRef] [PubMed]
- Perugrini, P.; Bleve, M.; Cortinovis, F.; Coplani, A. Biocellulose masks as delivery systems: A novel methodological approach to assure quality and safety. Cosmetics 2018, 5, 66. [Google Scholar] [CrossRef]
- Perugrini, P.; Bleve, M.; Redondi, R.; Cortinovis, F.; Colpani, A. In vivo evaluation of the effectiveness of biocellulose facial masks as active delivery systems to skin. J. Cosmet. Dermatol. 2019, 19, 725–735. [Google Scholar] [CrossRef]
- Malmir, S.; Atiyeh, K.; Mehrab, P.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 115835. [Google Scholar] [CrossRef]
- Li, Y.; Jang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yang, Y. Bacterial cellulose-hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2015, 3, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Tan, N.C.S.; Mahadevaswany, U.R.; Chanchareonsook, N.; Steele, T.; Lim, S. Bacterial cellulose adhesive composites for oral cavity applications. Carbohydr. Polym. 2021, 274, 118403. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).