Abstract
New hydroxy- and anilinoindanone derivatives 3 and 4 were synthesized starting from 3-hydroxybenzo[e]isoindolinone 1 via the addition of alkyllithium (s-BuLi, n-BuLi, MeLi or i-PrLi) to the carbonyl group, followed by lactam ring opening and, finally, an intramolecular cyclization leading to target compounds. The same starting material was used for the preparation of the new benzo[f]phthalazinone derivatives 12–16 through multi-step reactions. The target derivative 16 was obtained from the corresponding bromolactam 15 by the Buchwald–Hartwig amination. Structures of the obtained compounds were confirmed by the NMR spectra.
1. Introduction
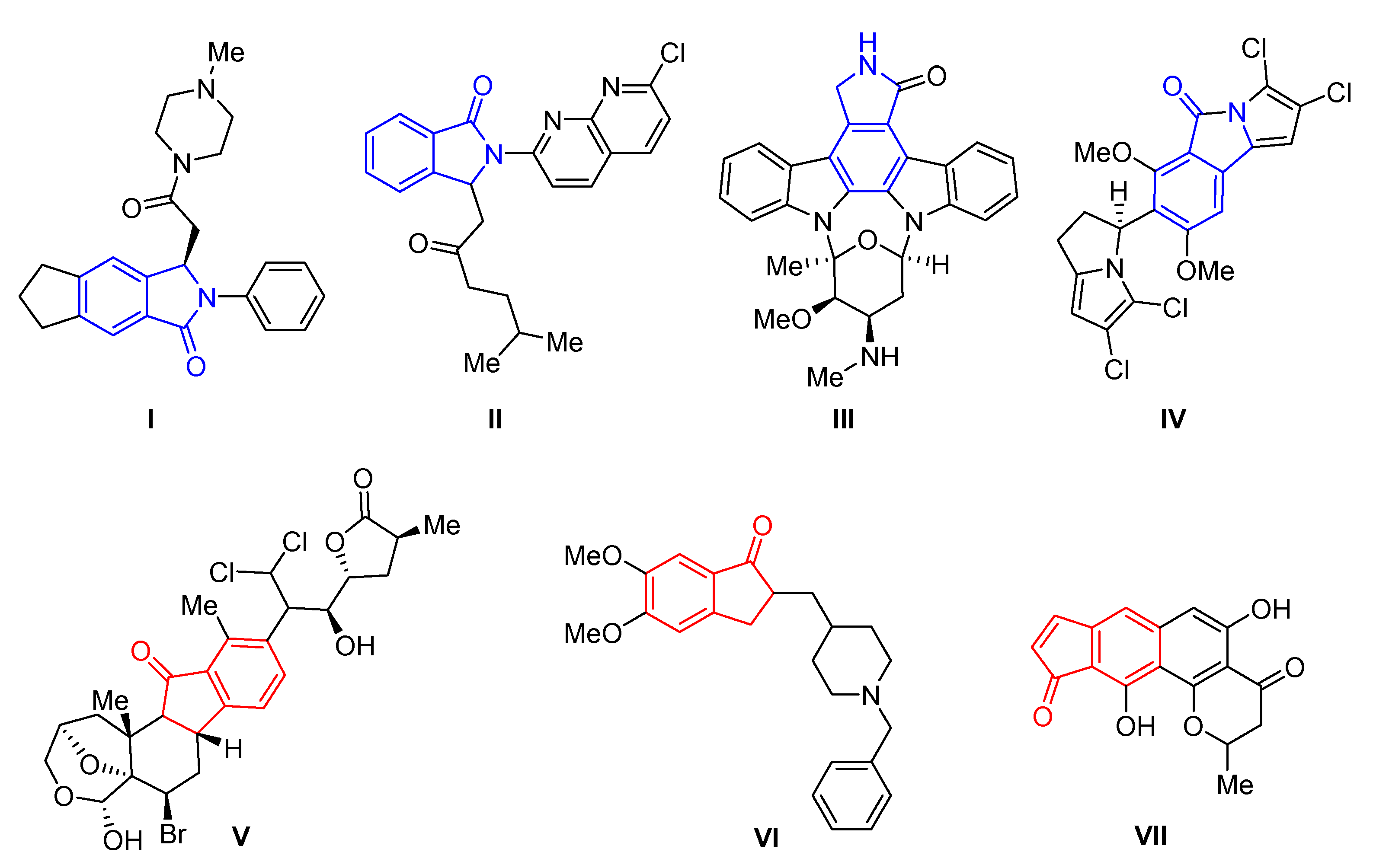
The isoindolinone framework is present as the core unit in many synthetic and naturally occurring derivatives with interesting biological properties [1,2,3]. The ones commonly known are the hypotensive and inhibitory platelet aggregation activity of the nonbenzodiazepine sedative and hypnotic drug JM-1232 (I, Figure 1) [4,5,6] as well as the anxiolytic agents Pagoclone (II) [7] and Staurosporine (III) [8]. Additionally, cytotoxicity activity against the HCT-116 human colon cancer cell line of the naturally occurring Chlorizidine A (IV) [9] was found. Cytotoxicity properties also exhibit Nakiterpiosin (V) [10,11], a metabolite found in marine sponge (activity toward the P388 murine leukemia cell line, GI50 = 10 ng/mL). Moreover, indanone moiety builds the structure of Donepezil (VI) [12], a drug useful in the treatment of Alzheimer’s disease (the AChE inhibitor) [13]. Among this active group of compounds, the Euplectin (VII) benzo[f]indanone derivative with activity against murine mastocytoma cells P-815 (IC50 = 1.67 µg/mL) [14] has also found its place.
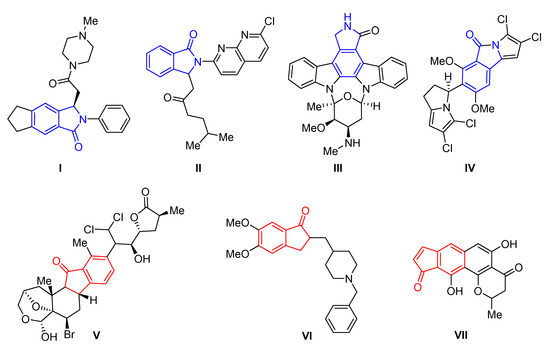
Figure 1.
Biologically active isoindolinone and indanone derivatives.
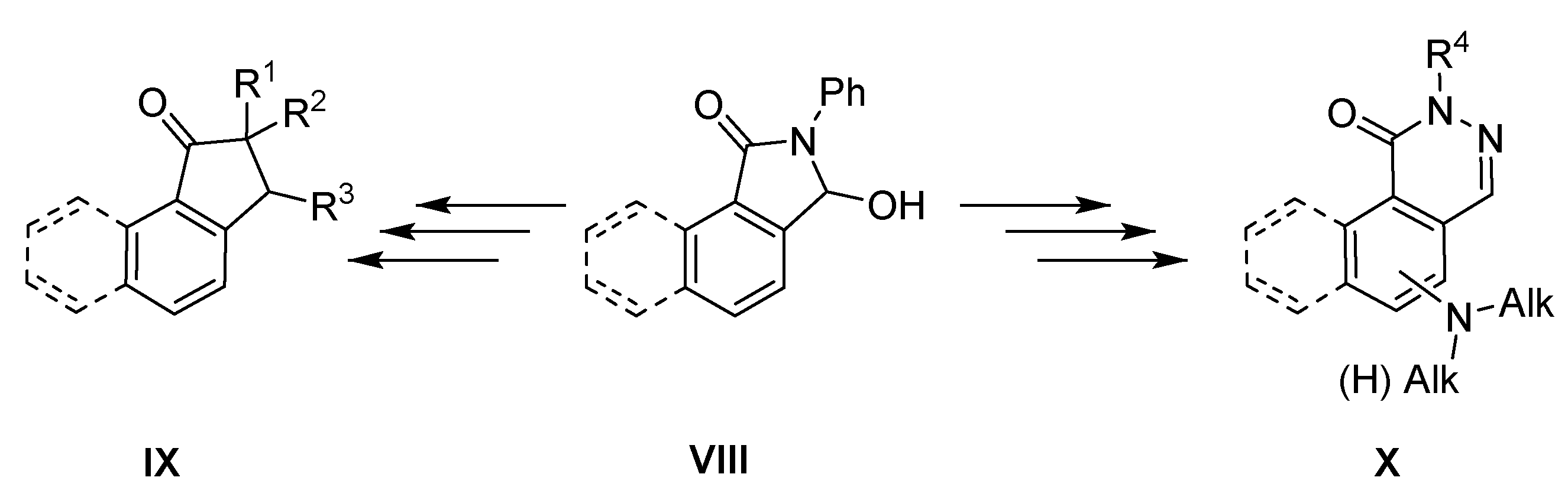
For a long time, we have been interested in the synthesis and functionalization of substituted isoindolinones [15,16]. In the present work, we focused on 3-hydroxybenzo[e]isoindol-1-one VIII and the possibilities of its conversion into new carbo- and heterocyclic systems, particularly into derivatives of indan-1-one IX and phthalazin-1-one X (Scheme 1).
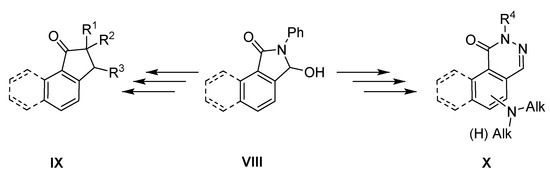
Scheme 1.
Planned synthetic route.
2. Results and Discussion
One of the methods of modifying the isoindolinone skeleton is the strategy based on the lithiation of the aromatic ring with alkyllithium, followed by reaction with the electrophile [17,18,19]. Based on this methodology, we wanted to widen the library of substituted benzoisoindolinones, which can be used to prepare new benzo[f]phthalazinone derivatives.
The starting 3-hydroxy-2-phenyl-1H-benzo[e]isoindol-1-one 1 was prepared by treating lithiated N-phenylnaphthalene-1-carboxamide (s-BuLi, THF, 0 °C) with DMF at 0 °C and, finally, quenching with MeOH. A feature of naphthalene carboxamides is their tendency to undergo an addition reaction of organolithium to the naphthalene ring [20,21]. The increase in the reaction temperature from −78 °C up to 0 °C resulted in a reduction in the amount of formed side products and the formation of the target compound 1 in a 60% yield. Unfortunately, under these conditions, about 10% of the starting amide was recovered.
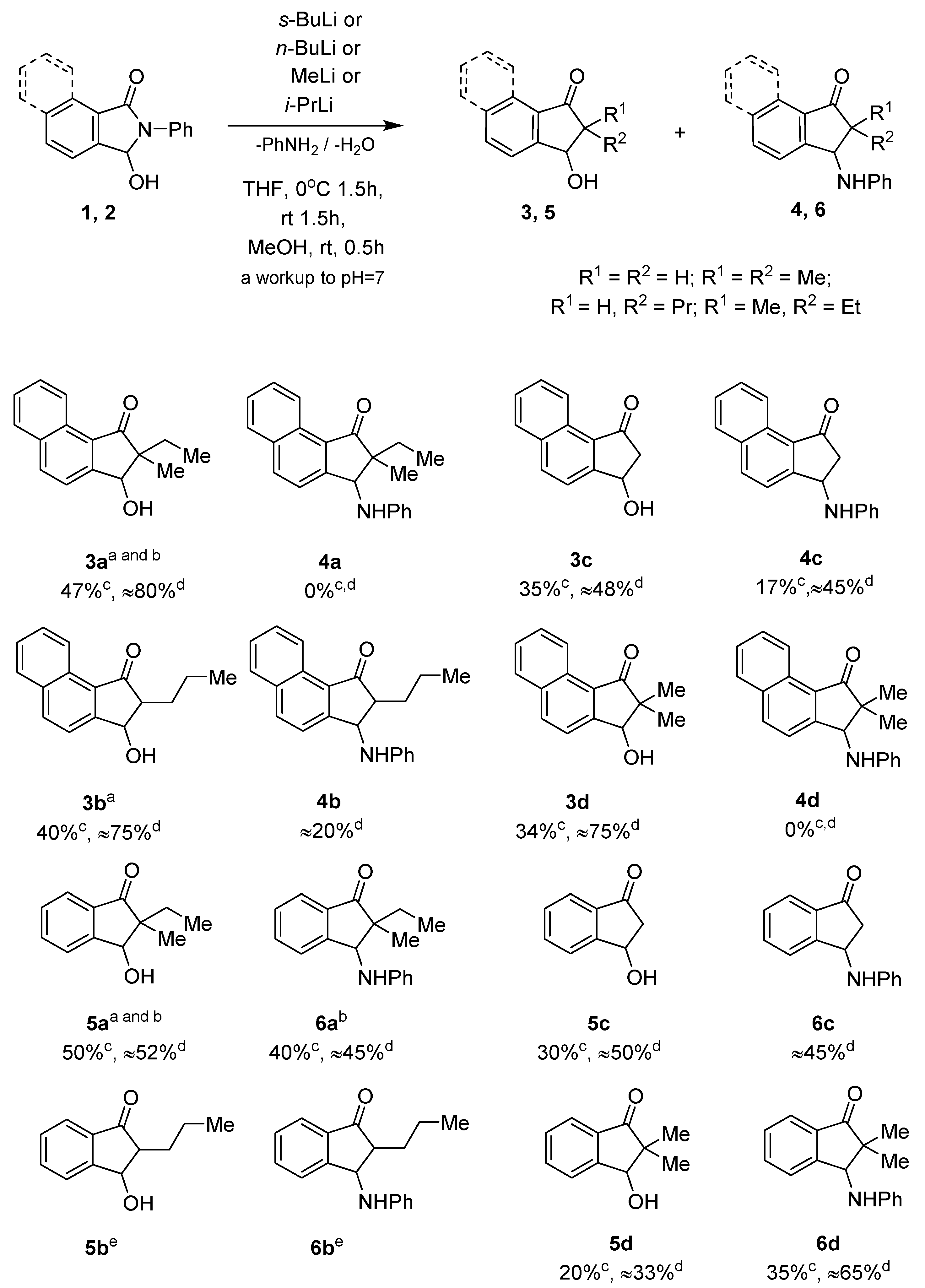
Unexpectedly, the treatment of benzoisoindolinone 1 with the 2.2 equiv. s-BuLi and subsequently with an electrophile, e.g., 1,2-dibromoethane or bromine [22,23,24], led to the formation of 3-hydroxyindanone 3a as a main product along with a small amount of the starting compound 1 (Scheme 2). Despite repeated attempts, 1H NMR analysis of the resulting post-reaction mixtures, after quenching by adding MeOH and a workup to pH ≈ 7, consistently indicated the conversion of 1 into an indanone-type derivative (with and without the addition of an electrophile). Furthermore, 1H NMR spectra showed the presence of aniline in the post-reaction mixture, which was due to the formation of keto-alcohol 3a from 1.

Scheme 2.
Reaction of isoindolinones 1, 2 with alkyllithium compounds. a Isolated as single diastereoisomers; b isolated as a mixture of diastereoisomers; c total yield of the isolated product after silica gel chromatography; d total yield determined by 1H NMR analysis of the crude reaction mixture; e observed in the 1H NMR spectrum of the crude reaction mixture.
Additionally, the reactions of 1 with other alkyllithium compounds, under the same reaction conditions as shown in Scheme 2, provided unexpected outcomes. In the case of i-PrLi, the course of the reaction with 1 was similar to that of s-BuLi, giving 3-hydroxyindanone 3d as a sole product with a yield of 34%. In turn, the treatment of 1 with n-BuLi or MeLi led to the formation of two main products: hydroxy and anilino derivatives: 3b, 4b (n-BuLi), and 3c, 4c (MeLi), respectively, which were observed in the 1H NMR spectra of the crude post-reaction mixtures and isolated by flash chromatography. Only in the case of compound 4b did its separation in its pure form end in failure.
On the other hand, the treatment of isoindolinone 2 with MeLi, i-PrLi, n-BuLi, or s-BuLi each time led to two main types of indanone derivatives, 5 and 6, in moderate yields (Scheme 2). The structures of compounds 3–6 were confirmed with the aid of spectroscopic analyses (for details, see Sections S1.1, S1.2, and S2.3.1 of the Supplementary Materials).
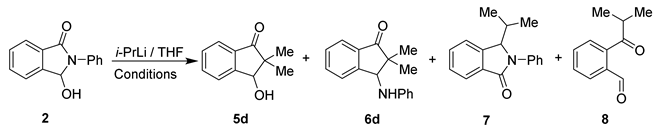
Taking into account our results, the above described, and the recent literature reports on the construction of the indan-1-one skeleton [25], we decided to check how reaction conditions, such as time, temperature, final quenching, and the pH of the post-reaction mixture, might affect the course of the reaction and products’ distribution. For this purpose, we investigated the reaction between isoindolinone 2 and i-PrLi (2.2 equiv.) in THF under various conditions (Table 1).

Table 1.
Effect of reaction conditions on products’ distribution.
The achieved results, summarized in Table 1, showed that the addition of i-PrLi to lactam 2 took place at each temperature. After about 10 min at 0 °C, both indanone derivatives 5d and 6d were present in the reaction mixture (1H NMR, Table 1, entry 1). Under these conditions, the molar amounts of indanones 5d and 6d were similar, although a slight advantage of anilinoindanone 6d could be found.
Stable molar ratio products, 5d and 6d, were achieved by extending the reaction time at 0 °C to 1.5 h (1H NMR: 5d/6d 0.47–0.48/1.0) and were not affected by slight changes in temperature or reaction time (Table 1, entries 2, 3, 4).
Lowering the reaction temperature to −78 °C or raising it to rt (Table 1, entries: 7, 8) did not significantly change the distribution of the products. However, when the reaction was carried out at −78 °C, a lot of unreacted 2 was observed.
The best degree of substrate conversion was obtained in the following cases: (1) i-PrLi, rt/1.5 h; (2) MeOH, rt/0.5 h (Table 1, entry 7) and (1) i-PrLi, 0 °C/1.5 h; (2) rt/1.5 h; (3) MeOH, rt/0.5 h (Table 1, entry 3).
Interestingly, the amount of the formed 3-hydroxyindanone 5d was reduced by extending the reaction time at rt (Table 1, entries 5, 6). Thus, it seems possible that the initially formed 5d existed in equilibrium with its open form and could be converted into the anilino derivative 6d.
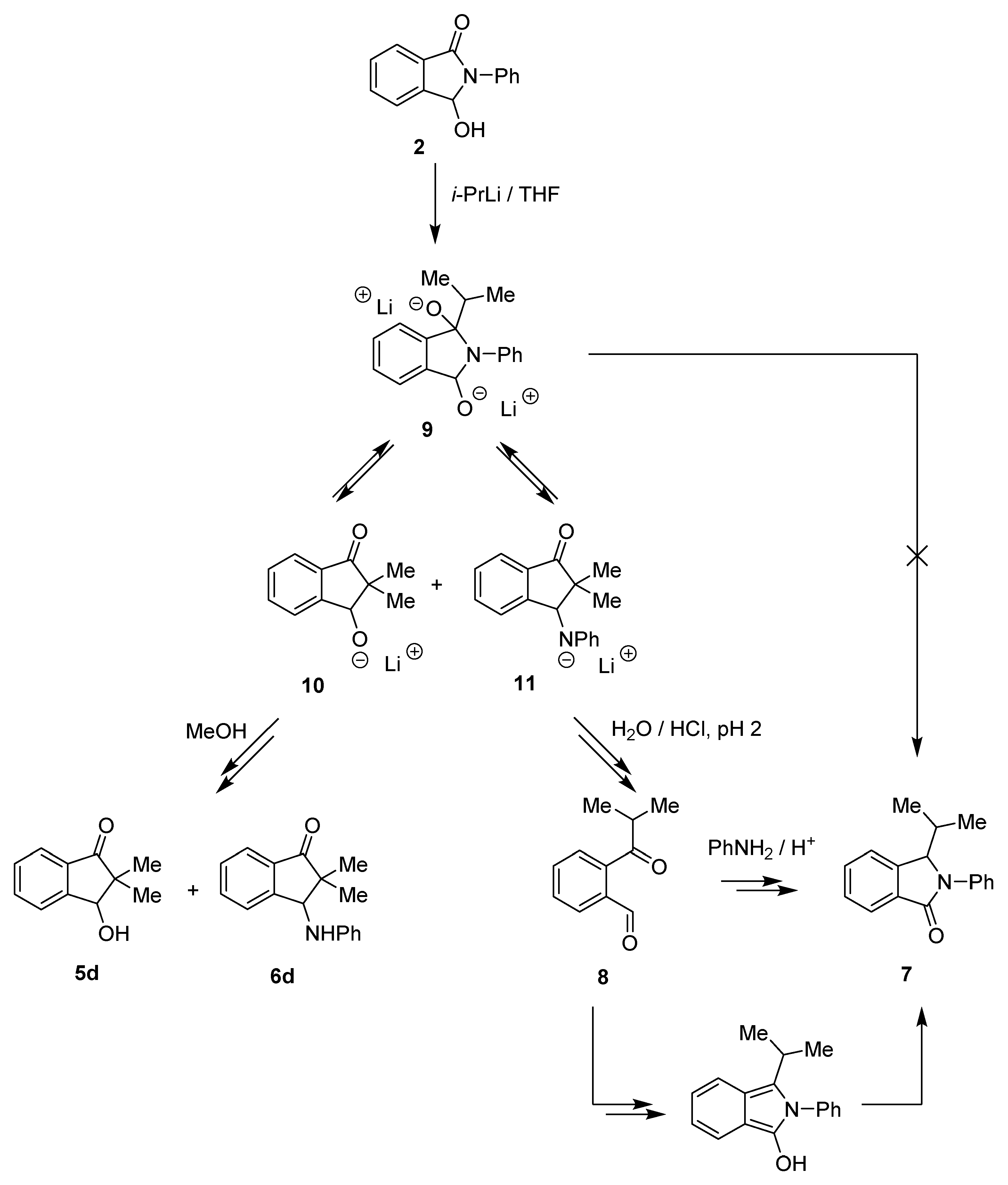
Based on an earlier publication [25], we also expected to obtain 2-phenyl-3-(propan-2-yl)-isoindol-1-one 7 (2 → 9 → 7, Scheme 3). Unfortunately, in all our tests (Table 1, entries 1–10 and Scheme 2) we did not observe even a trace of the formation of 3-alkyllactams type 7.
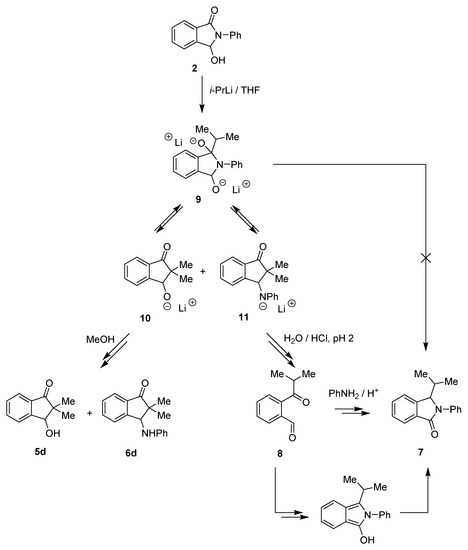
Scheme 3.
Reaction of isoindolinone 2 with isopropyllithium.
Therefore, we decided to conduct the experiment under the specific conditions described in the literature [25] (Table 1, entry 11). The reaction was performed without the presence of TMEDA and was quenched with water and acidified to pH ≈ 2. The crude mixture was examined using 1H NMR analysis, which showed the characteristic proton signal of the CHO group at 10.05 ppm, corresponding to ketoaldehyde 8 (Table 1). The fractions isolated after chromatographic purification were analyzed one more time using 1H NMR. The 1H NMR spectrum obtained for the sample of aldehyde 8 was identical to the ones available in the literature [26]. Additionally, an HRMS analysis was performed; thereby, the structure of compound 8 was definitively confirmed.
In our opinion, the final quenching of the reaction had a strong influence on the type of formed products and their distribution. An addition of water followed by acidification (HClaq 1:1) of the mixture to pH ≈ 2 led to 7 and 8 as main products (the 1H NMR spectrum also showed traces of 5d, 6d, and 2). On the other hand, the addition of methanol (without acidification) resulted in the formation of hydroxy- and anilino-indanones 5d and 6d without 7 and 8 (Table 1, Scheme 3).
The presented results suggested that isoindolinone 7 probably did not arise directly from 3-hydroxyisoinolinone 2 (via 9), as was proposed in [25]; but its formation may be the effect of the specific conditions under which the reaction was finalized and the condensation of aldehyde 8 (formed, e.g., from hydroxyindanone 5d by the retro aldol reaction) with aniline in the presence of acidic catalysts [27] (Scheme 3).
Presumably, the reaction between 3-hydroxyisoindolinones 1 and 2 and alkyllithiums can have several paths, and the choice of each of them is determined by the specific properties of the substrates and reaction conditions. At present, we have not discussed the detailed mechanism of the described reactions because that would be premature.
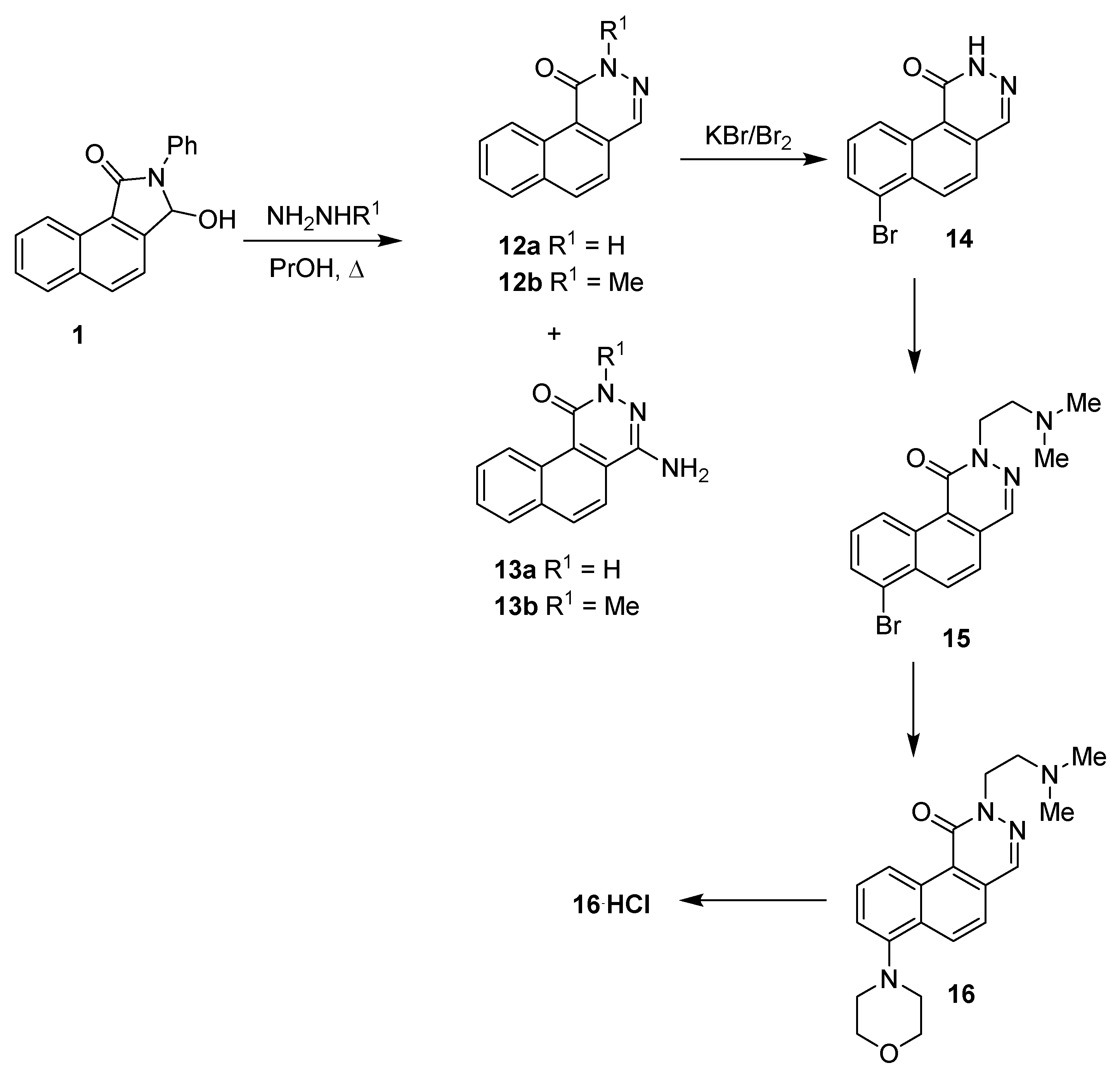
The second part of our research focused on the transformation of the benzo[e]isoindolinone skeleton into benzo[f]phthalazinone derivatives and their subsequent modifications. In the first stage, we conducted the heterocyclic ring expansion of 1 to benzo[f]phthalazinone 12 using hydrazine hydrates or methylhydrazine (Scheme 4) [28].
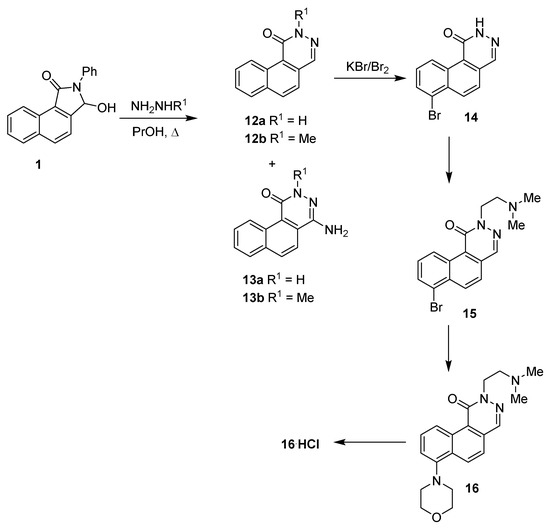
Scheme 4.
Synthesis of benzo[f]phthalazinone derivatives.
In general, the reaction between various substituted 3-hydroxyisoindolinones and hydrazine leads to the formation of phthalazinones, as sole products, with good yields [15,16]. Unexpectedly, the treatment of benzo[e]isoindolinone 1 with hydrazine hydrate resulted in the formation of benzo[f]phthalazinone 12a (29%) and 4-aminobenzo[f]phthalazinone 13a (25%) side by side.
A similar result was also achieved under the reaction of lactam 1 with methylhydrazine. Additionally, N-methylphthalazinone 12b (24%), the 4-amino- derivative 13b (9%), was isolated from the reaction mixture, too. The separation and purification process of both phthalazinone derivatives 12 and 13 turned out to be problematic due to their limited solubility.
The structures of derivatives 12 and 13 were characterized based on a comparison of the 1H NMR and 13C NMR spectra. The diagnostic value in the identification of phthalazinone derivatives had chemical shifts of the methine proton (N=CH) and NH2 protons of the amine substituent attached to carbon C-4. In the case of 12a, the C4-H proton signal was appreciably more shifted downfield (singlet at 8.51 ppm) than in the instance of the N-methyl derivative 12b (singlet at 8.24 ppm), while the protons of the NH2 groups of 13a,b were present as singlets in the range of 6.01–6.12 ppm.
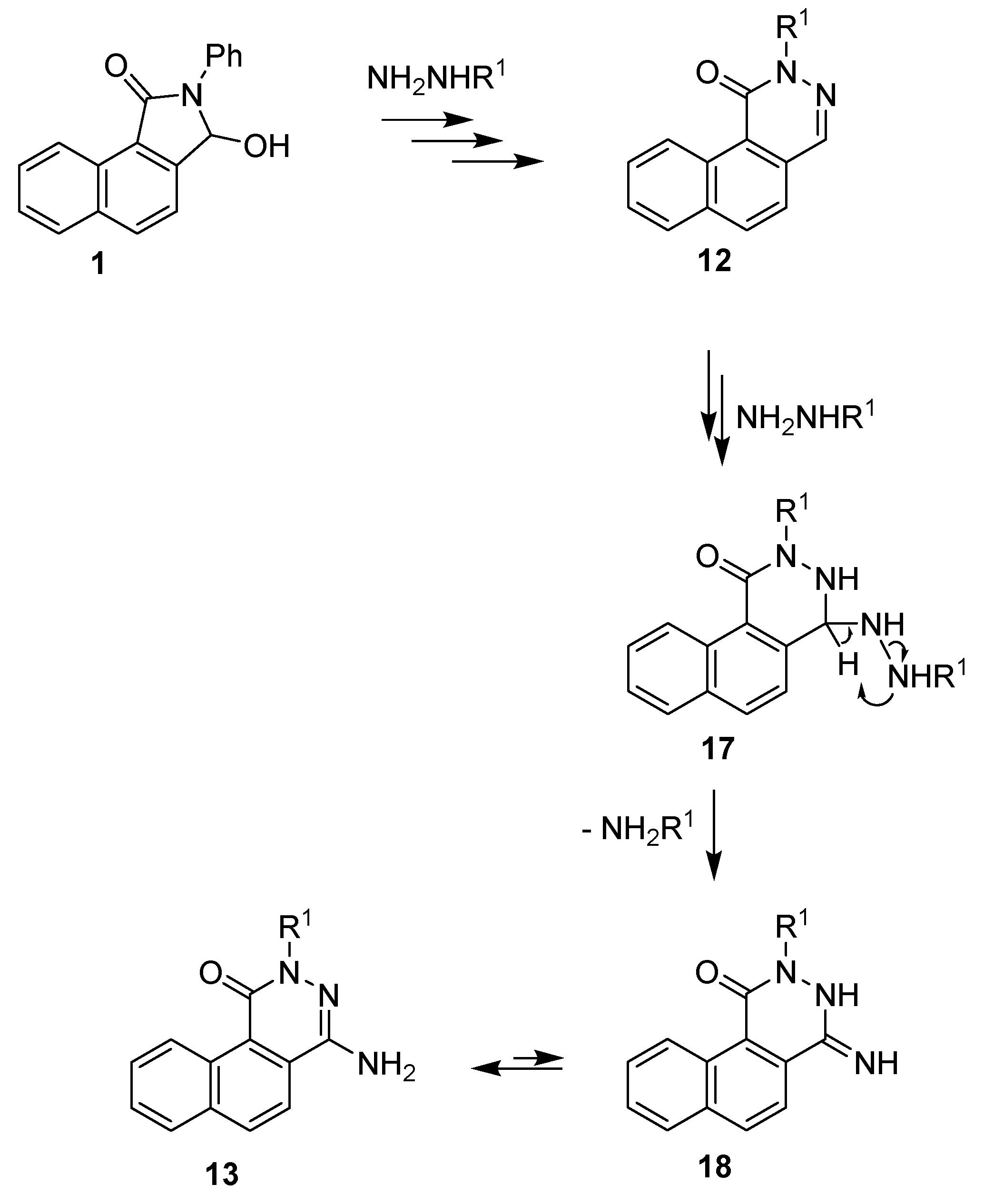
In our opinion, the observed formation of aminophthalazinones 13 was a result of the reaction between benzo[f]phthalazinone 12 and excess hydrazine (methylhydrazine). The proposed mechanism for the formation of 4-aminophthalazinones is presented in Scheme 5. The key step in the formation of 13 was the addition of hydrazine (methylhydrazine) to the azomethine bond of lactams 12 (12 → 17), followed by the N–N bond cleavage of hydrazine derivatives 17 and, finally, the elimination of ammonia (methylamine), leading to the amine-imine tautomeric system 13⇆18. Interestingly, the reaction did not occur when a stoichiometric amount of hydrazine was used.
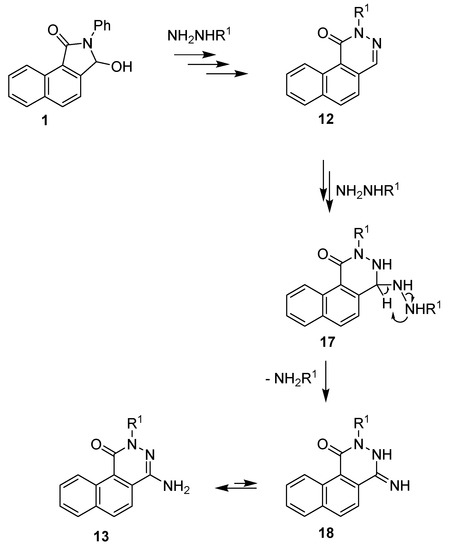
Scheme 5.
Proposed method of formation of 4-aminophthalazinones 13.
The usual methods used for the cleavage of the N–N bonds in hydrazine derivatives include, among other things, reduction with Zn/H+, SmI2, and Na/NH3; catalytic hydrogenolysis by Ni-RANEY; PtO2 and oxidative cleavage with meta-chloroperbenzoic acid; etc. [29]. Some years ago, the synthesis of amines starting from substituted hydrazines and naphthols only under thermal conditions was also described [30].
In our recent works, we have shown the possibilities of phthalazinone skeleton modifications via the Pd cross-coupling reaction of N-substituted 4-bromophthalazinones with selected amines and thiols (Buchwald–Hartwig-type coupling) [28,31]. Therefore, we first started with the conversion of lactam 12a into bromoderivative in a Br2-KBr acetate buffer solution. However, attempts to transform 12a into 4-bromoderivative ended in failure. It turned out that the introduction of the additional benzene ring, condensed with phthalazinone moiety, significantly changed the reactivity of the molecule, whereby 12a (Scheme 4) underwent bromination (KBr-Br2) [28] in the 7 position, leading to derivative 14, with a yield of 87%. In the next stage, compound 14, without an additional purification process, was treated with 2-chloroethyldimethylamine hydrochloride in the presence of MeONa in methanol, delivering the disubstituted benzo[f]phthalazinone 15 at an overall yield of 52% (12a → 14 → 15).
The position of the bromine atom in 14 and 15 was confirmed by 1D and 2D NMR (COSY, HMQC, HMBC) spectroscopy and additionally, by comparison with literature data [32] (for details, see Sections S1.3 and S2.4.2 of the Supplementary Materials).
The palladium cross-coupling reaction between bromolactam 15 and morpholine in the presence of the Pd/R-BINAP catalytic system was performed according to the methodology described by us recently [28]. This method led to product 16 in a 44% yield.
Unexpectedly, the 1D and 2D NMR spectroscopy studies of diamine 16 were complicated by the use of an unstable CDCl3, which partially decomposed during storage to form HCl [33,34,35,36,37,38]. This unplanned complication resulted in the formation of hydrochloride salt of diamine 16 (Scheme 4, compound 16•HCl) and prompted us to look more closely at the 1H and 13C NMR spectra of 16•HCl.
Interestingly, the 1H and 13C NMR spectroscopy of amine salts, especially active substances, in solvents such as CDCl3 or DMSO-d6, is a useful research tool for their analysis [35,36].
When comparing the 1H NMR spectra of diamine 16 and salt 16•HCl, attention is drawn to the widened singlet at 12.97 ppm, which is specific for the NH+ proton [35,38].
The difference of electronegativity between protonated and neutral nitrogen atoms causes chemical shifts of protons of methylene and methyl groups in the vicinity of NH+, which are shifted downfield (Table S1, Supplementary Materials) [35,36].
It is known from the literature [39] that typical 1H chemical shift values for the N,N-dimethylaminoethyl moiety of 2-substituted phthalazinone derivatives fall in the ranges of 4.18–4.63 ppm and 2.75–2.88 ppm for protons of two CH2 groups and 2.26–2.37 ppm for protons of Me groups. The same is the case with diamine 16.
The protons of the two methyl groups (NH+Me2) in the spectrum of 16•HCl were seen as a six-proton doublet at 2.93 ppm; for diamine 16, they were shown as a singlet at 2.37 ppm. In the case of the protons of ethylene moiety, the greatest changes in chemical shifts and multiplicity were visible for two protons of the methylene group closest to the protonated nitrogen atom. Their resonance signal was shifted downfield by 0.76 ppm in comparison to 16 (2.85 ppm).
On the other hand, the resonance signals of the protons of morpholin-4-yl moiety did not change their position and multiplicity (Table S1, Supplementary Materials), which may indicate that only the nitrogen atom of the NMe2 group was protonated. The absorptions of aromatic protons were slightly shifted downfield, without the C10-H.
Additionally, 1H and 13C NMR data analysis was performed for 2-[2-(dimethylamino)ethyl]phthalazin-1(2H)-one 19 [39] and its HCl salt (19•HCl, generated in situ in chloroform solution) (for details, see Sections S1.4 and S2.4.4 of the Supplementary Materials).
Based on the 13C chemical shift values of 19, its derivative 19•HCl, and also 2D NMR data (1H,13C HMQC), it was possible to assign resonance signals in the 13C NMR spectrum of 16•HCl. In the range of 118 to 161 ppm, all signals of the aromatic carbons, C=O (160.7 ppm), and N=C (138.6 ppm) groups were visible. Similarly, the signals of carbons of the morpholin-4-yl substituent were displayed at 67.5 and 54.0 ppm. The carbon resonance pics of the ethylene and methyl groups were of low intensity and often difficult to observe; finally, they were assigned from the 1H,13C HMQC spectrum. The signals appearing at 56.0 and 47.7 ppm were attributed to CH2NH+ and C(O)NCH2, respectively. In turn, the signal of carbon methyl groups appeared at 44.1 ppm.
3. Materials and Methods
3.1. General Information
Melting points were determined on a Boetius hot-stage apparatus and were uncorrected. 1H and 13C NMR spectra were recorded on a Bruker Advance III spectrometer at 600 MHz and 150 MHz, respectively. Chemical shifts (δH, δC) were quoted in parts per million (ppm), referenced to the signal of TMS or to the appropriate residual solvent peak (CDCl3 at 7.26 ppm or DMSO-d6 at 2.50 ppm for 1H NMR; CDCl3 at 77.16 ppm or DMSO-d6 at 39.52 ppm for 13C NMR) [40]. Coupling constants (J) were quoted in Hertz (Hz). The 2D homonuclear 1H and 1H COSY and heteronuclear 1H, 13C HMQC, and HMBC spectra were used to assign the proton and carbon signals. IR spectra were recorded on a Nexus FT-IR spectrometer.
LC/HRMS analyses were performed using an Agilent Technologies HPLC 1290 coupled to an Agilent Technologies 6550 Accurate Mass Q-TOF LC-MS mass spectrometer equipped with a JetStream Technology ion source housed in the Department of Pathophysiology, Medical University of Lublin, Poland. Internal mass calibration was enabled; reference ions of m/z = 121.0509 and 922.0098 were used.
The analytical thin layer chromatography tests (TLC) were carried out on Sigma-Aldrich (Supelco) silica gel plates (Kiselgel 60 F254, layer thickness 0.2 mm), and the spots were visualized using a UV lamp. The flash column chromatography purifications were performed on Fluka silica gel (Silica gel 60, 0.040–0.063 mm).
Alkyllithium solutions (s-butyllithium in cyclohexane (s-BuLi), n-butyllithium in hexanes (n-BuLi), methyllithium in diethyl ether (MeLi), isopropyllithium in pentane (i-PrLi); Sigma-Aldrich) were each time titrated before use [41].
All reactions with organolithium and organopalladium compounds were performed under an argon atmosphere using the standard Schlenk technique. THF and toluene were distilled from sodium benzophenone ketyl prior to use.
Commercially available reagents (hydrazine monohydrate, methylhydrazine, 2-chloro-N,N-dimethylethylamine hydrochloride, t-BuOK, tris(dibenzylideneacetone)-dipalladium(0) (Pd2(dba)3), (R)-2,2′-bis(diphenylphosphino)-1,1′-binaphthalene (R-BINAP)) were purchased from Sigma-Aldrich and were used without further purification.
N-Phenylnaphthalene-1-carboxamide [42,43,44], 3-hydroxy-2-phenyl-2,3-dihydro-1H-isoindol-1-one 2 [45], and 2-[2-(dimethylamino)ethyl]-2H-phthalazin-1-one 19 [39] were obtained as described previously; their characterization data were in agreement with the reported analysis. The characterization data of 2-(2-methylpropanoyl) benzaldehyde 8 [26] and 3-hydroxy-2,3-dihydro-1H-inden-1-one 5c [46] were in agreement with the already reported analysis.
3.2. Synthesis of 3-Hydroxy-2-phenyl-2,3-dihydro-1H-benzo[e]isoindol-1-one (1)
To the stirred at 0 °C under argon solution of N-phenylnaphthalene-1-carboxamide (4.04 × 10−3 mol) in THF (30 mL), n-BuLi in hexanes (8.89 × 10−3 mol) was added dropwise. The solution was held at 0 °C for 1 h. Then, DMF (2.68 × 10−2 mol) was added dropwise. The reaction mixture after 1 h at 0 °C was warmed to rt and stirred under these conditions for 2 h. After that, methanol (25 mL) was added, and stirring was continued for 0.5 h. Then, solvents were removed under reduced pressure. Water (25 mL) and DCM (25 mL) were added to the obtained residue, and the whole lot was neutralized with HClaq (1:1, v/v). The water layer was separated and extracted subsequently with DCM (3 × 15 mL). The organic phase was dried over MgSO4 and concentrated until dryness. The crude material was separated by flash chromatography (Hex-AcOEt 4:1, 3:1, 2:1).
3.3. Synthesis of 3-Hydroxy-2,3-dihydro-1H-inden-1-one 3 and 5 and 3-Anilino-2,3-dihydro-1H-inden-1-one 4 and 6 Derivatives
Under argon, to the solution of 3-hydroxyisoindolinone 1 or 2 (1.33 × 10−3 mol) in THF (20 mL) at 0 °C, the solution of alkyllithium compound (s-BuLi, n-BuLi, MeLi, or i-PrLi, 2.93 × 10−3 mol) was added dropwise. The solution was stirred at 0 °C for 1.5 h. Next, the reaction mixture was warmed to rt and stirred under these conditions for 1.5 h. After that, methanol (15 mL) was added and stirring was continued for 0.5 h. Then, solvents were removed under reduced pressure. Water (40 mL) and DCM (40 mL) were added to the obtained residue, and the whole lot was neutralized with HClaq (1:1, v/v). The water layer was separated and extracted subsequently with DCM (3 × 15 mL). The organic phase was dried over MgSO4 and concentrated until dryness. The crude material was separated by flash chromatography.
3.4. Synthesis of benzo[f]phthalazin-1(2H)-ones 12 and 4-amino-benzo[f]phthalazin-1(2H)-ones 13
Typical procedure [28].
A mixture of 3-hydroxyisoindolinone 1 (2.56 × 10−3 mol) and hydrazine monohydrate (3 mL) in propan-1-ol (15 mL) was heated with stirring under reflux for 38 h. After the completion of the reaction (TLC check), the mixture was cooled, and all volatile materials were removed under reduced pressure. To the residue, water was added and neutralized with acetic acid. The separated solid was collected by filtration, washed with water, dried by vacuum suction, and subjected to flash chromatography to obtain products 12 and 13.
3.5. Synthesis of 7-bromo-2-[2-(dimethylamino)ethyl]benzo[f]phthalazin-1(2H)-one (15)
Step 1. Bromination of benzo[f]phthalazinone 12a (analogous procedure as described in [28]).
To a suspension of benzophthalazinone 12a (2.45 × 10−4 mol) in the acetate buffer solution pH ≈ 5.8 (5 mL) was added potassium bromide (2.69 × 10−4 mol) and bromine (2.69 × 10−4 mol) at an ambient temperature. The mixture was then stirred for 6 h at an ambient temperature; next, the whole was heated to boiling and stirred for 30 h until the bromine color entirely disappeared. After cooling to an ambient temperature, the separated solid was collected by filtration and washed with water (2 mL). The crude product 14 (Scheme 4, yield: 58 mg, 87%) was used in the next step without additional purification.
Step 2. Alkylation of 7-bromobenzo[f]phthalazinone 14 (analogous procedure as described in [38]).
To the solution of sodium methoxide (6.32 × 10−4 mol) in dry methanol (15 mL) was added bromophthalazinone 14 (2.11 × 10−4 mol) and (2-chloroethyl)dimethyl ammonium chloride (4.22 × 10−4 mol). The mixture was heated at reflux for 30 h. Afterwards, the inorganic material was collected by filtration and washed with dry methanol; the filtrate was evaporated to dryness. The residue was subjected to flash chromatography (MeOH-CHCl3 1:1) to give the pure product 15.
3.6. Synthesis of 2-[2-(dimethylamino)ethyl]-7-(morpholin-4-yl)benzo[f]phthalazin-1(2H)-one (16)
Analogous procedure as described in [31].
The reaction was carried out under an argon atmosphere in an oven-dried resealable Schlenk flask. A resealable Schlenk flask was charged with Pd2(dba)3 (1 mol%), (R)-BINAP (15 mol%), and freshly distilled toluene (5 mL). The contents of the flask were stirred for 1 min. Then, morpholine (2.4 equiv.) was added, and the whole was again stirred and heated at ≈100 °C for 20 min. After this time, the reaction was cooled. Next, bromophthalazinone 15 (5.78 × 10−5 mol), t-BuOK (1.2 equiv.), and toluene (2 mL) were added. This mixture was then stirred and heated at ≈100 °C for 20 h. After this time, it was cooled and diluted with chloroform (2 mL). The solid was filtered off and washed with chloroform (2 mL), and the filtrate was concentrated. The product was purified by flash chromatography.
4. Conclusions
This article considers the possibilities for the use of the 3-hydroxybenzoisoindol-1-one skeleton to form new indan-1-one or phthalazin-1-one derivatives. We performed a preliminary analysis of synthetic pathways for the reaction of isoindolinones 1 and 2 with selected organolithium compounds. The predicted mechanism without details of the noted products’ formation was discussed. As confirmed by the 1H NMR spectra, in the case of naphthalene derivatives 1, the main products of the mentioned transformations were hydroxyindanones. In turn, after the reaction of isoindolinone 2 with organolithium compounds, in the 1H NMR spectra of crude reaction mixtures, two potential products were visible, amino- and hydroxyindanones, present in various ratios. The course of this process was not entirely clear, but our observations can suggest the existence of some specific equilibrium between both compounds.
Furthermore, the transformation of the benzoisoindolinone 1 skeleton to benzophthalazinone 12 or 13 derivatives was described. In the synthesis of these compounds, the key role was played by hydrazine, which was used in excess. Research also showed that Pd coupling of bromobenzophthalazinone 15 with morpholine can be performed by applying a Pd/R-BINAP catalytic system according to the method previously described by us.
It was concluded that this study has opened up the highly interesting option for the transformation of lactams 1 and 2 into cyclic hydroxy- and amino-ketones 3–6 and phthalazinones 12–16 with huge synthetic potential.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules27238319/s1. Table S1, 1H NMR chemical shifts of 16 and 16•HCl; Table S2, 1H NMR chemical shifts of 19 and 19•HCl; Table S3, 13C NMR chemical shifts of 19 and 19•HCl; Figure S1, The coupling constants between protons in naphthalene moiety of benzophthalazinones 14, 15 and benzoquinazolinone 20; Figures S2–S76, 1H, 13C, 2D NMR spectra of compounds 1, 3–8, 12–16•HCl and 19•HCl.
Author Contributions
Conceptualization, Z.M.; investigation, Z.M., M.N., E.F. and A.S.; writing—original draft preparation, Z.M., M.N. and E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the University of Lodz for partial financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Speck, K.; Magauer, T. The chemistry of isoindole natural products. Beilstein J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.R.; Raja, S. Indolinones as promising scaffold as kinase inhibitors: A review. Mini Rev. Med. Chem. 2012, 12, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Csende, F.; Porkolab, A. A review on antibacterial activity of some isoindole derivatives. Der Pharma. Chem. 2018, 10, 43–50. Available online: https://www.derpharmachemica.com/pharma-chemica/a-review-on-antibacterial-activity-of-some-isoindole-derivatives.pdf (accessed on 25 November 2022).
- Omura, S.; Sasaki, Y.; Iwai, Y.; Takeshima, H. Staurosporine, a potentially important gift from a microorganism. J. Antibiot. 1995, 48, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Nishiyama, T.; Yamada, Y. The antinociceptive effects and pharmacological properties of JM-1232(-): A novel isoindoline derivative. Anesth. Analg. 2009, 108, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, I.; Masayuki, S.; Yonamire, R.; Kazama, T. The effect of a new water-soluble sedative-hypnotic drug, JM-1232(−), on long-term potentiation in the CA1 region of the mouse hippocampus. Anesth. Analg. 2011, 113, 1043–1049. [Google Scholar] [CrossRef]
- de Wit, H.; Vieni, L.; Haig, G.M.; Hunt, T. Evaluation of the abuse potential of pagoclone, a partial GABAA agonist. J. Clin. Psychopharmacol. 2006, 26, 268–273. [Google Scholar] [CrossRef]
- Sorbera, L.A.; Leeson, P.A.; Silvestre, J.S.; Castaner, J. Pagoclone. Drugs Fut. 2001, 26, 651–657. [Google Scholar] [CrossRef]
- Alvarez-Mico, X.; Jensen, P.R.; Fenical, W.; Hughes, C.C. Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one containing alkaloid from a Marine Streptomyces sp. Org. Lett. 2013, 15, 988–991. [Google Scholar] [CrossRef]
- Teryuya, T.; Nakagawa, S.; Koyama, T.; Arimoto, H.; Kita, M.; Uemura, D. Nakiterpiosin and nakiterpiosinone, novel cytotoxic C-nor-D-homosteroids from the Okinawan sponge Terpios hoshinota. Tetrahderon 2004, 60, 6989–6993. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Q.; Chen, C. Synthesis and structure revision of nakiterpiosin. J. Am. Chem. Soc. 2009, 131, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007, 3, 303–333. Available online: https://www.dovepress.com/getfile.php?fileID=939 (accessed on 25 November 2022). [PubMed]
- Ernest-Russell, M.A.; Chai, C.L.L.; Wardlaw, J.H.; Elix, J.A. Euplectin and Coneuplectin, new naphthopyrones from the lichen Flavoparmelia euplecta. J. Nat. Prod. 2000, 63, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, J.Z.; Epsztajn, J.; Bakalarz, A.D.; Łajszczak, A.; Malinowski, Z. Application of organolithium and related reagents in synthesis. Part 221. Synthetic strategies bsed on ortho-aromatic metallation. A concise regiospecific conversion of benzoic acids into the 4-pyridyl-2H-phthalazin-1-ones. Synth. Commun. 1999, 29, 457–473. [Google Scholar] [CrossRef]
- Epsztajn, J.; Malinowski, Z.; Urbaniak, P.; Andrijewski, G. A practical approach for preparation of 2-[(dialkylamino)-methyl]-4-aryl-2H-phthalazin-1-ones via Mannich reaction of 4-aryl-2H-phthalazin-1-ones. Synth. Commun. 2005, 35, 179–192. [Google Scholar] [CrossRef]
- Jóźwiak, A.; Ciechańska, M. The behaviour of 2-substituted-3-hydroxyisoindolinones in the reaction with sec-butyllithium. J. Het. Chem. 2014, 51, 357–362. [Google Scholar] [CrossRef]
- Achmatowicz, M.; Thiel, O.R.; Wheeler, P.; Bernard, C.; Huang, J.; Larsen, R.D.; Faul, M.M. Practical synthesis of a p38 MAP kinase inhibitor. J. Org. Chem. 2009, 74, 795–809. [Google Scholar] [CrossRef]
- Metallinos, C.; Nerdinger, S.; Snieckus, V. N-Cumyl benzamide, sulfonamide, and aryl O-carbamate directed metalation groups. Mild hydrolytic lability for facile manipulation of directed ortho metalation derived aromatics. Org. Lett. 1999, 1, 1183–1186. [Google Scholar] [CrossRef]
- Clayden, J.; Frampton, C.S.; McCarthy, C.; Westlund, N. Perilithiation and the synthesis of 8-substituted-1-naphthamides. Tetrahedron 1999, 55, 14161–14184. [Google Scholar] [CrossRef]
- Eppley, R.L.; Dixon, J.A. The addition to and alkylation of naphthalene by tert-butyllithium. Kinetics and mechanism. J. Am. Chem. Soc. 1968, 90, 1606–1612. [Google Scholar] [CrossRef]
- Pinto, D.J.P.; Quan, M.L.; Smith II, L.M.; Orwat, M.J.; Gilligan, P.J. Bristol-Myers Squibb Co. Dipeptide Analogs as Coagulation Factor Inhibitors. WO Patent 2008/157162, 24 December 2008. Available online: https://patentimages.storage.googleapis.com/8c/99/dd/b1181fa9b12372/US8367709.pdf (accessed on 25 November 2022).
- Heiss, C.; Schlosser, M. Organometallic control over the regiospecificity of functionalization reactions: 1,2,3-trifluorobenzene and bromo derivatives thereof as substrates. Eur. J. Org. Chem. 2003, 2003, 447–451. [Google Scholar] [CrossRef]
- Novák, J.; Salemink, C.A. Cannabis xxiv. A new convenient synthesis of cannabinol. Tetrahedron Lett. 1982, 23, 253–254. [Google Scholar] [CrossRef]
- Ciechańska, M.; Jóźwiak, A.; Nazarski, R.B.; Skorupska, E.A. Unexpected rearrangement of dilithiated isoindoline-1,3-diols into 3-aminoindan-1-ones via N-lithioaminoarylcarbenes: A combined synthetic and computational study. J. Org. Chem. 2019, 84, 11425–11440. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.H.T.; Kim, B.; Dong, V.M. Phthalides by rhodium-catalyzed ketone hydroacylation. J. Am. Chem. Soc. 2009, 131, 15608–15609. [Google Scholar] [CrossRef] [PubMed]
- Augner, D.; Schmalz, H.-G. Biomimetic synthesis of isoindolinones related to the marilines. Synlett 2015, 26, 1395–1397. [Google Scholar] [CrossRef]
- Malinowski, Z.; Fornal, E.; Sierocińska, B.; Czeczko, R.; Nowak, M. Synthesis of 4-alkylsulfanylphthalazin-1(2H)-ones via palladium catalyzed sulfanylation of substituted 4-bromophthalazin-1(2H)-ones. Tetrahedron 2016, 72, 7942–7951. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Luo, M. Reduction of hydrazines to amines with aqueous solution of titanium(III) trichloride. Org. Biomol. Chem. 2011, 9, 4977–4982. [Google Scholar] [CrossRef]
- Jia, L.; Tang, Q.; Luo, M.; Zeng, X. Direct ortho-selective amination of 2-naphthol and its analogues with hydrazines. J. Org. Chem. 2018, 83, 5082–5091. [Google Scholar] [CrossRef]
- Malinowski, Z.; Fornal, E.; Sumara, A.; Kontek, R.; Bukowski, K.; Pasternak, B.; Sroczyński, D.; Kusz, J.; Małecka, M.; Nowak, M. Amino- and polyaminophthalazin-1(2H)-ones: Synthesis, coordination properties, and biological activity. Beilstein J. Org. Chem. 2021, 17, 558–568. [Google Scholar] [CrossRef]
- Nowak, M.; Malinowski, Z.; Fornal, E.; Jozwiak, A.; Parfieniuk, E.; Gajek, G.; Kontek, R. Substituted benzoquinazolinones. Part 2: Synthesis of amino-, and sulfanyl-derivatives of benzo[f]- and benzo[h]quinazolinones. Tetrahedron 2015, 71, 9463–9473. [Google Scholar] [CrossRef]
- Teipel, J.; Gottstein, V.; Hölzle, E.; Kaltenbach, K.; Lachenmeier, D.W.; Kuballa, T. An easy and reliable method for the mitigation of deuterated chloroform decomposition to stabilise susceptible NMR samples. Chemistry 2022, 4, 776–785. [Google Scholar] [CrossRef]
- Kawai, S. Discussion on decomposition of chloroform. Yakugaku Zasshi 1966, 86, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Badawi, H.M.; Förner, W.; Ali, S.A. The molecular structure and vibrational, 1H and 13C NMR spectra of lidocaine hydrochloride monohydrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Holzer, W. Unambiguous assignment of the 1H- and 13C-NMR spectra of propafenone and a thiophene analogue. Molecules 2001, 6, 796–802. [Google Scholar] [CrossRef]
- Morishima, I.; Yoshikawa, K.; Okada, K.; Yonezawa, T.; Goto, K. Conformational dependence of the inductive effect in the.sigma.-electron system as studied by carbon-13 nuclear magnetic resonance. J. Am. Chem. Soc. 1973, 95, 165–171. [Google Scholar] [CrossRef]
- Sarneski, J.E.; Surprenant, H.L.; Molen, F.K.; Rellley, C.N. Chemical shifts and protonation shifts in carbon-13 nuclear magnetic resonance studies of aqueous amines. Anal. Chem. 1975, 47, 2116–2124. [Google Scholar] [CrossRef]
- Pakulska, W.; Malinowski, Z.; Szcześniak, A.K.; Czarnecka, E.; Epsztajn, J. Synthesis and pharmacological evaluation of N-(dimethylamino)ethyl derivatives of benzo- and pyridopyridazinones. Arch. Pharm. 2009, 342, 41–47. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Kofron, W.G.; Baclawski, L.M. A convenient method for estimation of alkyllithium concentrations. J. Org. Chem. 1976, 41, 1879–1880. [Google Scholar] [CrossRef]
- Wangngae, S.; Duangkamol, C.; Pattarawarapan, M.; Phakhodee, W. Significance of reagent addition sequence in the amidation of carboxylic acids mediated by PPh3 and I2. RSC Adv. 2015, 5, 25789–25793. [Google Scholar] [CrossRef]
- Pan, C.; Wang, L.; Han, J. Palladium-Catalyzed Annulation of Arylbenzamides with Diaryliodonium Salts. Adv. Synth. Catal. 2022, 364, 268–273. [Google Scholar] [CrossRef]
- He, Z.; Yan, C.; Zhang, M.; Irfan, M.; Wang, Z.; Zeng, Z. Palladium-Catalyzed Desulfurative Hiyama Coupling of Thioureas to Achieve Amides via Selective C–N Bond Cleavage. Synthesis 2022, 54, 705–710. [Google Scholar] [CrossRef]
- Epsztajn, J.; Jóźwiak, A.; Szcześniak, A.K. Application of organolithium and related reagents in synthesis. Part 13. Synthetic strategies based on aromatic metallation. A concise regiospecific conversion of benzoic acids into 4-hydroxy-1-arylnaphthalenes. Tetrahedron 1993, 49, 929–938. [Google Scholar] [CrossRef]
- He, G.; Wu, C.; Zhou, J.; Yang, Q.; Zhang, C.; Zhou, Y.; Zhang, H.; Liu, H. A Method for Synthesis of 3-Hydroxy-1-indanones via Cu-Catalyzed Intramolecular Annulation Reactions. J. Org. Chem. 2018, 83, 13356–13362. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).