Steric and Electronic Effects in N-Heterocyclic Carbene Gold(III) Complexes: An Experimental and Computational Study
Abstract
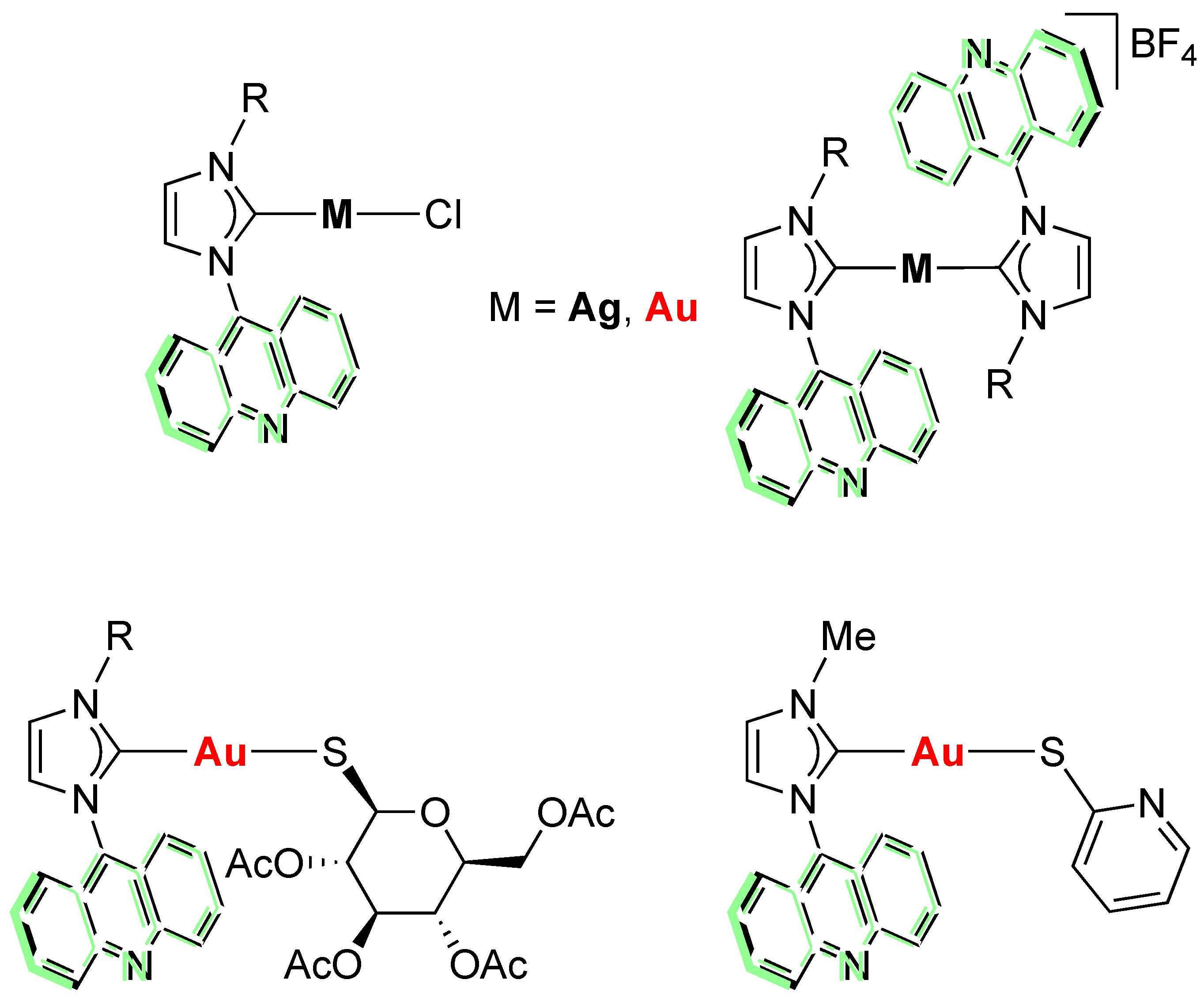
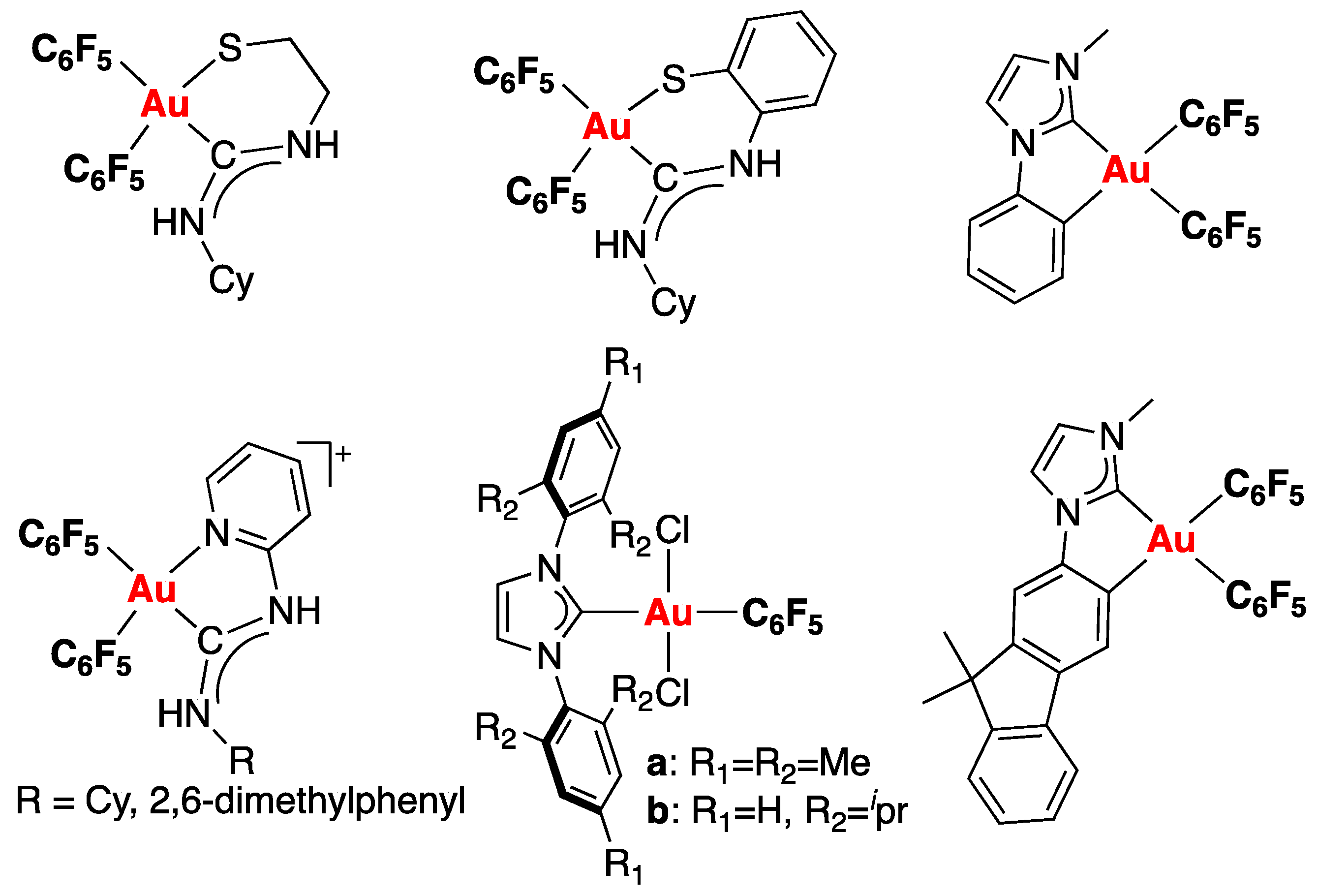
1. Introduction
2. Results and Discussion
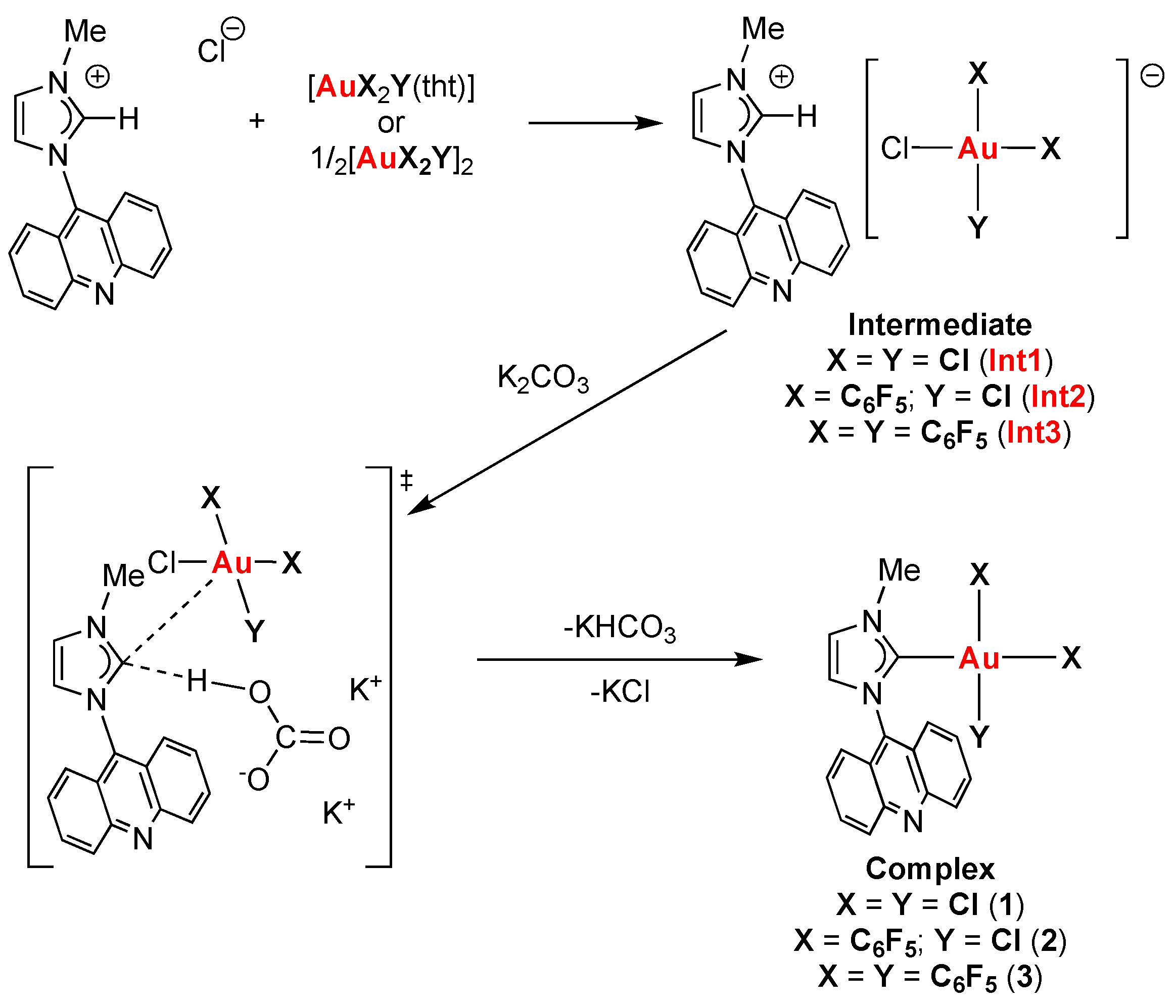
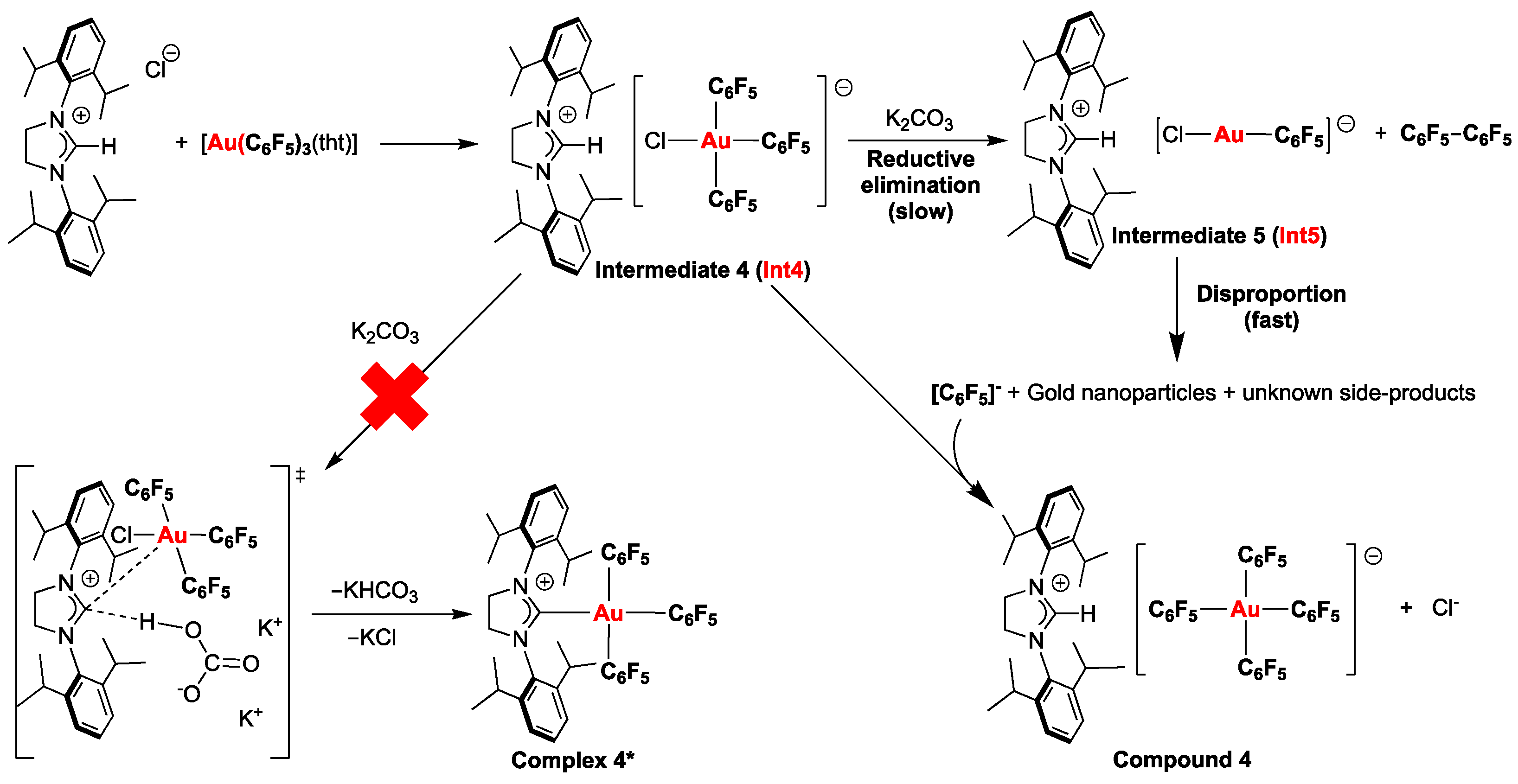
2.1. Synthesis of the Au(III) Complexes with NHC Ligands Derived from Acridine
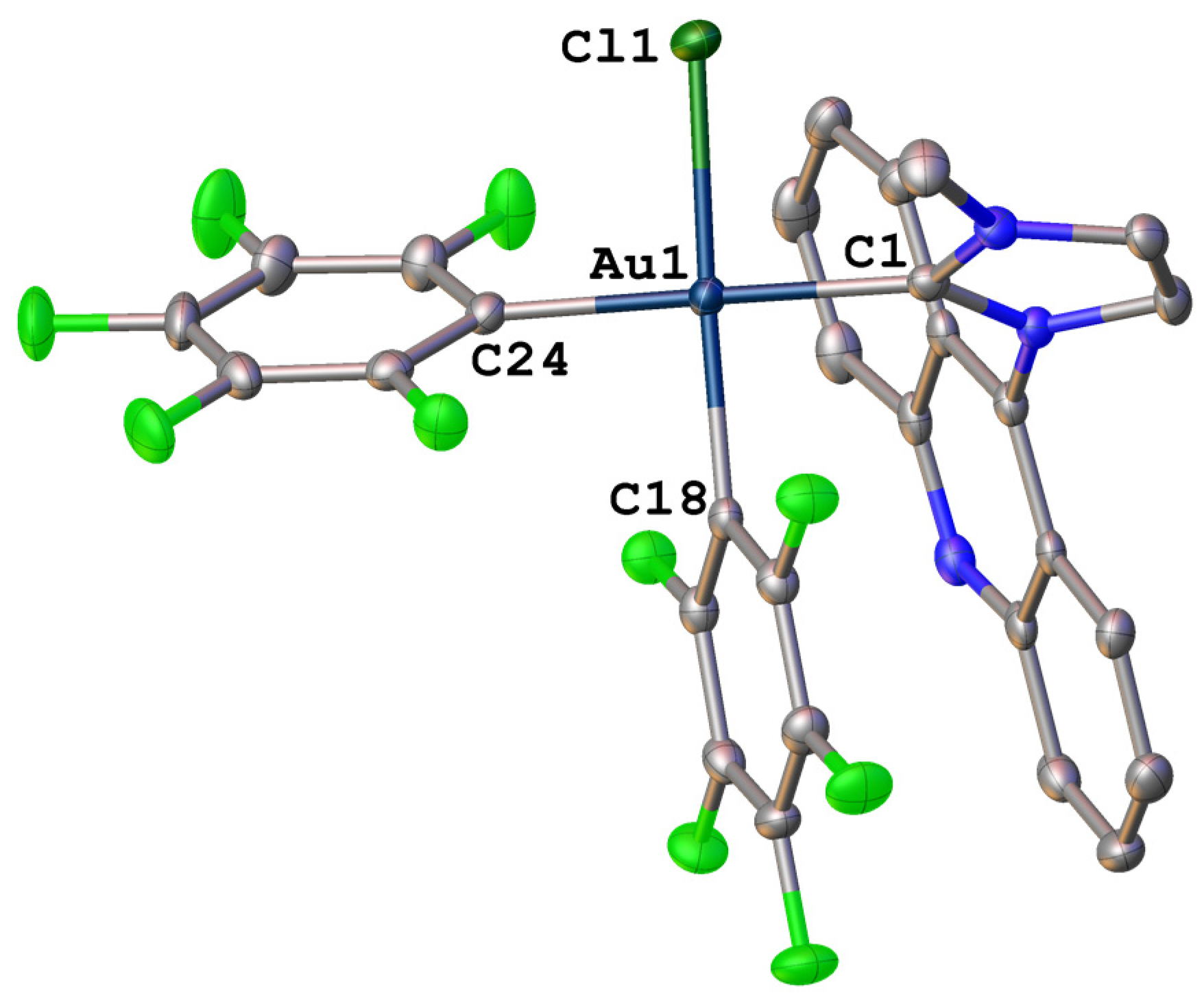
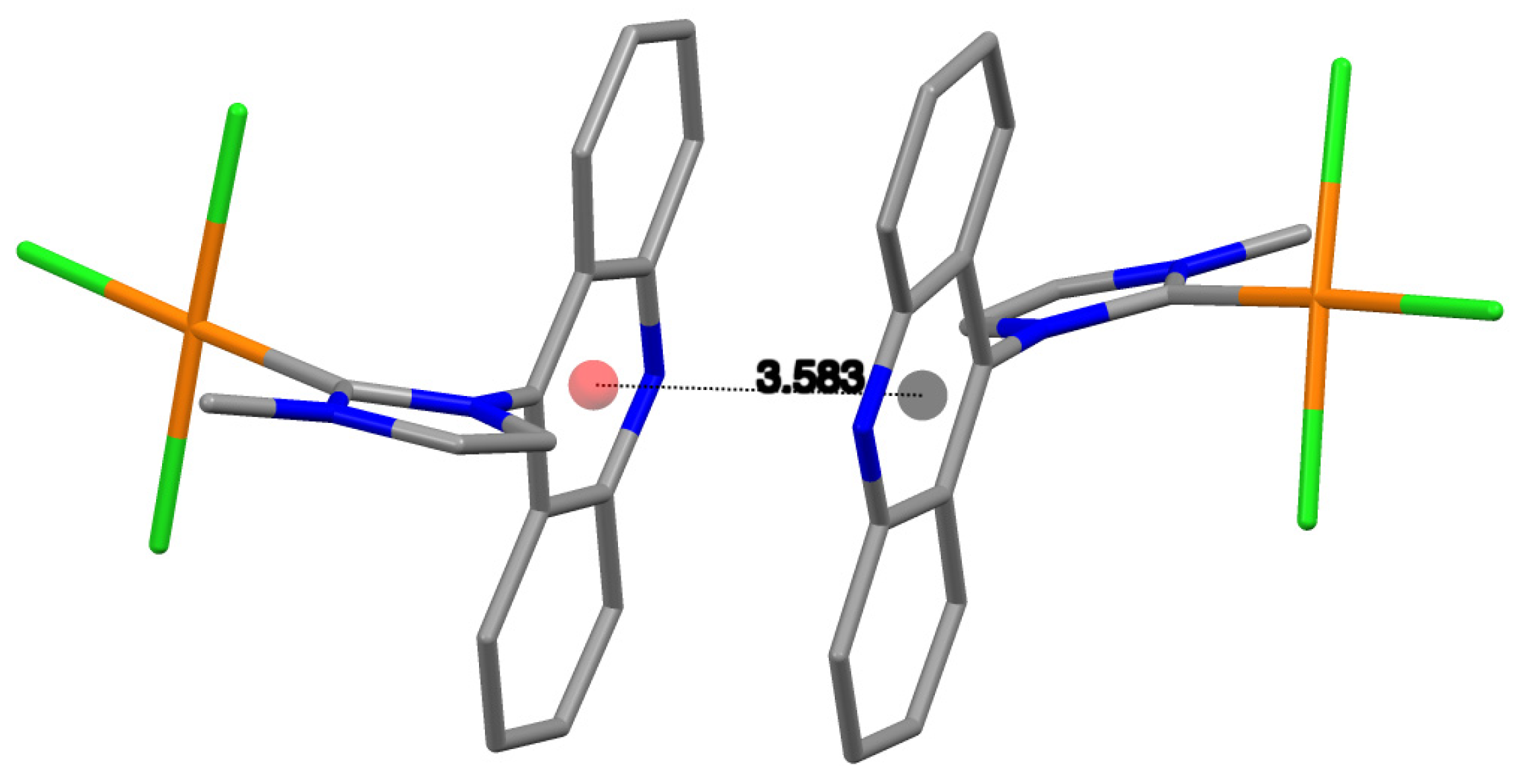
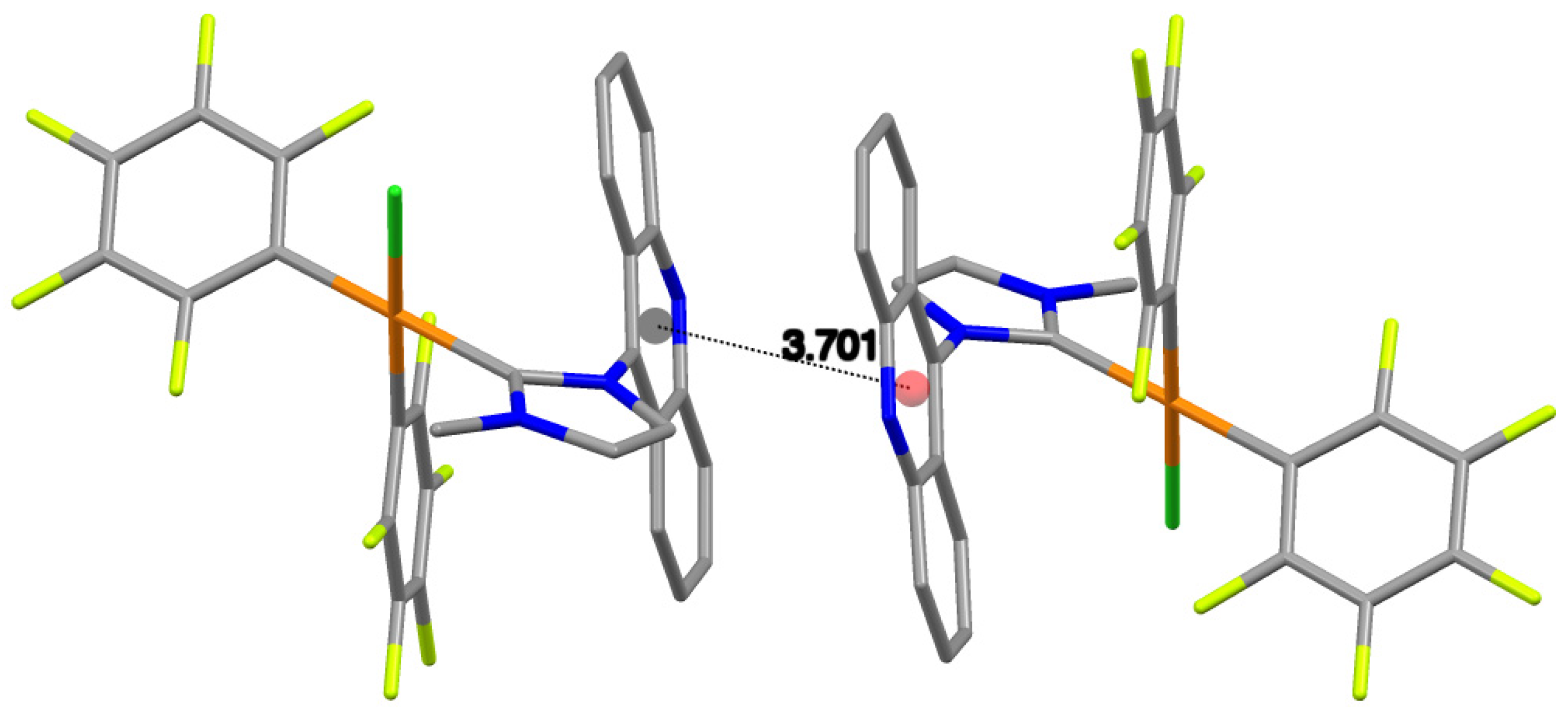
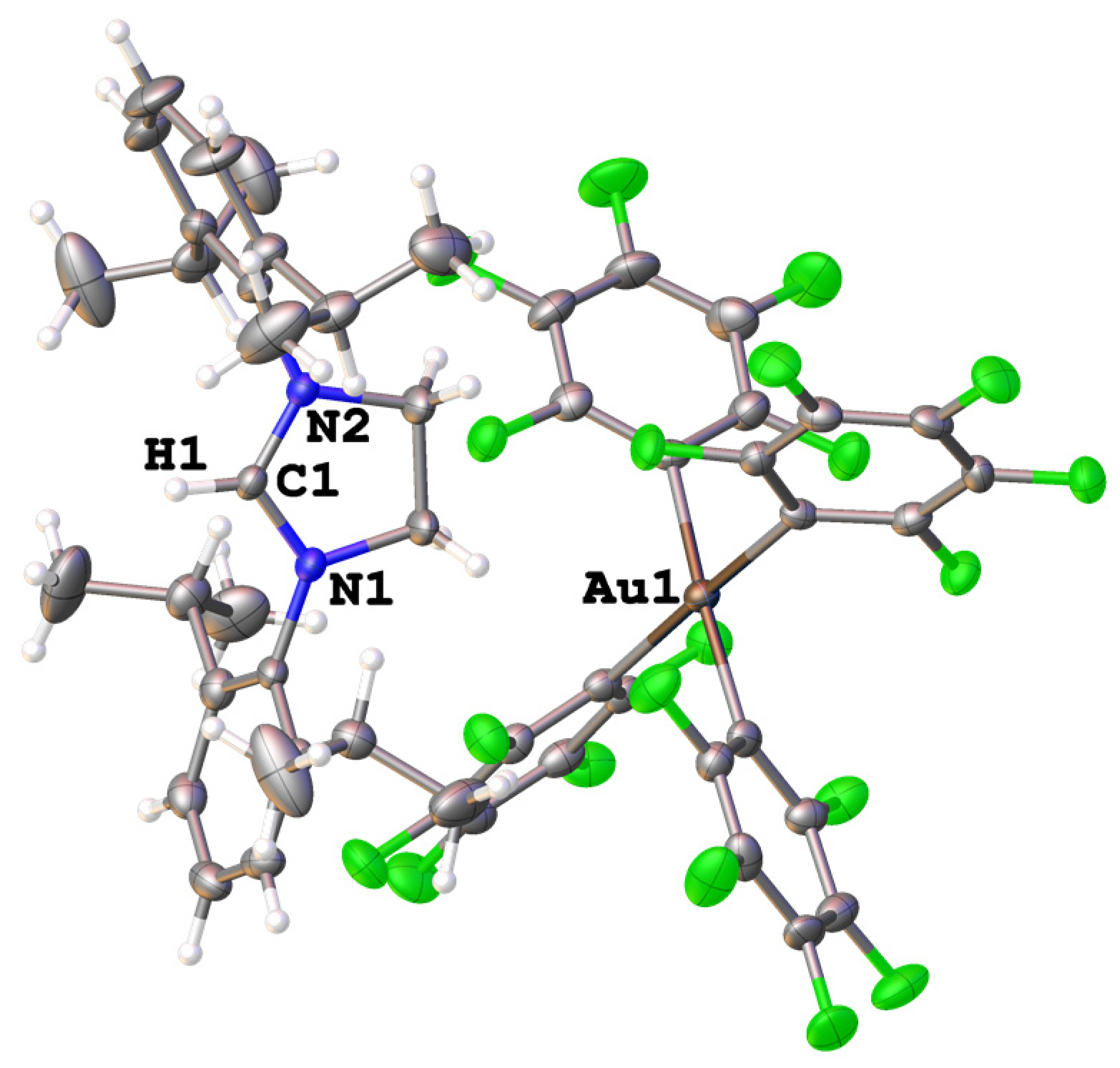
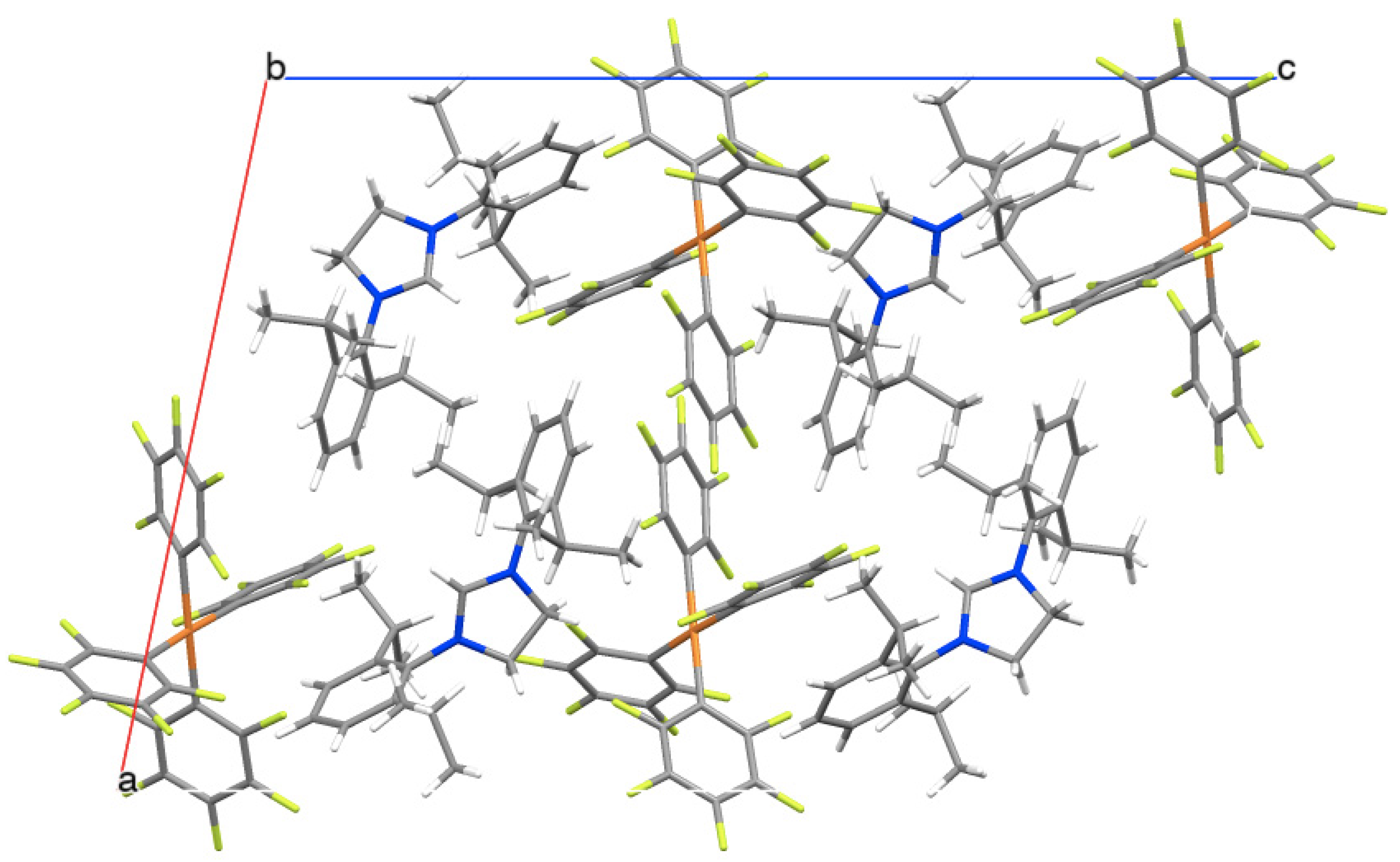
2.2. X-ray Structure Analysis
2.3. Synthesis of the 1,3-Bis-(2,6-diisopropylphenyl)imidazolinium tetrakis(pentafluorophenyl)aurate(III) Salt
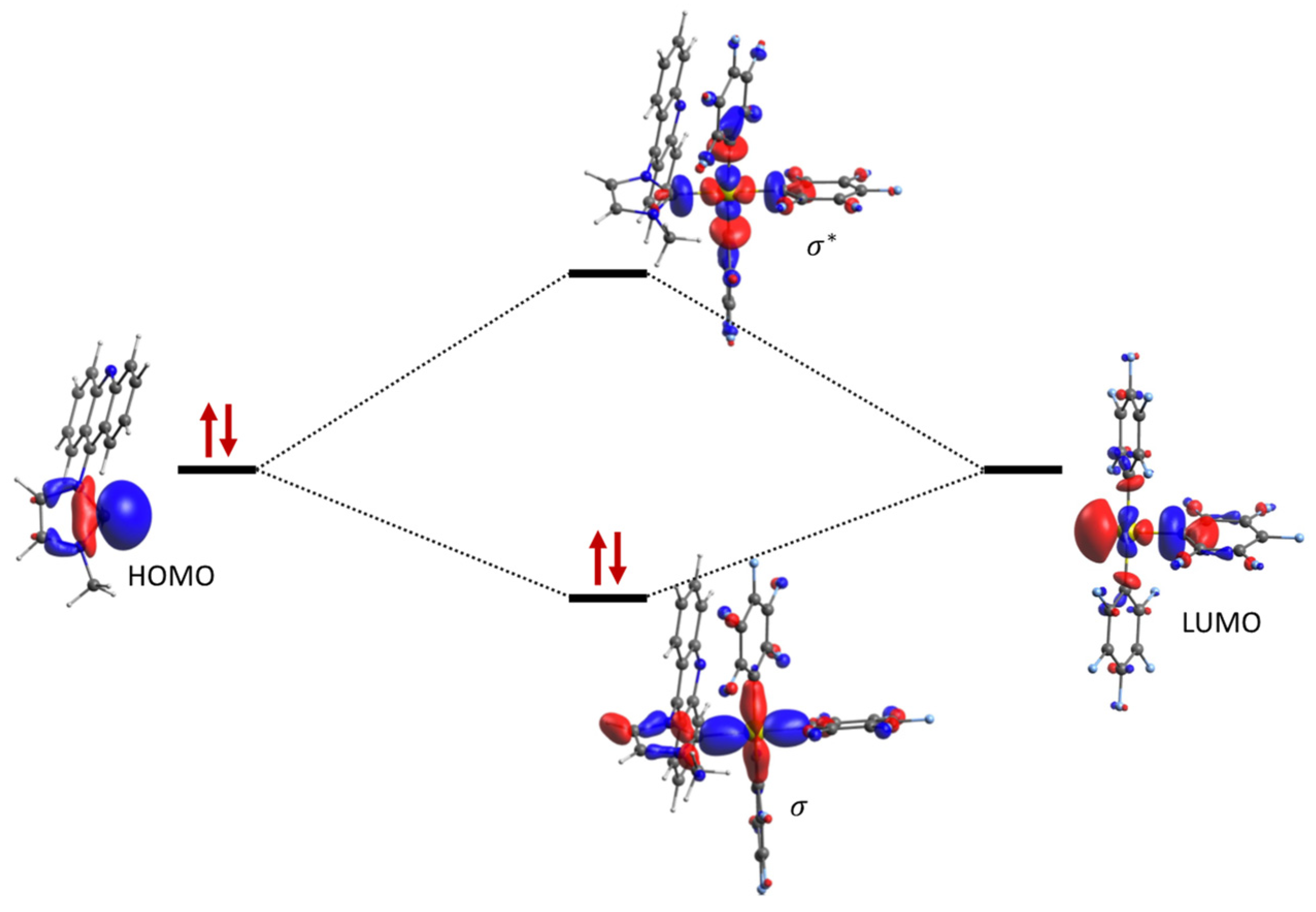
2.4. Computational Studies
3. Materials and Methods
3.1. General Measurements and Analysis Instrumentation
3.2. Crystallographic Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M.C. N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Ibáñez, S.; Poyatos, M.; Peris, E. N-Heterocyclic Carbenes: A Door Open to Supramolecular Organometallic Chemistry. Acc. Chem. Res. 2020, 53, 1401–1413. [Google Scholar] [CrossRef]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene-Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic Carbene-Silver Complexes: A New Class of Antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Williams, M.R.M.; Bertrand, B.; Fernandez-Cestau, J.; Waller, Z.A.E.; O’Connell, M.A.; Searcey, M.; Bochmann, M. Acridine-Decorated Cyclometallated Gold(III) Complexes: Synthesis and Anti-Tumour Investigations. Dalton Trans. 2018, 47, 13523–13534. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, M.C.; Laguna, A.; Visbal, R. N-Heterocyclic Carbene Coinage Metal Complexes as Intense Blue-Green Emitters. Organometallics 2012, 31, 7146–7157. [Google Scholar] [CrossRef]
- Visbal, R.; Fernández-Moreira, V.; Marzo, I.; Laguna, A.; Gimeno, M.C. Cytotoxicity and Biodistribution Studies of Luminescent Au(i) and Ag(i) N-Heterocyclic Carbenes. Searching for New Biological Targets. Dalton Trans. 2016, 45, 15026–15033. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent Advances in Gold-NHC Complexes with Biological Properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Linder, T.; Sundermeyer, J. Three Novel Anions Based on Pentafluorophenyl Amine Combined with Two New Synthetic Strategies for the Synthesis of Highly Lipophilic Ionic Liquids. Chem. Commun. 2009, 20, 2914–2916. [Google Scholar] [CrossRef]
- Bojan, V.R.; López-de-Luzuriaga, J.M.; Manso, E.; Monge, M.; Olmos, M.E. Metal-Induced Phosphorescence in (Pentafluorophenyl)Gold(III) Complexes. Organometallics 2011, 30, 4486–4489. [Google Scholar] [CrossRef]
- Romanov, A.S.; Bochmann, M. Gold(I) and Gold(III) Complexes of Cyclic (Alkyl)(Amino)Carbenes. Organometallics 2015, 34, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Mankad, N.P.; Toste, F.D. C(Sp3)-F Reductive Elimination from Alkylgold(III) Fluoride Complexes. Chem. Sci. 2012, 3, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Ahlsten, N.; Perry, G.J.P.; Cambeiro, X.C.; Boorman, T.C.; Larrosa, I. A Silver-Free System for the Direct C-H Auration of Arenes and Heteroarenes from Gold Chloride Complexes. Catal. Sci. Technol. 2013, 3, 2892–2897. [Google Scholar] [CrossRef]
- Kaub, C.; Lebedkin, S.; Li, A.; Kruppa, S.V.; Strebert, P.H.; Kappes, M.M.; Riehn, C.; Roesky, P.W. Bimetallic D10-Metal Complexes of a Bipyridine Substituted N-Heterocyclic Carbene. Chem. A Eur. J. 2018, 24, 6094–6104. [Google Scholar] [CrossRef]
- Bestgen, S.; Gamer, M.T.; Lebedkin, S.; Kappes, M.M.; Roesky, P.W. Di- And Trinuclear Gold Complexes of Diphenylphosphinoethyl-Functionalised Imidazolium Salts and Their N-Heterocyclic Carbenes: Synthesis and Photophysical Properties. Chem. A Eur. J. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Phillips, N.; Dodson, T.; Tirfoin, R.; Bates, J.I.; Aldridge, S. Expanded-Ring N-Heterocyclic Carbenes for the Stabilization of Highly Electrophilic Gold(I) Cations. Chem. A Eur. J. 2014, 20, 16721–16731. [Google Scholar] [CrossRef] [PubMed]
- Simler, T.; Möbius, K.; Müller, K.; Feuerstein, T.J.; Gamer, M.T.; Lebedkin, S.; Kappes, M.M.; Roesky, P.W. Mono- and Dinuclear Coinage Metal Complexes Supported by an Imino-Pyridine-NHC Ligand: Structural and Photophysical Studies. Organometallics 2019, 38, 3649–3661. [Google Scholar] [CrossRef]
- Crespo, J.; Guari, Y.; Ibarra, A.; Larionova, J.; Lasanta, T.; Laurencin, D.; López-De-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Richeter, S. Ultrasmall NHC-Coated Gold Nanoparticles Obtained through Solvent Free Thermolysis of Organometallic Au(i) Complexes. Dalton Trans. 2014, 43, 15713–15718. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lum, C.T.; Lok, C.N.; Zhang, J.J.; Che, C.M. Chemical Biology of Anticancer Gold(III) and Gold(I) Complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- Hofer, M.; Gomez-Bengoa, E.; Nevado, C. A Neutral Gold(III)-Boron Transmetalation. Organometallics 2014, 33, 1328–1332. [Google Scholar] [CrossRef]
- Feuerstein, W.; Holzer, C.; Gui, X.; Neumeier, L.; Klopper, W.; Breher, F. Synthesis of New Donor-Substituted Biphenyls: Pre-Ligands for Highly Luminescent (C^C^D) Gold(III) Pincer Complexes. Chem. A Eur. J. 2020, 26, 17156–17164. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.J.; Pistner, A.J.; Yap, G.P.A.; Lutterman, D.A.; Rosenthal, J. Thermal versus Photochemical Reductive Elimination of Aryl Chlorides from NHC-Gold Complexes. Organometallics 2013, 32, 5026–5029. [Google Scholar] [CrossRef]
- Pažický, M.; Loos, A.; Ferreira, M.J.; Serra, D.; Vinokurov, N.; Rominger, F.; Jäkel, C.; Hashmi, A.S.K.; Limbach, M. Synthesis, Reactivity, and Electrochemical Studies of Gold(I) and Gold(III) Complexes Supported by N-Heterocyclic Carbenes and Their Application in Catalysis. Organometallics 2010, 29, 4448–4458. [Google Scholar] [CrossRef]
- Von Arx, T.; Szentkuti, A.; Zehnder, T.N.; Blacque, O.; Venkatesan, K. Stable N-Heterocyclic Carbene (NHC) Cyclometalated (C^C) Gold(III) Complexes as Blue-Blue Green Phosphorescence Emitters. J. Mater. Chem. C 2017, 5, 3765–3769. [Google Scholar] [CrossRef]
- Montanel-Pérez, S.; Izaga, A.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. Synthesis of Gold(III) Complexes with Bidentate Amino-Thiolate Ligands as Precursors of Novel Bifunctional Acyclic Diaminocarbenes. ACS Omega 2018, 3, 13097–13103. [Google Scholar] [CrossRef] [PubMed]
- Montanel-Pérez, S.; Elizalde, R.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. Synthesis of Bioactive N-Acyclic Gold(I) and Gold(III) Diamino Carbenes with Different Ancillary Ligands. Eur. J. Inorg. Chem. 2019, 2019, 4273–4281. [Google Scholar] [CrossRef]
- Montanel-Pérez, S.; Herrera, R.P.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. The Fluxional Amine Gold(III) Complex as an Excellent Catalyst and Precursor of Biologically Active Acyclic Carbenes. Dalton Trans. 2015, 44, 9052–9062. [Google Scholar] [CrossRef] [PubMed]
- Tzouras, N.V.; Nahra, F.; Falivene, L.; Cavallo, L.; Saab, M.; van Hecke, K.; Collado, A.; Collett, C.J.; Smith, A.D.; Cazin, C.S.J.; et al. A Mechanistically and Operationally Simple Route to Metal–N-Heterocyclic Carbene (NHC) Complexes. Chem. A Eur. J. 2020, 26, 4515–4519. [Google Scholar] [CrossRef]
- Elangovan, A.; Chiu, H.H.; Yang, S.W.; Ho, T.I. Arylethynylacridines: Electrochemiluminescence and Photophysical Properties. Org. Biomol. Chem. 2004, 2, 3113–3118. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on N-n Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000, 18, 3885–3896. [Google Scholar] [CrossRef]
- Liu, C.-S.; Chen, P.-Q.; Yang, E.-C.; Tian, J.-L.; Bu, X.-H.; Li, Z.-M.; Sun, H.-W.; Lin, Z. Silver(I) Complexes in Coordination Supramolecular System with Bulky Acridine-Based Ligands: Syntheses, Crystal Structures, and Theoretical Investigations on C−H···Ag Close Interaction. Inorg. Chem. 2006, 45, 5812–5821. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Chen, X.Y.; Huo, S.M.; Ma, Y.R.; Zeng, R.H. Catena-Poly[Silver(I)-μ-Acridine-9-Carboxyl-Ato-Κ3 N:O,O′]. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m1482. [Google Scholar] [CrossRef]
- Poater, A.; Cosenza, B.; Correa, A.; Giudice, S.; Ragone, F.; Scarano, V.; Cavallo, L. SambVca: A Web Application for the Calculation of the Buried Volume of N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2009, 2009, 1759–1766. [Google Scholar] [CrossRef]
- Hofer, M.; Nevado, C. Cross-Coupling of Arene-Gold(III) Complexes. Tetrahedron 2013, 69, 5751–5757. [Google Scholar] [CrossRef]
- Basova, T.V.; Hassan, A.; Morozova, N.B. Chemistry of Gold(I, III) Complexes with Organic Ligands as Potential MOCVD Precursors for Fabrication of Thin Metallic Films and Nanoparticles. Coord. Chem. Rev. 2019, 380, 58–82. [Google Scholar] [CrossRef]
- Ballarin, B.; Busetto, L.; Cristina Cassani, M.; Femoni, C. A New Gold(III)-Aminoethyl Imidazolium Aurate Salt: Synthesis, Characterization and Reactivity. Inorg. Chim. Acta 2010, 363, 2055–2064. [Google Scholar] [CrossRef]
- Ceamanos, C.; Gimeno, M.C.; Jayaswal, M.N.; Laguna, A. Heterometallic Complexes with the Mono and Disubstituted [(4-Pyridylamino)Carbonyl]Ferrocene Ligands. J. Organomet. Chem. 2012, 713, 169–177. [Google Scholar] [CrossRef]
- Coetzee, J.; Gabrielli, W.F.; Coetzee, K.; Schuster, O.; Nogai, S.D.; Cronje, S.; Raubenheimer, H.G. Structural Studies of Gold(I, II, and III) Compounds with Pentafluorophenyl and Tetrahydrothiophene Ligands. Angew. Chem. Int. Ed. 2007, 46, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Nevado, C. Unexpected Outcomes of the Oxidation of (Pentafluorophenyl) Triphenylphosphanegold(I). Eur. J. Inorg. Chem. 2012, 2012, 1338–1341. [Google Scholar] [CrossRef]
- Murray, H.H.; Fackler, J.P.; Porter, L.C.; Briggs, D.A.; Guerra, M.A.; Lagow, R.J. Synthesis and X-Ray Crystal Structures of [Au(CH2)2PPh2]2(CF3)2, [Au(CH2)2PPh2]2(C6F5)2, and [PPN][Au(C6F5)4]: Two Dinuclear Gold(II) Ylide Complexes Containing Alkyl and Aryl Ligands and a Tetrakis(Pentafluorophenyl)Aurate(III) Anion Complex. Inorg. Chem. 1987, 26, 357–363. [Google Scholar] [CrossRef]
- Schlueter, J.A.; Geiser, U.; Wang, H.H.; VanZile, M.L.; Fox, S.B.; Williams, J.M.; Laguna, A.; Laguna, M.; Naumann, D.; Roy, T. Electrochemical Synthesis and Crystallization of a Novel Tetraarylaurate Anion: Synthesis, Structure, and Physical Properties of (BEDT-TTF)Au(C6Cl5)4. Inorg. Chem. 1997, 36, 4265–4269. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- De Marco, R.; Dal Grande, M.; Baron, M.; Orian, L.; Graiff, C.; Achard, T.; Bellemin-Laponnaz, S.; Pöthig, A.; Tubaro, C. Synthesis, Structural Characterization and Antiproliferative Activity of Gold(I) and Gold(III) Complexes Bearing Thioether-Functionalized N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2021, 2021, 4196–4206. [Google Scholar] [CrossRef]
- Allen, E.A.; Wilkinson, W. The Vibrational Spectra of Some Dialkyl Sulphide Complexes of Gold(III) and Gold(I) Halides. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 2257–2262. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Laguna, M.; Abad, M. Synthesis and Reactions of Di-μ-Halo- or -Pseudohalotetrakis(Pentafluorophenyl)Digold(III). J. Organomet. Chem. 1983, 249, 437–443. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M. (Tetrahydrothiophene)Gold(I) or Gold(III) Complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Area Detector Adsorption Correction; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Agilent Technologies. CrysAlisPro, Version 1.171.35.11 Multiscan Absorption Correction with SCALE3 ABSPACK Scaling Algorithm; Agilent Technologies: Yarnton, UK, 2011. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Azofra, L.M.; Veenboer, R.M.P.; Falivene, L.; Vummaleti, S.V.C.; Poater, A.; Nolan, S.P.; Cavallo, L. Quantifying Electronic Similarities between NHC-Gold(i) Complexes and Their Isolobal Imidazolium Precursors. Phys. Chem. Chem. Phys. 2019, 21, 15615–15622. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Vishwakarma, R.; Bariya, P.K. Quantum Chemical Insight into C-H⋯F Bonding Interactions between Noncovalently Bonded Ion-Pairs in N-Heterocyclic Carbene Complexes of Gold(I) [(NHC*)2Au]+[PF6]− and Gold(III) [(NHC*)2AuCl2]+[PF6]−. J. Organomet. Chem. 2015, 795, 34–39. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent Structures and Interactions by Density Functional Theory with Small Atomic Orbital Basis Sets. J. Chem. Phys. 2015, 143, 54107. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Kruse, H.; Grimme, S. A Geometrical Correction for the Inter- and Intra-Molecular Basis Set Superposition Error in Hartree-Fock and Density Functional Theory Calculations for Large Systems. J. Chem. Phys. 2012, 136, 154101. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]

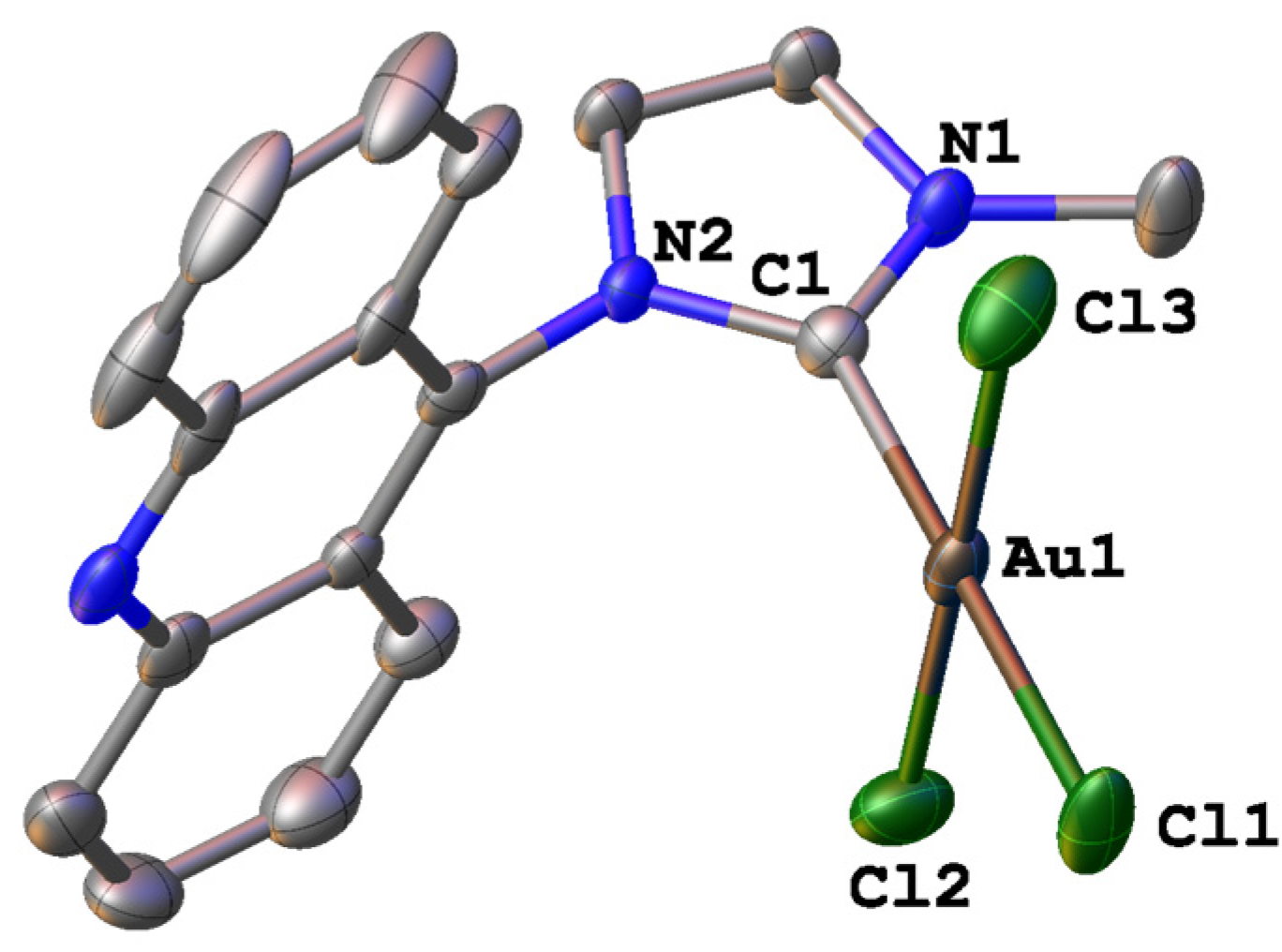
| Compound | 1 | 2 |
|---|---|---|
| Au–Ccarb | 2.007 (6) | 2.047 (2) |
| Au–Xtrans | 2.3198 (15) | 2.053 (2) |
| Au–Cl(1) | 2.3198 (15) | 2.3323 (6) |
| Ccarb–Au–Xtrans | 178.74 (17) | 179.03 (8) |
| torsion angle [a] | 89.82 | 65.78 |
| interplanar distance [b] | 3.517 | 3.504 |
| Centroid–centroid distance | 3.583 | 3.701 |
| aromatic contact | py-py | py-py |
| Reaction to | Intermediate | Int5 | Product |
|---|---|---|---|
| 3 | −47.95 (Int3) | - | −126.11 |
| 4 | −47.95 (Int4) | −69.15 | −90.38 |
| 4* | −47.95 (Int4) | - | −114.58 |
| State | λexp | λcal | Oscillator Strength | Dominant Transitions [a] | Nature |
|---|---|---|---|---|---|
| 11A | 360 | 337 | 0.1629 | 94% πacr1→πacr2 | IL |
| 131A | 250 | 223 | 1.0082 | 38% πacr1→5dx2-y2 11% πpfp1→πacr2 | LMCT LL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosero-Mafla, M.A.; Zapata-Rivera, J.; Gimeno, M.C.; Visbal, R. Steric and Electronic Effects in N-Heterocyclic Carbene Gold(III) Complexes: An Experimental and Computational Study. Molecules 2022, 27, 8289. https://doi.org/10.3390/molecules27238289
Rosero-Mafla MA, Zapata-Rivera J, Gimeno MC, Visbal R. Steric and Electronic Effects in N-Heterocyclic Carbene Gold(III) Complexes: An Experimental and Computational Study. Molecules. 2022; 27(23):8289. https://doi.org/10.3390/molecules27238289
Chicago/Turabian StyleRosero-Mafla, Miguel A., Jhon Zapata-Rivera, M. Concepción Gimeno, and Renso Visbal. 2022. "Steric and Electronic Effects in N-Heterocyclic Carbene Gold(III) Complexes: An Experimental and Computational Study" Molecules 27, no. 23: 8289. https://doi.org/10.3390/molecules27238289
APA StyleRosero-Mafla, M. A., Zapata-Rivera, J., Gimeno, M. C., & Visbal, R. (2022). Steric and Electronic Effects in N-Heterocyclic Carbene Gold(III) Complexes: An Experimental and Computational Study. Molecules, 27(23), 8289. https://doi.org/10.3390/molecules27238289








