Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation
Abstract
1. Introduction
2. Results and Discussion
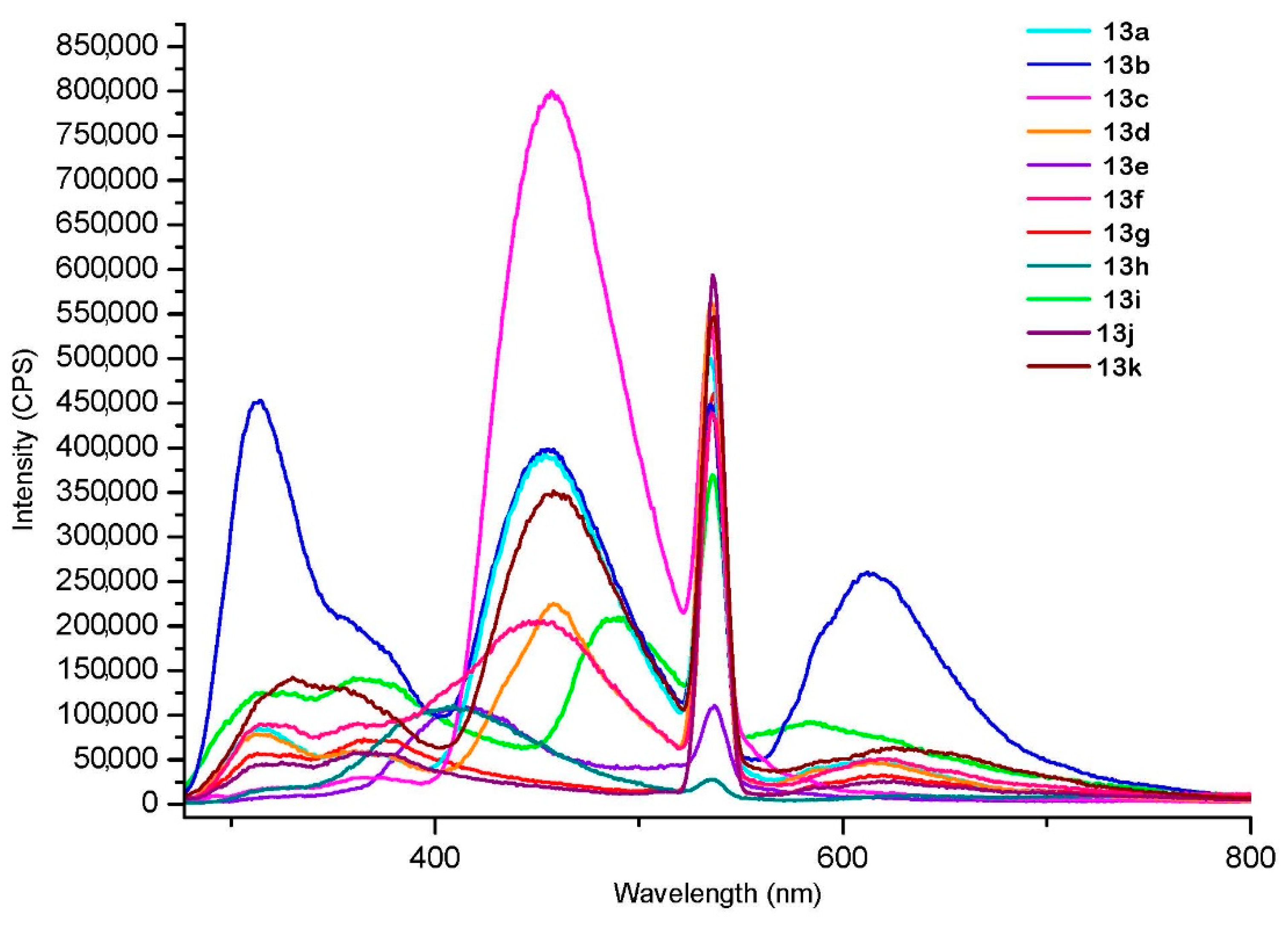
3. Fluorescence
4. Pharmacology
4.1. Methodology
4.2. Experimental Section
4.2.1. General
4.2.2. General Procedure for the Synthesis of Compounds 13a–k, 14c, j, k
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Azmi, A.; Elassar, A.A.Z.; Booth, B.L. The chemistry of diaminomaleonitrile and its utility in heterocyclic synthesis. Tetrahedron 2003, 59, 2749–2763. [Google Scholar] [CrossRef]
- Al-Azmi, A. Recent developments in the chemistry of diaminomaleonitrile. Curr. Org. Synth. 2015, 12, 110–135. [Google Scholar] [CrossRef]
- Al-Azmi, A. Pyrazolo[1,5-a]pyrimidines: A close look into their synthesis and applications. Curr. Org. Synth. 2019, 23, 1–23. [Google Scholar] [CrossRef]
- Sato, N. Pyrazines, and their Benzo Derivatives. Compr. Heterocycl. Chem. III 2008, 8, 273–331. [Google Scholar]
- Watanabe, K.; Iguchi, K.; Fujimori, K. Clavulazine, a New Marine Pyrazine Congener from the Okinawan Soft Coral Clavularia viridis. Heterocycles 1998, 49, 269–274. [Google Scholar] [CrossRef]
- de Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Maiuolo, L.; Algieri, V.; Olivito, F.; de Nino, A. Recent Developments on 1,3-Dipolar Cycloaddition Reactions by Catalysis in Green Solvents. Catalysts 2020, 10, 65. [Google Scholar] [CrossRef]
- Osborne, J.D.; Matthews, T.P.; McHardy, T.; Proisy, N.; Cheung, K.M.; Lainchbury, M.; Brown, N.; Walton, M.I.; Eve, P.D.; Boxall, K.J.; et al. Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J. Med. Chem. 2016, 59, 5221–5237. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Yoshida, S.; Yoshimatsu, M.; Aoyama, K.; Kosugi, Y.; Kojima, T.; Morishita, N.; Dougan, D.R.; Snell, G.P.; Imamura, S.; et al. Design and Synthesis of Potent Inhibitor of Apoptosis (IAP) Proteins Antagonists Bearing an Octahydropyrrolo[1,2-a]pyrazine Scaffold as a Novel Proline Mimetic. J. Med. Chem. 2013, 56, 1228–1246. [Google Scholar]
- Lainchbury, M.; Matthews, T.P.; McHardy, T.; Boxall, K.J.; Walton, M.I.; Eve, P.D.; Hayes, A.; Valenti, M.R.; de Haven Brandon, A.K.; Box, G.; et al. Discovery of 3-alkoxyamino-5-(pyridin-2-ylamino)pyrazine-2-carbonitriles as selective, orally bioavailable CHK1 inhibitors. J. Med. Chem. 2012, 55, 10229–10240. [Google Scholar] [CrossRef] [PubMed]
- Buron, F.; Ple, N.; Turck, A.; Queguiner, G. Synthesis of Pyrazine Alcaloids from Botryllus leachi. Diazines 43. J. Org. Chem. 2005, 70, 2616. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Aithagani, S.K.; Yadav, M.; Singh, V.P.; Vishwakarma, R.A. Iron-catalyzed Cross-Coupling of Electron-Deficient Heterocycles and Quinone with Organoboron Species via Innate C–H Functionalization: Application in Total Synthesis of Pyrazine Alkaloid Botryllazine A. J. Org. Chem. 2013, 78, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Chrovian, C.C.; Soyode-Johnson, A.; Ao, H.; Bacani, J.M.; Carruthers, N.I.; Lord, B.; Nguyen, L.; Rech, J.C.; Wang, Q.; Bhattacharya, A.; et al. Novel Phenyl-Substituted 5,6-Dihydro-[1,2,4]triazolo[4,3-a]pyrazine P2X7 Antagonists with Robust Target Engagement in Rat Brain. ACS Chem. Neurosci. 2016, 7, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chlupacova, M.K.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, O.; Dolezal, M.; Paterova, P.; Kubicek, V.; Pesko, M.; Kunes, J.; Coffey, A.; Guo, J.; Kralova, K. N-Substituted 5-Amino-6-methylpyrazine-2,3-dicarbonitriles: Microwave-Assisted Synthesis and Biological Properties. Molecules 2014, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal Role of Pyrazine Ring in Medicinally Important Compounds: A Review. Mini Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Üngören, S.H.; Dilekoğlu, E.; Koca, I. Synthesis of pyrazine-2,3-dicarbonitrile and 1,2,4-triazine-5(4H)-one derivatives from furan-2,3-diones. Chin. Chem. Lett. 2013, 24, 1130–1133. [Google Scholar] [CrossRef]
- Wang, F.; Wei, M.; Duan, X.; Liu, X.; Yao, S.; Wang, J.; Zhu, H.; Chen, C.; Gu, L.; Zhang, Y. Identification, synthesis and biological evaluation of pyrazine ring compounds from Talaromyces minioluteus (Penicillium minioluteum). Org. Chem. Front. 2020, 7, 3616–3624. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Wang, X.-L.; Liu, W.; Yang, Y.-S.; Tang, J.-F.; Zhu, H.-L. Design, synthesis and biological evaluation of heterocyclic azoles derivatives containing pyrazine moiety as potential telomerase inhibitors. Bioorganic Med. Chem. 2012, 20, 6356–6365. [Google Scholar] [CrossRef]
- Mortzfeld, F.; Hashem, C.; Vrankova, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Milić, J.V.; Schaack, C.; Hellou, N.; Isenrich, F.; Gershoni-Poranne, R.; Neshchadin, D.; Egloff, S.; Trapp, N.; Ruhlmann, L.; Boudon, C.; et al. Light-Responsive Pyrazine-Based Systems: Probing Aromatic Diarylethene Photocyclization. J. Phys. Chem. C 2018, 122, 19100–19109. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tour, J.M. Synthesis of Highly Functionalized Pyrazines by Ortho-Lithiation Reactions. Pyrazine Ladder Polymers. J. Am. Chem. Soc. 1999, 121, 8783–8790. [Google Scholar] [CrossRef]
- Lee, J.Y.; Aoyama, T.; Uchiyama, M.; Matsumoto, S. Synthesis and properties of liquid pyrazine dyes. Dyes Pigm. 2020, 174, 108030. [Google Scholar] [CrossRef]
- Kobak, R.Z.U.; Öztürk, E.S.; Koca, A.; Gül, A. The synthesis and cyclotetramerisation reactions of aryloxy-, arylalkyloxy-substituted pyrazine-2,3-dicarbonitriles and spectroelectrochemical properties of octakis(hexyloxy)-pyrazinoporphyrazine. Dyes Pigm. 2010, 86, 115–122. [Google Scholar] [CrossRef]
- Yavari, I.; Pashazadeh, R.; Hosseinpour, R. Synthesis of Functionalized Pyrazines and Quinoxalines from Nef-Isocyanide Adducts and 1,2-Diamines. Helvetica Chim. Acta. 2012, 95, 169–172. [Google Scholar] [CrossRef]
- Talebizadeh, M.; Abbasinejad, M.A.; Darehkordi, A. A Simple One-pot Three-component Synthesis of Dihydrobenzo[4,5]imidazo[2,1-b]thiazol-3-ols by Reaction of Acyl Chlorides, Isocyanides and 2-Mercaptobenzimidazoles. J. Heterocycl. Chem. 2018, 55, 2737–2743. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Moghimi, J. A novel isocyanide-based three-component reaction: Synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. J. Org. Chem. 2007, 72, 6309–6311. [Google Scholar] [CrossRef] [PubMed]
- Novakova, V.; Hladík, P.; Filandrová, T.; Zajícová, I.; Krepsová, V.; Miletin, M.; Lenčo, J.; Zimcik, P. Structural factors influencing the intramolecular charge transfer and photoinduced electron transfer in tetrapyrazinoporphyrazines. Phys. Chem. Chem. Phys. 2014, 16, 5440–5446. [Google Scholar] [CrossRef][Green Version]
- Ahmad, M.; Perveen, Z.; Bortoluzzi, A.J.; Hameed, S.; Shah, M.R.; Tariq, M.; Din, G.; Muhammad, T.; Siddique, M.; Anwar, M. Synthesis, and characterization of novel iminobenzoates with terminal pyrazine moieties. Chem. Cent. J. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Moloudi, M.; Kabirifard, H.; Piri, S.; Naghizadeh, E. Synthesis of 2,3-dicyanopyrazine and ethyl 5-amino-4,6-dicyanobiphenyl-3-carboxylate derivatives from ethyl aroylpyruvates. Heterocycl Comm. 2018, 24, 99–102. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Long, G.; Wang, Y.; Zhao, J.; Li, D.; Li, J.; Ganguly, R.; Li, Y.; Sun, H.; et al. Synthesis, structure, physical properties, and OLED application of pyrazine–triphenylamine fused conjugated compounds. RSC Adv. 2015, 5, 63080–63086. [Google Scholar] [CrossRef]


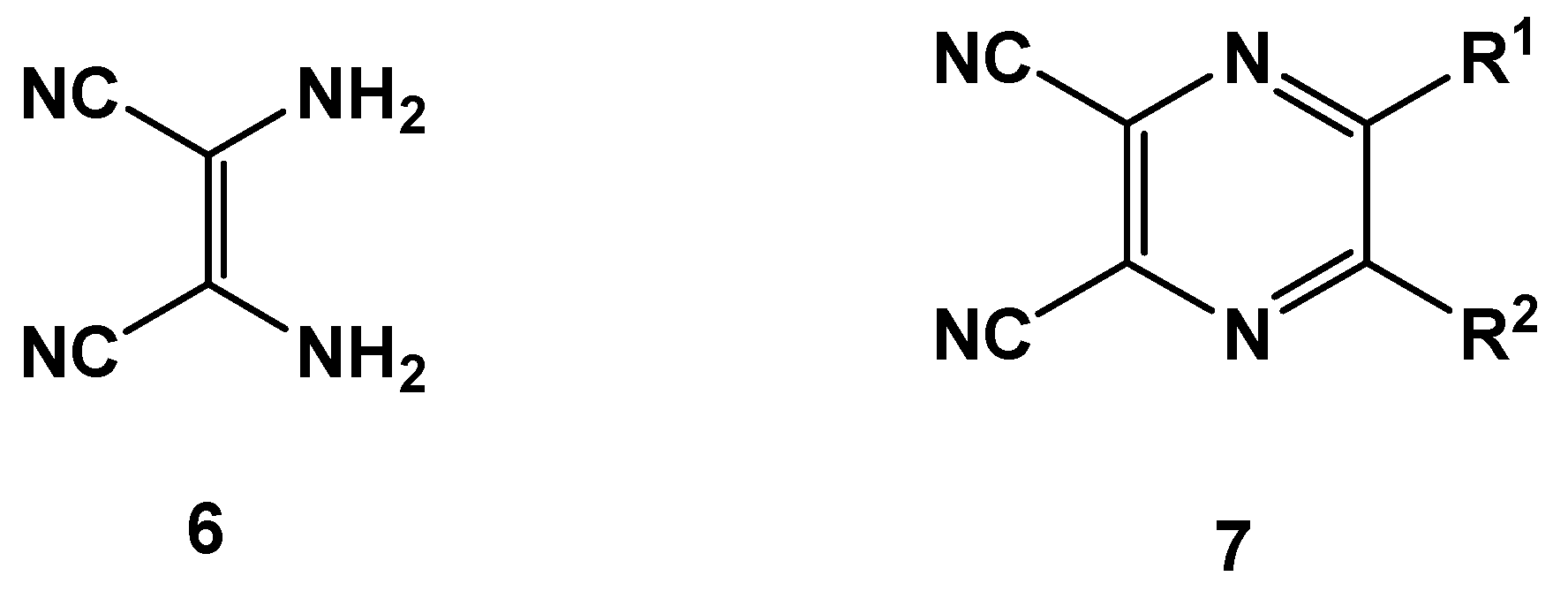
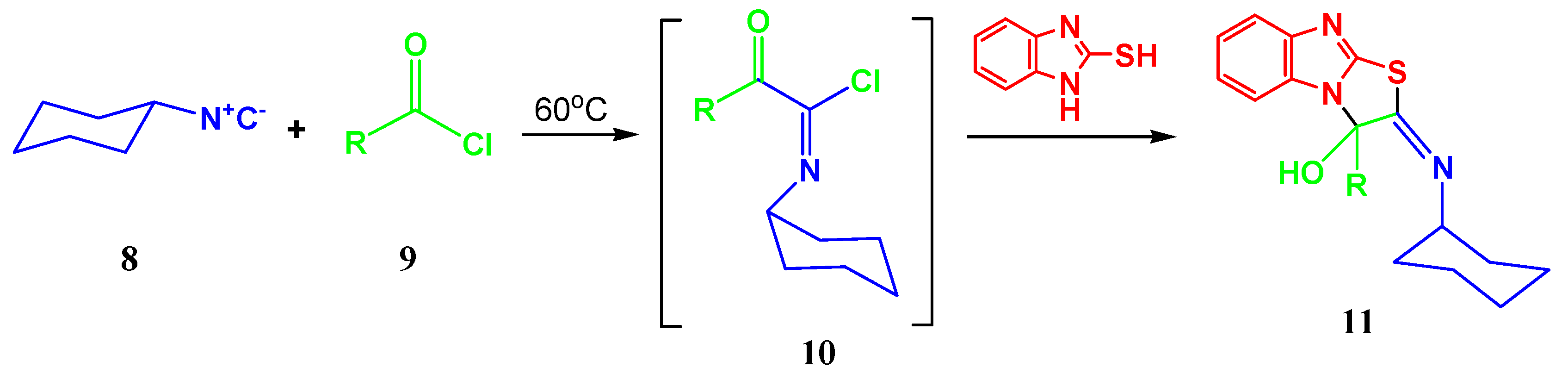
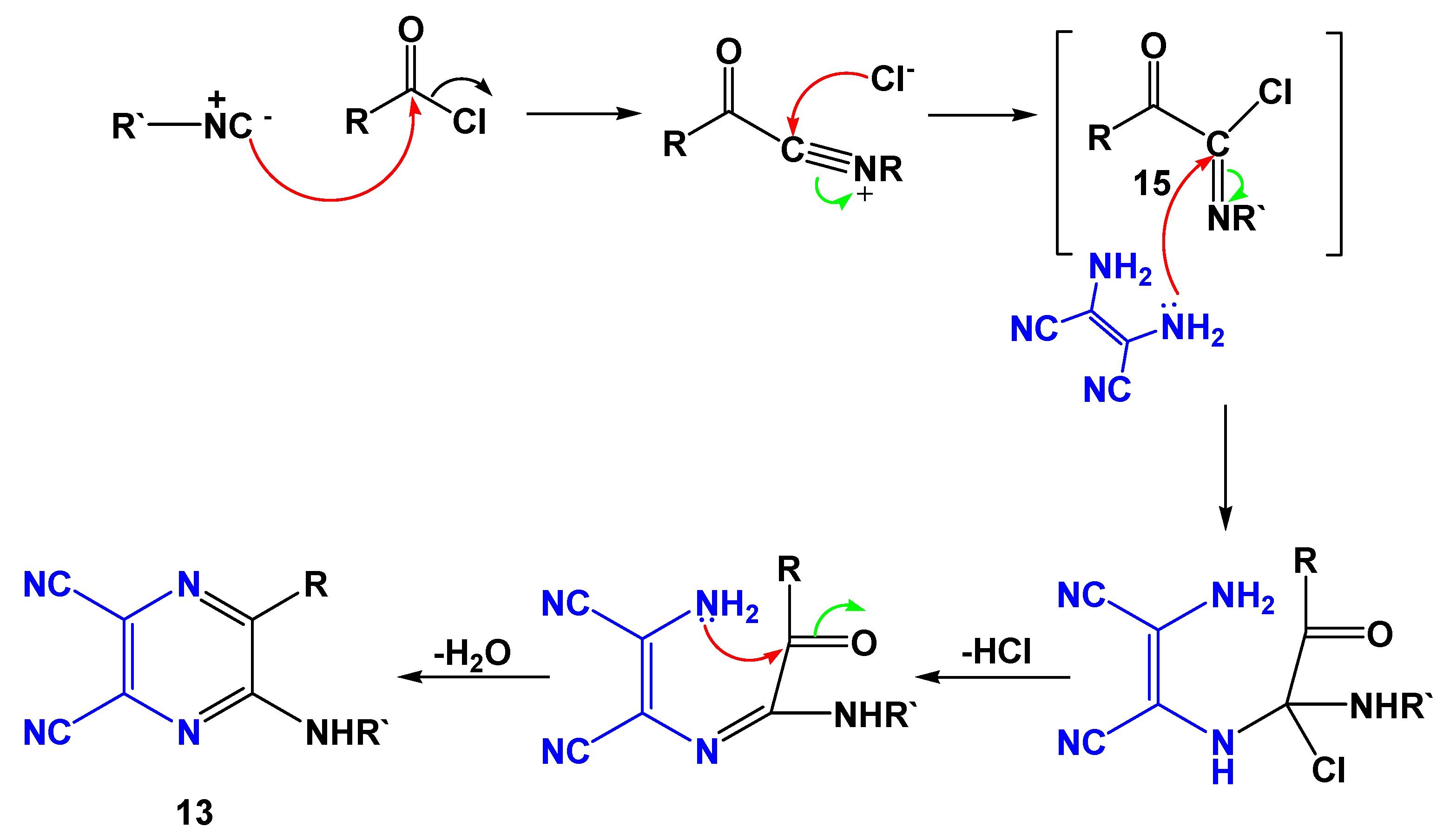
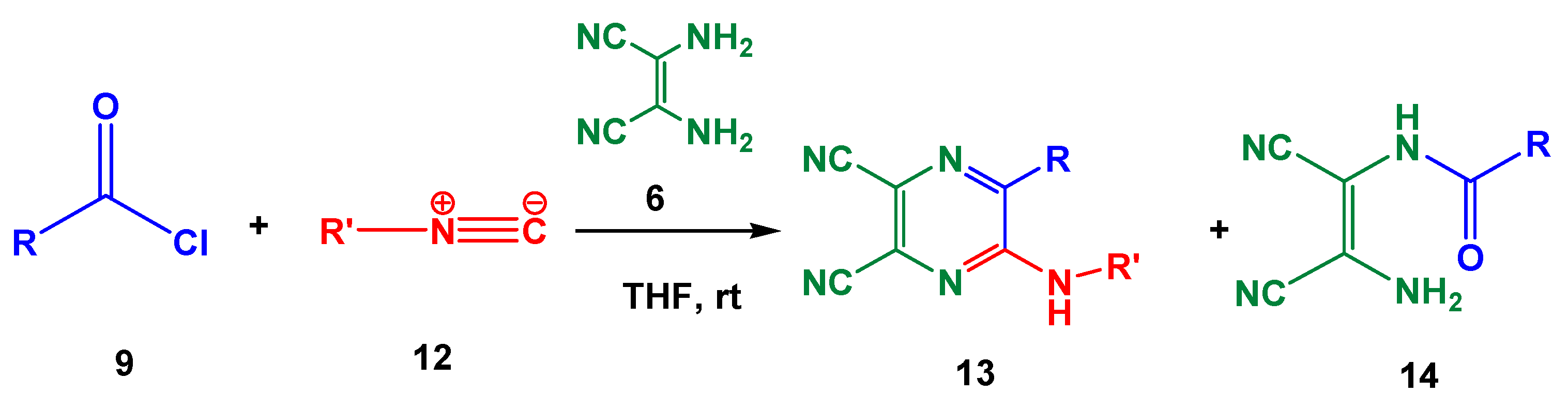
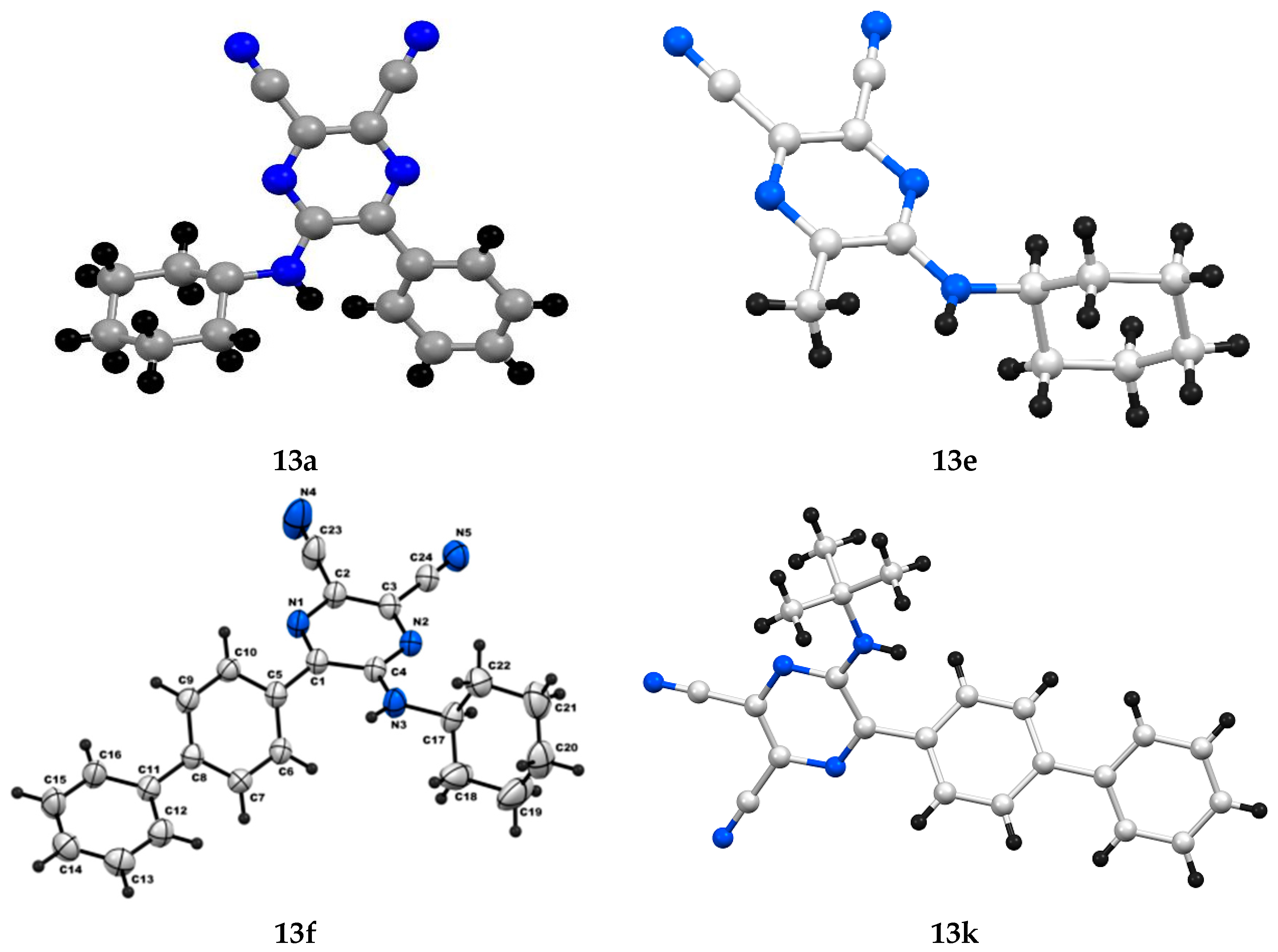
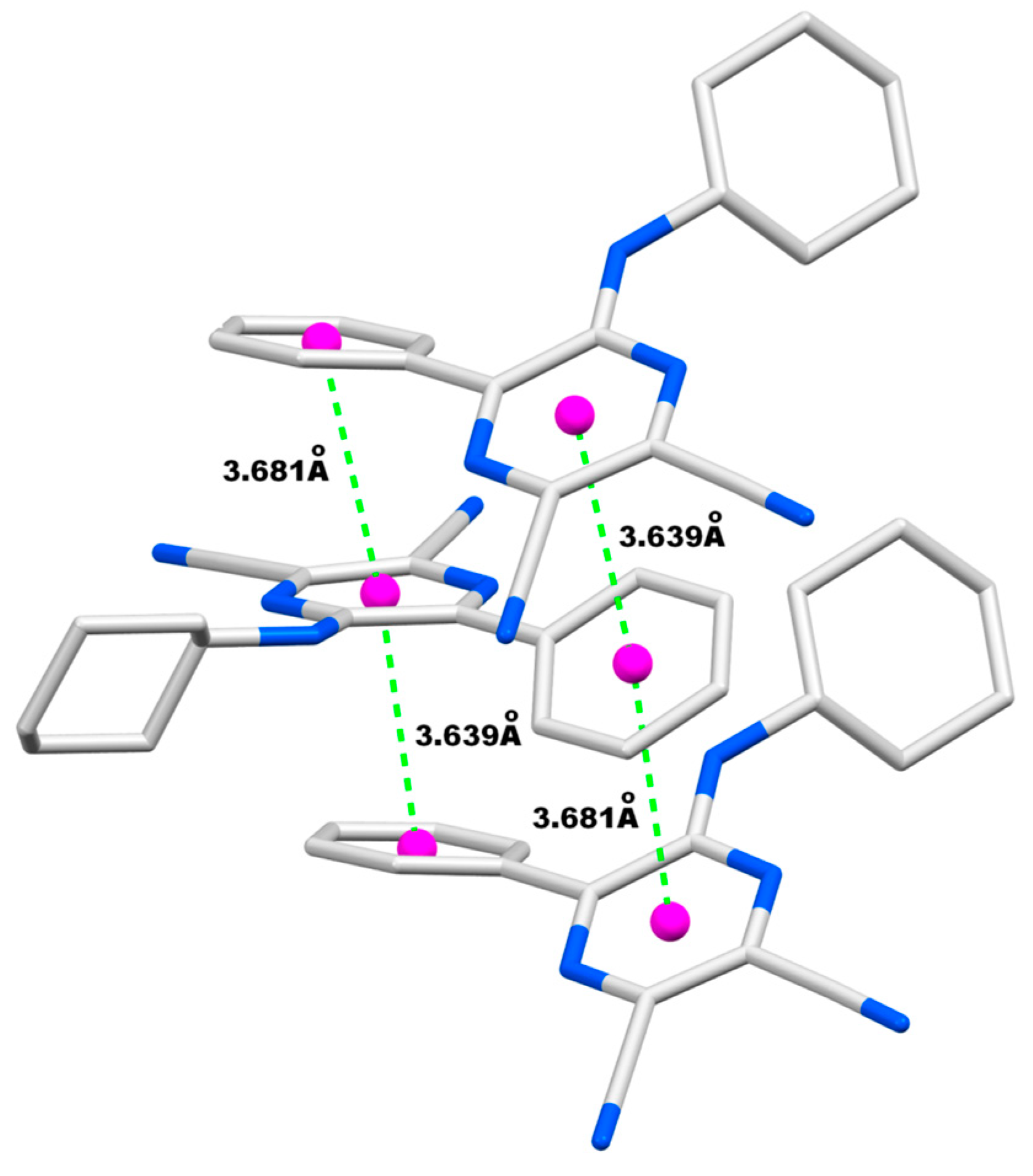

| Entry | R | R’ | 13 | Yield of 13% | 14 | Yield of 14% |
|---|---|---|---|---|---|---|
| 1 | C6H5 | Cyclohexyl | a | 88 | a | 8 |
| 2 | 4-CH3C6H4 | Cyclohexyl | b | 78 | - | |
| 3 | 4-ClC6H4 | Cyclohexyl | c | 83 | c | 10 |
| 4 | 4-CH3OC6H4 | Cyclohexyl | d | 80 | d | 10 |
| 5 | CH3 | Cyclohexyl | e | 72 | - | |
| 6 | 4-C6H5C6H4 | Cyclohexyl | f | 40 | f | 40 |
| 7 | CF3Py | Cyclohexyl | g | 87 | - | |
| 8 | CH3 | t-Bu | h | 37 | - | |
| 9 | C6H5 | t-Bu | i | 39 | i | 10 |
| 10 | 4-CH3OC6H4 | t-Bu | j | 41 | j | 15 |
| 11 | 4-C6H5C6H4 | t-Bu | k | 43 | k | 45 |
| Compound | Excitation at 267 nm λmax (Intensity in CPS) | Excitation at 303 nm λmax (Intensity in CPS) | Excitation at 373 nm λmax (Intensity in CPS) |
|---|---|---|---|
| 13a | 454 (4 × 105) | 454 (2.5 × 106) | 454 (3 × 106) |
| 13b | 455 (4 × 105) * | 455 (2.5 × 106) | 455 (3.7 × 106) |
| 13c | 457 (8 × 105) | 458 (4 × 106) | 457 (6 × 106) |
| 13d | 458 (2.5 × 105) | 459 (1.3 × 106) | 459 (2.5 × 106) |
| 13e | 414 (1.1 × 105) | 413 (2 × 106) | 421 (0.5 × 106) |
| 13f | 450 (2 × 105) | 456 (1.1 × 106) | 445 (2.3 × 106) |
| 13g | 358 (0.8 × 105) | 352 (0.25 × 106) | 421 (0.2 × 106) |
| 13h | 408 (1.1 × 105) | 406 (1.2 × 106) | 422 (0.4 × 106) |
| 13i | 490 (2.5× 105) | 486 (0.8 × 106) | 487 (0.97 × 106) |
| 13j | 368 (0.6 × 105) | 359 (0.17 × 106) | 420 (0.2 × 106) |
| 13k | 456 (3.5 × 105) | 461 (1.9× 106) | 458 (3.5 × 106) |
| Sample (1 mg/mL) | Inhibition Zone for Diameter (mm) for Bacteria, Mean (Min–Max) SD | |||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | |
| 13a | 0 | 12.7 (12–15) 0.9 | 0 | 0 |
| 13b | 0 | 0 | 0 | 0 |
| 13c | 0 | 0 | 0 | 0 |
| 13d | 0 | 13.4 (12–18) 2.3 | 0 | 0 |
| 13e | 0 | 12.7 (11–13) 0.2 | 0 | 0 |
| 13f | 0 | 11.5 (11–12) 0.2 | 0 | 0 |
| 13g | 0 | 0 | 0 | 0 |
| 13h | 0 | 0 | 0 | 0 |
| 13i | 0 | 0 | 0 | 0 |
| 13j | 0 | 0 | 0 | 0 |
| 13k | 0 | 0 | 0 | 0 |
| (DMSO) | 0 | 0 | 0 | 0 |
| * Ampicillin | 34.33 (34–35) 0.5 | nil | 22.66 (22–23) 0.5 | 33.66 (33–34) 0.5 |
| * Kanamycin | 25.33 (25–26) 0.5 | 12.33 (12–13) 0.5 | 25.33 (25–26) 0.5 | 31 (31)0 |
| * Penicillin G | 23.66 (23–24) 0.5 | nil | 21.66 (21–23)1.1 | 33.66 (33–35) 1.1 |
| ** Cycloheximide | NA | NA | NA | NA |
| Sample (1 mg/mL) | Inhibition Zone for Diameter (mm) for Yeast, Mean (Min–Max) SD | |
|---|---|---|
| C. albicans | S. cerevisiae | |
| 13a | 0 | 0 |
| 13b | 9.5(9–10)0.5 | 0 |
| 13c | 0 | 0 |
| 13d | 0 | 0 |
| 13e | 0 | 0 |
| 13f | 8.6(8–10)0.6 | 0 |
| 13g | 0 | 0 |
| 13h | 0 | 0 |
| 13i | 0 | 0 |
| 13j | 0 | 0 |
| 13k | 0 | 0 |
| Negative control (DMSO) | 0 | 0 |
| * Ampicillin | NA | NA |
| * Kanamycin | NA | NA |
| * Penicillin G | NA | NA |
| ** Cycloheximide | 0 | 52.33 (51–53) 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Azmi, A.; John, E. Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules 2022, 27, 8278. https://doi.org/10.3390/molecules27238278
Al-Azmi A, John E. Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules. 2022; 27(23):8278. https://doi.org/10.3390/molecules27238278
Chicago/Turabian StyleAl-Azmi, Amal, and Elizabeth John. 2022. "Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation" Molecules 27, no. 23: 8278. https://doi.org/10.3390/molecules27238278
APA StyleAl-Azmi, A., & John, E. (2022). Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules, 27(23), 8278. https://doi.org/10.3390/molecules27238278





