Abstract
Based on some homodrimane carboxylic acids and their acyl chlorides, a series of fourteen 2-homodrimenyl-1,3-benzothiazoles, N-homodrimenoyl-2-amino-1,3-benzothiazoles, 4′-methyl-homodrimenoyl anilides and 4′-methyl-homodrimenthioyl anilides were synthesized and their biological activities were evaluated on five species of fungi (Aspergillus niger, Fusarium solani, Penicillium chrysogenum, P. frequentans, and Alternaria alternata) and two strains of bacteria (Bacillus sp. and Pseudomonas aeruginosa). The synthesis involved the decarboxylative cyclization, condensation and thionation of the said acids, anhydrides or their derivatives with 2-aminothiophenol, 2-aminobenzothiazole, p-toluidine and Lawesson’s reagent. As a result, together with the desired compounds, some unexpected products 8, 25, and 27 were obtained, and the structures and mechanisms for their formation have been proposed. Compounds 4, 9, and 25 showed higher antifungal and antibacterial activity compared to the standards caspofungin (MIC = 1.5 μg/mL) and kanamycin (MIC = 3.0 μg/mL), while compound 8 had comparable activities. In addition, compounds 6, 17, and 27 showed selective antifungal activity at MIC = 2.0, 0.25, and 1.0 μg/mL, respectively.
1. Introduction
Natural labdane-type diterpenes isolated from terrestrial plants and marine sources are still interesting objects of study due to a wide range of their biological activities [1]. Some of them are obtained from sources in sufficient amounts to be used as precursors for the synthesis of natural analogs, special purpose compounds, or pharmaceutical agents with remarkable properties [2].
The chemistry of 1,3-benzothiazole and its 2-substituted derivatives has become a separate area of research due to a high degree of structural diversity, which generates a wide variety of their applications or pharmacological activities [3,4,5].
A wide variety of reagents and methods which lead to 2-substituted 1,3-benzothiazoles are known. One of the most requested methods of their synthesis involves the cyclocondensation of aromatic aldehydes or others carbonyl compounds such as carboxylic acids, esters, acyl halides, etc., with o-aminophenol [6,7] or its disulfides [8]. Frequently, for the conversion of the resulting amides into the corresponding thioamides, Lawesson’s reagent is used, but the course of reactions and their yields strongly depend on the structure of substrates [9].
The synthesis of terpeno-heterocyclic hybrid compounds with a cumulative biological potential is a new direction of organic chemistry that has emerged in the last decade. Research in this field has been successful, a large number of molecular hybrids containing both terpene and diazine [10,11], 1,2,4-triazole and carbazole [12,13], azaheterocyclic [14,15], hydrazinecarbothioamide and 1,2,4-triazole [16], 1,3,4-oxadiazole and 1,3,4-thiadiazole [17], thiosemicarbazone and 1,3-thiazole [18] units were reported, many of which showed excellent antifungal and/or antibacterial activity.
In continuation of our work aimed at the preparation of hybrid terpeno-heterocyclic compounds, herein, we report the result of the synthesis of novel homodrimane sesquiterpenoids bearing 2-substituted 1,3-benzothiazole, N-substituted 2-amino-1,3-benzothiazole and N-substituted p-toluidine units and their antimicrobial properties evaluation.
2. Results and Discussion
2.1. Synthesis and Characterization
According to the synthesis strategy of the desired compounds, at first, the intermediate carboxylic acids were obtained from commercial (+)-sclareolide (1). It was converted to methoxyester 2 in two steps, with an overall yield of 25%, applying the known procedure [19], followed by the saponification into acid 3 in 89% yield. Starting from sclareolide (1) carboxylic acids 5 and 7 were obtained in five and six steps, with overall yields of 81% and 62%, respectively [13,20] (Scheme 1).
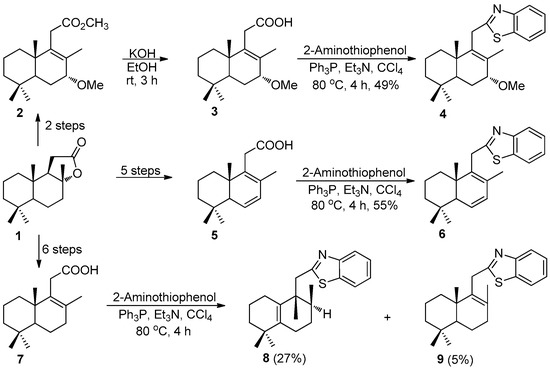
Scheme 1.
Synthesis of 2-homodrimenyl-1,3-benzothiazoles 4, 6, 8, and 9 from carboxylic acids 3, 5, 7 and 2-aminothiophenol.
Further, the one-pot decarboxylative cyclization reactions of acids 3, 5, and 7 with 2-aminothiophenol promoted by triphenylphosphine and triethylamine [21] were performed under reflux for 4 h, which after silica gel chromatography afforded 2-homodrimenyl-1,3-benzothiazoles 4, 6, 8, and 9, in the yields as depicted in Scheme 1.
The structures of intermediary compounds as well as final products were confirmed by 1H, 13C, 15N, and 2D NMR spectroscopy and HRMS analysis. The formation of the desired hybrid compounds 4, 6, 8, and 9 was proved, first of all, with the presence of signals attributed to aromatic protons from a common 2-substituted-1,3-benzothiazole unit in a range of 7.32–7.96 ppm. In addition, some individual signals such as a singlet corresponding to protons of C7-bonded methoxy group at 3.39 ppm, or doublet of doublets of C6- and C7-bonded protons at 5.88 and 5.95 ppm confirmed the presence of a terpene unit. Those structures were fully confirmed by the carbon spectral data.
It should be noted that, in the case of acid 7, surprisingly, in addition to the desired compound 9, obtained with a yield of only 5%, the compound 8 with an unexpected structure was afforded as a major reaction product, in 27% total yield. The rearrangement of the carbon skeleton of compound 8 was confirmed by a shift of some signals in the 1H NMR spectrum compared to the starting acid 7, e.g., by singlet signals of the C8- and C9-bonded methyl groups at 0.92 and 1.05 ppm and the appearance of new multiplet signals of the C8-bonded proton at 1.54–1.56 ppm. The 13C NMR spectra confirmed this by signals of the C8 (34.5 ppm) and C9 (42.1 ppm), those at 130.8 and 139.4 ppm being attributed to C10 and C5, respectively.
The NMR data of compound 8 have been assigned on the basis of their 1D (1H, 13C, DEPT-135°) and 2D homo- (1H/13C HSQC, 1H/13C HMBC and 1H/1H COSY-45°) correlation spectra. An analysis of the 1H, 13C, 1H/1H COSY and 1H/13C HSQC NMR spectra suggested the presence of two isolated spin systems: CH2CH2CH2 (C1 to C3) and CH2CH2CH (C6 to C8) (Figure 1). The rearranged carbon framework of compound 8 was indisputable according to a detailed analysis of its 1H/13C HMBC spectrum. Thus, the observed correlations of H2-C2 with two sp2 hybridized carbons (C5, δC 139.4 and C10, δC 130.8) were indicative of the Δ5,10 double bond localization, which was also supported by the correlations of H3-C18/C5, H3-C9/C10 and H2-C11/C10. The migration of H3-C20 methyl from the C10 to C9 position was ascertained by the H3-C20/C10, H3-C20/C8 and H3-C20/C9 and the H3-C20/C11 cross-peaks in the HMBC spectrum.
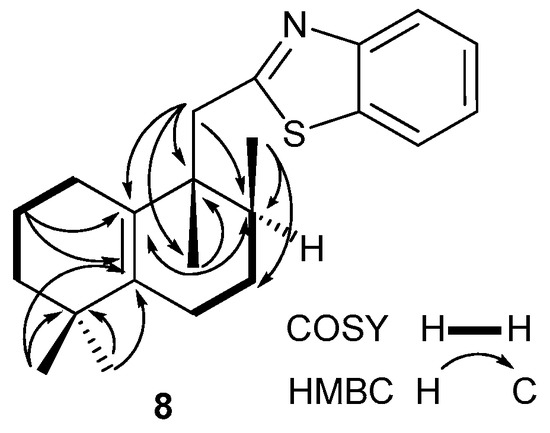
Figure 1.
Selected COSY and HMBC correlations for compound 8.
The rearrangement of 2-homodrimenyl-1,3-benzothiazole 8 carbon skeleton can be explained by the following reaction pathway (Scheme 2). The reaction started with formation of triphenylphosphonium chloride as product of Ph3P and CCl4 interaction, followed by its condensation with carboxylic acid which led to acylphosphonium intermediate 10. Next, the nucleophilic attack of the amino group to the carbonyl gave the intermediate amide 11 and triphenylphosphine oxide. Then, the nucleophilic attack of the deprotonated sulfur atom led to the unstable cyclic intermediate 12. Next, the formation of compound 8 was a result of the elimination reaction, which led to the desired 2-homodrimenyl-1,3-benzothiazole 9 that by protonation gave carbocation 13. The latter suffered a rearrangement of the carbon skeleton as a result of the C10-bonded methyl group migration to C9, followed by both C5 deprotonation and C5-C10 double bond formation.
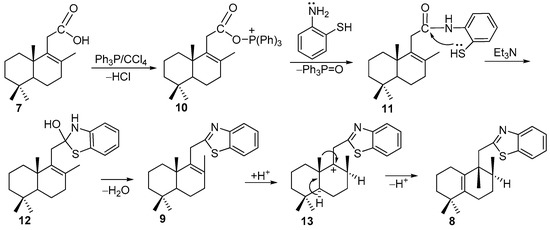
Scheme 2.
A plausible reaction pathway of formation of compound 8.
Next, a series of new N-homodrimenoyl-2-amino-1,3-benzothiazoles were prepared, starting from the intermediate carboxylic acids 3, 5, 7, and 20, via their acyl chlorides 14, 16, 18, and 21, generated in situ in 25–65% yields. It should be mentioned that the acid 20 was obtained from the commercially available (+)-sclareolide (1) in 6 steps, with an overall yield of 60%, according to the known method [11]. The desired N-substituted 2-amino-1,3-benzothiazoles 15, 17, 19, and 22 were obtained with yields between 40–84% by acylation of 2-amino-1,3-benzothiazole with the mentioned sesquiterpene acyl chlorides under the mentioned conditions (Scheme 3).
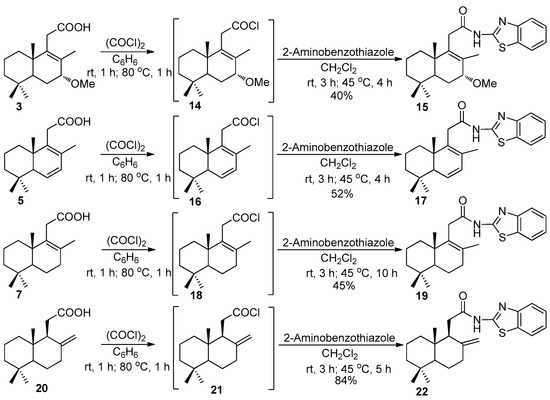
Scheme 3.
Synthesis of N-substituted 2-amino-1,3-aminobenzothiazoles 15, 17, 19, and 22 from carboxylic acids 3, 5, 7, 20 and 2-aminobenzothiazole.
According to the NMR spectra, the hybrids involved both heterocyclic and terpene units, and their accurate masses were confirmed by a high-resolution mass spectrometry (HRMS) analysis. All proton spectra of compounds 15, 17, 19, and 22, include the signals of aromatic protons in a range of 7.30–7.82 ppm, together with the signals specific for terpene unit such as singlets of C8-bonded methyl groups at 1.69–1.82 ppm and C7-bonded methoxy group at 3.40 ppm, C6- and C7-bonded protons at 3.50, 5.93, and 5.94 ppm, singlets of C17-exomethylene group at 4.39 and 4.73 ppm, and broad singlets of amidic protons in a range of 9.73–11.26 ppm. The structures of the reported N-substituted 2-amino-1,3-benzothiazoles were additionally confirmed by the 13C NMR spectra.
Then, effort was devoted to prepare 2-substituted 6-methyl-benzothiazoles starting from the carboxylic acids 3 and 5, as well as, from acyl chlorides 14, 16, 18 using p-toluidine. The one-pot condensation of homodrimane acyl chlorides 14, 16, and 18, generated in situ from acids 3, 5 and 7 (see Scheme 3) with p-toluidine, yielded amides 23, 26, and 28 in 50–54% yields (Scheme 4).
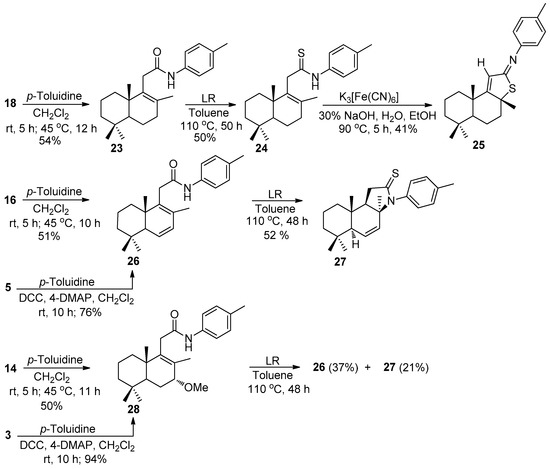
Scheme 4.
Synthesis of unexpected compounds 25 and 27.
An attempt to perform the direct amidation of acids 3 and 5 with p-toluidine in the presence of N,N′-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (4-DMAP) gave better results, because the yields of amides 26 and 28 increased up to 76% and 94%, respectively (Scheme 4). Together with the signals of protons from terpene units, the proton spectra of amides 23, 26, and 28 contained the singlets of C4′-bonded methyl in a range of 2.30–2.32 ppm and doublets of aromatic protons from 7.11 ppm to 7.37 ppm, and a broad singlet of the amidic proton at 7.53–7.70 ppm. The structures of the mentioned compounds were fully confirmed by the 13C NMR spectra.
After that, amides 23, 26, and 28 were submitted to the thionation reaction using Lawesson’s reagent (LR) in toluene [22]. In the case of amide 23, a reaction occurred, and thioamide 24 was obtained in 50% yield (Scheme 4). Its structure was confirmed by the NMR and HRMS analyses. In the 1H and 13C NMR spectra, down field shifts of the amidic proton to 9.03 ppm and of C12 to 200.9 ppm compared to the initial amide 23 were observed.
Heterocyclization of thioamide 24 performed in the presence of potassium ferricyanide under basic conditions (30% NaOH) [22] did not lead to the desired 2-substituted 6-methyl-benzothiazole and gave an unexpected compound 25 in 41% yield (Scheme 4). Its structure was elucidated based on the NMR and HRMS spectra. Comparing with the initial thioamide 24, its 1H NMR spectra did not contain the signals of an amidic proton and of one of C11-bonded proton, but the singlets of a C8-bonded methyl group and one of C11-H were shifted to 1.72 and 6.19 ppm, respectively. The same is true of the carbon spectrum, where some signals are strongly shifted, e.g., C8 (62.9 ppm), C11 (123.6 ppm), C9 (170.4 ppm), and C12 (176.6 ppm).
The analysis of the structure of compound 25 by 1H, 13C, 1H/1H COSY and 1H/13C HSQC NMR spectra suggested the presence of two isolated spin systems: CH2CH2CH2 (C1 to C3) and CHCH2CH2 (C5 to C7) (Figure 2). In the 1H/13C HMBC spectrum correlations of H-C11 with quaternary carbons (C9, δC 170.4 and C12, δC 176.6) were observed, which was also supported by the correlations of H-C11/C8. In addition, the correlation of H3-C17/C12 confirms the formation of the 5-membered heterocycle.
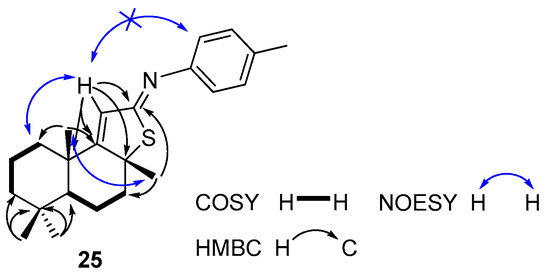
Figure 2.
Selected COSY, HMBC, and NOESY correlations for compound 25.
In the NOESY spectrum of compound 25, there is no NOE correlation between C11-H and C2′-H from the aromatic ring (Figure 2) that clearly indicates the E-configuration for C12 = N- double bond.
The thionation of amide 26 under the same conditions occurred and gave an unexpected cyclic thioamide 27 in 52% yield. The formation of the N-C8 bond was confirmed by the absence of an amidic proton signal, a shift of the singlet signal of C8-CH3 to 1.56 ppm and by the appearance of the C9-H doublet at 1.88 ppm. The upfield chemical shift of C8 and C9 atoms to 59.2 and 58.2 ppm and a downfield shifted signal of C12 (175.9 ppm) in the 13C NMR spectra also confirmed the structure of compound 27.
Structural analysis of compound 27 by 1H, 13C, 1H/1H COSY and 1H/13C HSQC NMR spectra suggested the presence of two isolated spin systems: CH2CH2CH2 (C1 to C3) and CH2CHCH (C5 to C7) (Figure 3). The rearranged carbon framework of compound 27 was indisputable by a detailed analysis of its 1H/13C HMBC spectrum. Thus, the observed correlations of H-C5 with two sp2 hybridized carbons (C6, δC 126.8 and C7, δC 130.6) were indicative of the Δ6,7 double bond localization, which was also supported by the correlations of H3-C17/C7. The relative configuration at C17 was deduced from the absence of H3-C17/H3-C10 NOESY correlation. The position of N has been confirmed by the 1H/15N HMBC spectra and was supported by the correlations of H2-C11/N and H-C2′/N cross-peaks (Figure 3).
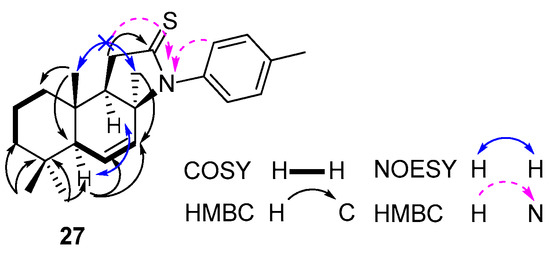
Figure 3.
Selected COSY, HMBC, NOESY, and HMBC correlations for compound 27.
However, in the case of amide 28, from the thionation reaction mixture, the same amides 26 and 27 were isolated in 37% and 21% yields, respectively (Scheme 4). Their spectral data were in accordance with those obtained earlier.
Returning to the compound 25, it can be said that its formation in place of the desired benzothiazole is due to the reaction conditions and the molecular structure of thioamide 24 which permits the existence of a tautomeric thioketo-enothiol 24<—>29 system. In the basic medium, the tautomer 29 easily generated enothiolate which due to the activation by hexacyanofferate ions attacked the C8-C9 double bond and generated the intermediate carbocation 30 (Scheme 5). In such a way, the formation the new C8-S bond occurred simultaneously with the one C11-proton elimination giving compound 25.
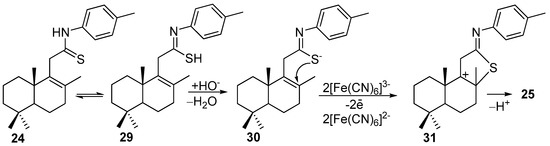
Scheme 5.
Plausible reaction pathway for formation of compound 25.
The formation of compound 27 can be explained by a sequence of transformations depicted in Scheme 6. In this case, the Lawesson’s reagent played a double role. The first role is to interact with axial the C7–bonded methoxy group of the amide 28, stimulating its elimination and generating the carbocation 32 which by deprotonation offered amide 26 (see Scheme 4 and Scheme 6). On the other hand, the thionation with Lawesson’s reagent gave an intermediate carbocation 33 which suffered a cyclization followed by deprotonation into cyclic thioamide 27. Note that there are several resonance structures, but from our point of view, intermediates 32 and 33 are more stable.
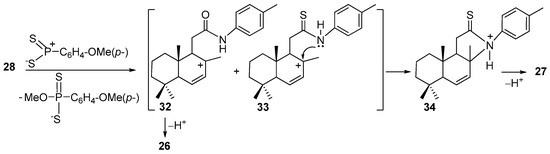
Scheme 6.
Plausible reaction pathway for formation of compound 27.
2.2. Antimicrobial Activity
All synthesized compounds were subjected to preliminary screening for their in vitro antifungal and antibacterial activities [23] against pure cultures of fungal species Aspergillus niger, Fusarium solani, Penicillium chrysogenum, Penicillium frequentans, and Alternaria alternata and both Gram-positive Bacillus sp. and Gram-negative Pseudomonas aeruginosa bacteria strains. The obtained minimum inhibitory concentration (MIC) values revealed that compounds 4 and 17 possessed a high nonselective antifungal (MIC 0.094 and 0.25 µg/mL, respectively) activity (Table 1, entries 1 and 6) in comparison with caspofungin. Moreover, compounds 6, 8, 9, 25, and 27 possessed a promising antifungal activity (Table 1, entries 2–4, 11 and 13) at MIC in a range from 0.95 to 2 µg/mL, vs. the same standard. At the same time, compounds 4 and 25 possessed high nonselective antibacterial (MIC 0.75 and 1.5 µg/mL, respectively) activities (Table 1, entries 1 and 11) relative to the standard kanamycin. Compounds 8, 9, and 17 possessed a moderate antibacterial activity (Table 1, entries 3, 4, and 11). As to compounds 15, 19, 22, 23, 24, 26, and 28, they were biologically inactive.

Table 1.
In vitro antifungal and antibacterial activities of compounds 4, 6, 8, 9, 15, 17, 19, 22–28.
In conclusion, it can be mentioned that the greatest antimicrobial activity was presented by homodrimane sesquiterpenoids bearing benzothiazole units, as well as those containing the NCS fragment, rigidly bound in space with the involvement of another ring, which sterically creates a stable bond similar to that in benzothiazole.
3. Materials and Methods
3.1. Synthesis and Characterization
The IR spectra were recorded on a Spectrum 100 FT-IR spectrometer (Perkin-Elmer, Shelton, CT, USA) using an ATR technique. The 1H, 13C, and 15N NMR (400, 100, and 40 MHz, respectively) and COSY, 1H–13C HSQC, 1H–13C HMBC, DEPT, and 1H–15N HSQC, 1H–15N HMBC spectra were acquired on a Bruker Avance DRX 400 spectrometer (Bruker BioSpin, Rheinstetten, Germany) in CDCl3 (NMR spectra for all the compounds are available online, see the Supplementary Materials). The 1H NMR chemical shifts were reported relative to the residual solvent protons as internal standards (7.26 ppm). The solvent carbon atoms served as internal standard for the 13C NMR spectra (77.0 ppm). The 15N NMR spectra were obtained using MeNO2 (380.5 ppm) and urea (73.4 ppm) as internal standards. Optical rotations measurements were performed on a Jasco DIP-370 polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA) with a 10 cm microcell. Melting points were determined on a Boetius (VEB Analytik, DDR) hot stage apparatus and were not uncorrected. The progress of reactions and purity of products were examined by TLC on Merck silica gel 60 plates, eluent CH2Cl2 or a mixture of CH2Cl2–MeOH, 99:1; 49:1. Visualization was achieved by the treatment with conc. H2SO4 and heating at 80 °C or using an UV lamp (254 or 365 nm). All solvents were purified and dried by standard techniques prior to use.
Compound 3: Compound 2 (294 mg, 1 mmol) was dissolved in EtOH (10 mL) and solid KOH (615 mg, 11 mmol) was added. The resulting mixture was heated at 50 °C for 3 h, and then, 2/3 of alcohol was distilled under reduced pressure on a rotary evaporator. The residue was diluted with H2O (20 mL), acidified with 40% HCl (20 mL) and extracted with Et2O (3 × 20 mL). The organic layer was washed with H2O (30 mL), dried over anhydrous Na2SO4 and concentrated, giving compound 3 (249 mg, 89% yield) as a colorless oil. +50.6 (c 2.4, CHCl3). IR spectrum, ν, cm−1: 736, 1070, 1376, 1459, 1626, 1704, 2927. 1H NMR (400 MHz, CDCl3) δ 0.82 (3H, s, 10-CH3), 0.87 (3H, s, 4-CH3), 0.88 (3H, s, 4-CH3), 1.09–1.15 (2H, m, CH2), 1.16–1.19 (1H, m, CH2), 1.35–1.58 (5H, m, H-5, 2CH2), 1.64 (3H, s, 8-CH3), 1.91–1.94 (1H, m, CH2), 3.01 (2H, d, J = 7.0 Hz, H-11), 3.32 (3H, s, 7-CH3), 3.43 (1H, d, J = 2.6 Hz, H-7). 13C NMR (100 MHz, CDCl3) δ 17.7 (C-17), 17.9 (C-20), 18.7 (C-2), 21.5 (C-18), 22.5 (C-6), 32.7 (C-19), 32.8 (C-4), 33.0 (C-11), 35.9 (C-1), 39.3 (C-10), 41.1 (C-3), 45.7 (7-OCH3), 56.4 (C-5), 79.4 (C-7), 130.0 (C-8), 139.1 (C-9), 171.9 (C-12). HRMS (ESI) calculated for C17H28O3 [M + H]+, 280.4035. Found: 280.4127.
Compounds 4, 6, 8, and 9 (General method).
To an ice bath-cooled solution of Ph3P (786 mg, 3 mmol) and Et3N (0.16 mL, 1.2 mmol) dissolved in CCl4 (7 mL), one of the acids 3 (280 mg, 1 mmol), 5 (248 mg, 1 mmol) or 7 (250 mg, 1 mmol) was added. After 10 min of stirring, the solution of 2-aminothiophenol (150 mg, 1.2 mmol) dissolved in CCl4 (3 mL) was added and the reaction mixture was refluxed under stirring for 4 h. The solvents were removed under a reduced pressure on a rotary evaporator to dryness and crude reaction products were subjected to silica gel flash chromatography (CH2Cl2).
Compound 4. (180 mg, 49%), colorless oil. 78.3 (c 0.6, CHCl3). IR spectrum, ν, cm−1: 729, 758, 1080, 1373, 1437, 1456, 1509, 1707, 1759, 2926. 1H NMR (400 MHz, CDCl3) δ 0.86 (3H, s, 10-CH3), 0.93 (3H, s, 4-CH3), 0.99 (3H, s, 4-CH3), 1.09–1.16 (2H, m, CH2), 1.23–1.27 (1H, m, CH2), 1.35–1.40 (2H, m, CH2), 1.51–1.55 (3H, m, H-5, CH2), 1.83 (3H, s, 8-CH3), 2.01–2.05 (1H, m, CH2), 3.43 (3H, s, 7-CH3), 3.51 (1H, t, J = 2.7 Hz, 7-CH), 3.88 (2H, t, J = 17.4 Hz, H-11), 7.31 (1H, dt, J = 7.5, 1.0 Hz, H-6′), 7.43 (1H, dt, J = 7.7, 1.0 Hz, H-7′), 7.78 (1H, d, J = 7.8 Hz, H-5′), 7.93 (1H, d, J = 8.0 Hz, C-8′). 13C NMR (100 MHz, CDCl3) δ 18.6 (C-20 and C-17), 18.7 (C-2), 21.7 (C-18), 22.7 (C-6), 32.8 (C-19), 32.8 (C-11), 32.9 (C-4), 36.0 (C-1), 39.9 (C-10), 41.2 (C-3), 46.0 (7-OCH3), 56.9 (C-5), 79.3 (C-7), 121.5 (C-5′), 122.3 (C-8′), 124.4 (C-6′), 125.7 (C-7′), 131.1 (C-8), 135.1 (C-9), 142.5 (C-4′), 153.4 (C-9′), 173.6 (C-2′). 15N NMR (400 MHz, CDCl3) δ 301. HRMS (ESI) calculated for C23H31NOS [M + H]+, 369.5667. Found: 369.5693.
Compound 6. (185 mg, 55%), yellow oil. −260.94 (c 0.59, CHCl3). IR spectrum, ν, cm−1: 729, 757, 1369, 1456, 1508, 1726, 2924. 1H NMR (400 MHz, CDCl3) δ 0.88 (3H, s, 10-CH3), 0.95 (3H, s, 4-CH3), 0.96 (3H, s, 4-CH3), 1.10–1.82 (6H, m, 3CH2), 1.86 (3H, s, 8-CH3), 2.15 (1H, t, J = 2.9 Hz, H-5), 3.85 (1H, d, J = 16.7 Hz, H-11), 3.96 (1H, d, J = 16.7 Hz, H-11), 5.88 (1H, dd, J = 9.5 Hz, J = 2.5 Hz, H-6), 5.95 (1H, dd, J = 9.5, 3.0 Hz, H-7); 7.33 (1H, ddd, J = 7.5, 7.2 Hz, J = 1.1 Hz, H-6′), 7.44 (1H, ddd, J = 8.1, 7.2, 1.2 Hz, H-7′), 7.82 (1H, dm, J = 8.3 Hz, H-5′), 7.96 (1H, dm, J = 8.1 Hz, H-8′). 13C NMR (100 MHz, CDCl3) δ 15.5 (C-20), 18.5 (C-8), 18.8 (C-2), 22.7 (C-18), 32.1 (C-11), 32.3 (C-19), 32.9 (C-4), 35.1 (C-1), 39.2 (C-10), 40.8 (C-3), 52.9 (C-5), 121.4 (C-5′), 122.4 (C-8′), 124.5 (C-6′), 125.7 (C-7′), 128.7 (C-6), 129.1 (C-7), 135.4 (C-8), 139.9 (C-9), 139.9 (C-9′), 153.6 (C-4′), 174.0 (C-2′). 15N NMR (400 MHz, CDCl3) δ 303. HRMS (ESI) calculated for C22H27NS [M + H]+, 337.1864. Found: 337.1949.
Compound 8. (92 mg, 27%), mp 58–59 °C, 7.63 (c 3.2, CHCl3). IR spectrum, ν, cm−1: 737, 763, 1122, 1311, 1377, 1433, 1454, 1505, 1713, 2920, 3059. 1H NMR (400 MHz, CDCl3) δ 0.92 (3H, d, J = 6.8 Hz, 8-CH3), 1.01 (3H, s, 4-CH3), 1.02 (3H, s, 4-CH3), 1.05 (3H, s, 9-CH3), 1.30–1.40 (2H, m, CH2), 1.46–1.63 (5H, m, H-8, 2CH2), 1.87–2.04 (2H, m, H-6), 2.17–2.22 (2H, m, CH2), 3.27 (2H, d, J = 3.9 Hz, H-11), 7.32 (1H, td, J = 7.8, 1.2 Hz, H-6′), 7.42 (1H, td, J = 7.0, 1.2 Hz, H-7′), 7.83 (1H, dm, J = 8.0 Hz, H-5′), 7.96 (1H, d, J = 8.0 Hz, H-8′). 13C NMR (100 MHz, CDCl3) δ 16.2 (C-17), 20.1 (C-2), 21.4 (C-20), 24.9 (C-6), 26.6 (C-1), 27.0 (C-7), 27.8 (C-18), 28.3 (C-19), 34.5 (C-8); 34.7 (C-4), 39.6 (C-3), 42.1 (C-11, C-9), 121.3 (C-5′), 122.5 (C-8′), 124.5 (C-6′), 125.6 (C-7′), 130.8 (C-10), 135.8 (C-9′), 139.4 (C-5), 152.1 (C-4′), 169.5 (C-2′). 15N NMR (400 MHz, CDCl3) δ 305. HRMS (ESI) calculated for C22H29NS [M + H]+, 339.2021. Found: 339.2106.
Compound 9. (17 mg, 5%), yellow oil. +28.62 (c 0.2, CHCl3). IR spectrum, ν, cm−1: 729, 757, 1014, 1124, 1146, 1293, 1376, 1435, 1456, 1506, 1577, 1694, 2865, 2926. 1H NMR (400 MHz, CDCl3) δ 0.84 (3H, s, 4-CH3), 0.90 (3H, s, 4-CH3), 1.02 (3H, s, 10-CH3), 1.05–1.11 (2H, m, CH2), 1.25–1.40 (3H, m, H-5, CH2), 1.45–1.60 (4H, m, 2CH2), 1.70 (3H, s, 8-CH3), 2.09–2.21 (2H, m, H-7), 3.83 (1H, d, J = 17.2 Hz, H-11), 3.90 (1H, d, J = 17.2 Hz, H-11), 7.32 (1H, ddd, J = 7.9 Hz, J = 7.3, 1.1 Hz, H-6′), 7.43 (1H, ddd, J = 8.2, 7.3, 1.3 Hz, H-7′), 7.81 (1H, ddd, J = 7.9, 1.3, 0.5 Hz, H-5′), 7.94 (1H, d, J = 8.2 Hz, H-8′). 13C NMR (100 MHz, CDCl3) δ 18.8 (C-6), 18.9 (C-2), 20.3 (C-20), 20.7 (C-17), 21.6 (C-18), 32.8 (C-11), 33.2 (C-19), 33.3 (C-4); 33.6 (C-7), 36.6 (C-1), 39.1 (C-10), 41.6 (C-3), 51.8 (C-5), 121.3 (C-5′), 122.4 (C-8′), 124.3 (C-6′), 125.6 (C-7′), 131.2 (C-8), 135.4 (C-9′), 137.7 (C-9), 153.6 (C-4′), 175.4 (C-2′). 15N NMR (400 MHz, CDCl3) δ 299. HRMS (ESI) calculated for C22H29NS [M + H]+, 339.2021. Found: 339.2107.
Compounds 15, 17, 19, and 22 (General method).
The solution of one of the acids 3 (280 mg, 1 mmol), 5 (248 mg, 1 mmol), 7 (250 mg, 1 mmol) or 20 (250 mg, 1 mmol) dissolved in anhydrous C6H6 (5 mL) was treated with a solution of (COCl)2 (0.95 mL, 11 mmol) dissolved in C6H6 (2.5 mL). The reaction mixture was stirred at room temperature for 1 h and subsequently refluxed for 1 h. The C6H6 and excess of (COCl)2 were removed at a reduced pressure on a rotary evaporator. Next, 2-aminobenzothiazole (225 mg, 1.5 mmol) was added to the solution of an acyl chloride 14, 16, 18 or 21 in CH2Cl2 (10 mL), and the resulting mixtures were stirred at r.t. for 3 h, then refluxed for 4–10 h. After cooling, the precipitates were filtered off, washed with CH2Cl2, and the filtrates were concentrated to dryness at a reduced pressure on a rotary evaporator. The crude reaction products were purified by silica gel flash chromatography (1 → 2% MeOH-CH2Cl2).
Compound 15. (164 mg, 40%), white crystals, mp 93–94 °C. 99.96 (c 1.4, CHCl3). IR spectrum, ν, cm−1: 728, 755, 1076, 1341, 1264, 1441, 1538, 1600, 1697, 2926, 3182. 1H NMR (400 MHz, CDCl3) δ 0.85 (3H, s, 10-CH3), 0.91 (3H, s, 4-CH3), 0.93 (3H, s, 4-CH3); 1.09–1.20 (2H, m, CH2), 1.37–1.58 (5H, m, H-5, 2CH2), 1.62–1.66 (1H, m, CH2), 1.77 (3H, s, 8-CH3), 2.01 (1H, d, J = 13.0 Hz, CH2), 3.22 (1H, d, J = 17.4 Hz, H-11), 3.32 (1H, d, J = 17.4 Hz, H-11), 3.40 (3H, s, 7-CH3), 3.50 (1H, d, J = 5.6 Hz, H-7), 7.30 (1H, dt, J = 7.5, 0.9 Hz, H-6′), 7.42 (1H, dt, J = 7.6, 1.0 Hz, H-7′), 7.77 (1H, d, J = 8.0 Hz, H-5′), 7.80 (1H, d, J = 7.7 Hz, H-8′), 9.91 (1H, br.s, NH). 13C NMR (100 MHz, CDCl3) δ 18.2 (C-20), 18.3 (C-17), 18.7 (C-2), 21.6 (C-18), 22.4 (C-6), 32.7 (C-19), 32.8 (C-4), 35.7 (C-11), 35.8 (C-1), 39.6 (C-10), 40.9 (C-3), 45.5 (7-CH3), 56.8 (C-5), 78.9 (C-7), 120.8 (C-5′), 121.3 (C-8′), 123.9 (C-6′), 126.2 (C-7′), 132.1 (C-4′), 132.7 (C-8), 138.9 (C-9), 148.1 (C-9′), 158.2 (C-2′), 169.6 (C = O). 15N NMR (400 MHz, CDCl3) δ 140, 259. HRMS (ESI) calculated for C24H32N2O2S [M-H]-, 412.2184. Found: 412.2112.
Compound 17. (23 mg, 52%), white crystals, mp 83–84 °C. −172.5 (c 0.1, CHCl3). IR spectrum, ν, cm−1: 728, 755, 908, 1147, 1267, 1343, 1442, 1536, 1599, 1702, 2925, 3178. 1H NMR (400 MHz, CDCl3) δ 0.84 (3H, s, 10-CH3), 0.96 (6H, s, 4-CH3, 4-CH3), 1.15–1.80 (6H, m, 3CH2), 1.83 (3H, s, 8-CH3), 2.10 (1H, t, J = 2.2 Hz, H-5), 3.21 (1H, d, J = 17.3 Hz, H-11), 3.42 (1H, d, J = 17.0 Hz, H-11), 5.93 (1H, d, J = 1.8 Hz, H-6), 5.94 (1H, d, J = 2.2 Hz, H-6), 7.31 (1H, dt, J = 1.0, 0.8 Hz, H-6′), 7.44 (1H, dt, J = 1.2, 1.0 Hz, H-7′), 7.75 (1H, d, J = 8.2 Hz, H-5′), 7.82 (1H, dd, J = 7.87, 0.47 Hz, H-8′), 9.74 (1H, br.s, NH). 13C NMR (100 MHz, CDCl3) δ 15.1 (C-20), 18. (C-17), 18.7 (C-2), 22.8 (C-18), 32.2 (C-19), 33.0 (C-4), 35.0 (C-1), 35.3 (C-11), 39.1 (C-10), 40.6 (C-3), 52.9 (C-5), 120.7 (C-5′), 121.4 (C-8′), 124.0 (C-6′), 126.3 (C-7′), 128.8 (C-6), 129.6 (C-7), 130.9 (C-9), 132.1 (C-4′), 135.7 (C-8), 148.0 (C-9′), 157.9 (C-2′), 169.9 (C-12). 15N NMR (400 MHz, CDCl3) δ 139, 254. HRMS (ESI) calculated for C23H28N2OS [M + H]+, 380.1922. Found: 380.2011.
Compound 19. (171 mg, 45%), white crystals, mp 84–85 °C. +102.5 (c 2.0, CHCl3). IR spectrum, ν, cm−1: 727, 755, 883, 907, 975, 1018, 1152, 1267, 1334, 1379, 1443, 1533, 1600, 1704, 1773, 2927, 3175, 3365. 1H NMR (400 MHz, CDCl3) δ 0.80 (3H, s, 4-CH3), 0.84 (3H, s, 4-CH3), 0.88 (3H, s, 10-CH3), 0.95–1.18 (4H, m, 2CH2), 1.27 (1H, dd, J = 12.6 Hz, J = 1.9 Hz, H-5), 1.33–1.57 (4H, m, 2CH2), 1.67 (3H, s, 8-CH3), 2.09 (1H, dd, J = 18.0 Hz, J = 6.4 Hz, H-7), 2.27 (1H, ddd, J = 18.4 Hz, J = 11.2 Hz, J = 7.4 Hz, H-7), 3.12 (1H, d, J = 17.6 Hz, H-11), 3.33 (1H, d, J = 17.6 Hz, H-11), 7.30 (1H, ddd, J = 8.0 Hz, J = 7.3 Hz, J = 1.0 Hz, H-7′), 7.42 (1H, ddd, J = 8.0 Hz, J = 7.3 Hz, J = 1.2 Hz, H-6′), 7.79 (1H, d, J = 8.0 Hz, H-5′), 7.81 (1H, ddd, J = 8.0 Hz, J = 1.2 Hz, J = 0.6 Hz, H-8′), 9.73 (1H, br.s., NH). 13C NMR (100 MHz, CDCl3) δ 18.7 (C-6), 18.8 (C-2), 19.7 (C-20), 20.4 (C-17), 21.6 (C-18), 33.1 (C-19), 33.3 (C-4), 33.5 (C-7), 35.9 (C-11), 36.3 (C-1), 38.8 (C-10), 41.3 (C-3), 51.5 (C-5), 120.7 (C-5′), 121.5 (C-8′), 124.0 (C-7′), 126.3 (C-6′), 132.1 (C-9′), 133.0 (C-9), 134.2 (C-8), 148.0 (C-4′), 158.4 (C-2′), 170.4 (C-12). 15N NMR (400 MHz, CDCl3) δ 137, 257. HRMS (ESI) calculated for C23H30N2OS [M + H]+, 382.2079. Found: 382.2165.
Compound 22. (320 mg, 84%), white crystals, mp 99–100 °C. −34.2 (c 2.0, CHCl3). IR spectrum, ν, cm−1: 750, 884, 998, 1018, 1135, 1157, 1215, 1270, 1292, 1324, 1332, 1365, 1383, 1443, 1457, 1542, 1599, 1644, 1697, 2932, 3063, 3178. 1H NMR (400 MHz, CDCl3) δ 0.46 (3H, s, 10-CH3), 0.76 (3H, s, 4-CH3), 0.84 (3H, s, 4-CH3), 0.99–1.18 (3H, m, H-5, CH2), 1.25 (1H, dd, J = 13.0, 4.4 Hz, H-6), 1.30–1.54 (4H, m, 2CH2), 1.68 (1H, dm, J = 13.0 Hz, H-6), 2.05 (1H, td, J = 13.0, 5.1 Hz, H-7), 2.33 (1H, ddd, J = 13.0, 4.0, 2.1 Hz, H-7), 2.45 (1H, dd, J = 11.2, 10.3 Hz, H-9), 2.47 (1H, dd, J = 6.2, 10.3 Hz, H-11), 2.63 (1H, dd, J = 26.2, 11.2 Hz, H-11), 4.39 (1H, s, 8-CH2), 4.73 (1H, s, 8-CH2), 7.33 (1H, ddd, J = 8.1, 7.1, 1.0 Hz, H-7′), 7.44 (1H, ddd, J = 8.2, 7.1, Hz, 1.1 Hz, H-6′), 7.81 (1H, dd, J = 8.2, 1.0 Hz, H-5′), 7.84 (1H, dd, J = 8.1, 1.1 Hz, H-8′), 11.26 (1H, br.s., NH). 13C NMR (100 MHz, CDCl3) δ 14.3 (C-20), 19.2 (C-2); 21.6 (C-18), 23.9 (C-6), 32.8 (C-11), 33.4 (C-4), 33.5 (C-19), 37.5 (C-7), 38.9 (C-1), 39.0 (C-10), 41.8 (C-3), 52.2 (C-9), 55.0 (C-5), 106.5 (8-CH2), 120.6 (C-5′), 121.7 (C-8′), 123.9 (C-7′), 126.3 (C-6′), 132.1 (C-9′), 147.8 (C-4′), 148.7 (C-8), 159.6 (C-2′), 171.8 (C-12). 15N NMR (400 MHz, CDCl3) δ 251 (C = N). HRMS (ESI) calculated for C23H30N2OS [M + H]+, 382.2079. Found: 382.2166.
Compounds 23, 26, and 28. (Typical procedure).
Method 1. The solution of one of the acids 3 (280 mg, 1 mmol), 5 (248 mg, 1 mmol) or 7 (250 mg, 1 mmol) dissolved in anhydrous C6H6 (5 mL) was treated with a solution of (COCl)2 (0.95 mL, 11 mmol) dissolved in C6H6 (2.5 mL). The reaction mixture was stirred at room temperature for 1 h, and additionally refluxed for 1 h. The C6H6 and excess of (COCl)2 were removed at reduced pressure on a rotary evaporator. Next, p-toluidine (160 mg, 1.5 mmol) was added to the solutions of acyl chlorides 14, 16 or 18 obtained in situ in CH2Cl2 (10 mL), and the resulting mixtures were stirred at r.t. for 5 h, and refluxed for 10–12 h. After cooling, the precipitate was filtered off, washed with CH2Cl2, and the filtrate was concentrated to dryness on a rotary evaporator. The crude reaction products were purified by silica gel flash chromatography (1→2% MeOH-CH2Cl2) to give products 23, 26, and 28.
Method 2. A solution of DCC (412 mg, 2 mmol), 4-DMAP (244 mg, 2 mmol), p-toluidine (214 mg, 2 mmol) and an acid 3 (280 mg, 1 mmol) or 5 (248 mg, 1 mmol) dissolved in CH2Cl2 (8 mL) was stirred for 10 h at room temperature. After the reaction period, the mixture was filtered, and the solvent was removed under a reduced pressure on a rotary evaporator to give the crude product which was purified by silica gel flash chromatography (CH2Cl2) to give compounds 26 and 28.
Compound 23. (183 mg, 54%), white crystals, mp 152–153 °C. +132.64 (c 1.0, CHCl3). IR spectrum, ν, cm−1: 733, 818, 908, 1171, 1248, 1346, 1405, 1458, 1516, 1603, 1661, 2867, 2927, 3293. 1H NMR (400 MHz, CDCl3) σσ 0.85 (3H, s, 4-CH3), 0.92 (3H, s, 4-CH3), 0.99 (3H, s, 10-CH3), 1.04–1.10 (2H, m, CH2), 1.17 (1H, dd, J = 12.6, 1.9 Hz, H-5), 1.40–1.62 (4H, m, 2CH2), 1.68 (3H, s, 8-CH3), 1.71–1.82 (2H, m, CH2), 2.08–2.26 (2H, m, H-7), 2.31 (3H, s, 4′-CH3), 3.04 (1H, d, J = 17.5 Hz, H-11), 3.22 (1H, d, J = 17.5 Hz, H-11), 7.12 (2H, d, J = 8.2 Hz, H-3′ and H-5′), 7.35 (2H, d, J = 8.3 Hz, H-2′ and H-6′), 7.53 (1H, br.s, NH). 13C NMR (100 MHz, CDCl3) δ 18.8 (C-6), 18.9 (C-2), 20.1 (C-20), 20.3 (C-17), 21.7 (C-18), 29.9 (C-7′), 33.3 (C-19), 33.4 (C-4), 33.7 (C-7), 36δ.3 (C-1), 37.2 (C-11), 39.0 (C-10), 41.6 (C-3), 52.4 (C-5), 119.9 (C-2′ and C-6′), 129.5 (C-3′ and C-5′), 132.1 (C-8), 133.9 (C-4′), 135.3 (C-1′), 136.4 (C-9), 169.6 (C-12). 15N NMR (400 MHz, CDCl3) δ 127. HRMS (ESI) calculated for C23H33NO [M + H]+, 339.2562. Found: 339.2649.
Compound 26. (256 mg, 76%), white crystals, mp 69–70 °C, −155.56 (c 0.69, CHCl3). IR spectrum, ν, cm−1: 817, 1177, 1607, 1245, 1351, 1454, 1513, 1542, 1653, 2927, 3292. 1H NMR (400 MHz, CDCl3) δ 0.87 (3H, s, 10-CH3), 0.97 (3H, s, 4-CH3), 0.99 (3H, s, 4-CH3), 1.10–1.62 (6H, m, 3CH2), 1.83 (3H, s, 8-CH3), 2.04 (1H, t, J = 2.5 Hz, H-5), 2.32 (3H, s, 4′-CH3), 3.08 (1H, d, J = 16.9 Hz, H-11), 3.31 (1H, d, J = 16.9 Hz, H-11),5.92 (1H, dd, J = 9.4, 2.4 Hz, H-6), 5.97 (1H, dd, J = 9.5, 2.7 Hz, H-7), 7.14 (2H, d, J = 8.2 Hz, H-2′ and H-6′), 7.37 (2H, d, J = 8.4 Hz, H-3′ and H-5′), 7.66 (1H, s, NH). 13C NMR (100 MHz, CDCl3) δ 15.0 (C-17), 18.4 (C-20), 18.7 (C-2); 20.8 (C-7′); 22.7 (C-18), 32.4 (C-19), 33.0 (C-4), 34.8 (C-1), 36.5 (C-11), 39.1 (C-10), 40.8 (C-3), 53.6 (C-5), 119.9 (C-2′ and C-6′), 128.9 (C-6), 129.4 (C-7), 129.7 (C-3′and C-5′), 129.9 (C-8), 135.1 (C-9), 138.1 (C-1′), 169.0 (C-12). 15N NMR (400 MHz, CDCl3) δ 125. HRMS (ESI) calculated for C23H31NO [M + H]+, 337.2406. Found: 337.2492.
Compound 28. (346 mg, 94%), white solid, mp 187–188 °C, +70.2 (c 0.39, CHCl3). IR spectrum, ν, cm−1: 816, 1086, 1243, 1310, 1448, 1536, 1571, 1624, 2928, 3322. 1H NMR (400 MHz, CDCl3) δ 0.87 (3H, s, 10-CH3), 0.93 (3H, s, 4-CH3), 0.95 (3H, s, 4-CH3), 1.10–1.19 (2H, m, CH2), 1.39–1.60 (5H, m, H-5 and 2CH2), 1.78 (3H, s, 8-CH3), 2.00–2.04 (2H, m, CH2), 2.30 (3H, s, 4′-CH3), 3.05 (1H, d, J = 17.6 Hz, H-11), 3.22 (1H, d, J = 17.6 Hz, H-11), 3.39 (3H, s, 7-CH3), 3.48 (1H, d, J = 2.6 Hz, H-7), 7.11 (2H, d, J = 8.2 Hz, H-2′ and H-6′), 7.35 (2H, d, J = 8.4 Hz, H-3′ and H-5′), 7.70 (1H, s, NH). 13C NMR (100 MHz, CDCl3) δ 18.2 (C-20), 18.4 (C-2), 18.6 (C-17), 20.8 (C-7′), 21.6 (C-18), 22.4 (C-6), 32.8 (C-19), 32.9 (C-4), 35.4 (C-1), 37.0 (C-11), 39.7 (C-10), 41.2 (C-3), 45.9 (7-OCH3), 56.8 (C-5), 79.0 (C-7), 120.1 (C-2′ and C-6′), 129.3 (C-3′ and 5′), 132.0 (C-8), 133.3 (C-4′), 135.1 (C-9), 140.8 (C-1′), 168.6 (C-12). 15N NMR (400 MHz, CDCl3) δ 128. HRMS (ESI) calculated for C24H35NO2 [M-31]+, 369.2668. Found: 338.2492.
Compounds 24 and 27. (Typical procedure)
To a solution of one of the amides 23 (339 mg, 1 mmol), 26 (337 mg, 1 mmol) or 28 (369 mg, 1 mmol) dissolved in toluene (8 mL), Lawesson’s reagent (203 mg, 0.5 mmol) was added and the reaction mixture was refluxed for 48–50 h. Then, the mixture was filtered, and the solvent was removed under a reduced pressure on a rotary evaporator to afford the crude reaction product, which was purified by silica gel flash column chromatography (1% MeOH-CH2Cl2).
Compound 24. (177 mg, 50%), white solid, mp 104–105 °C. +45.63 (c 0.5, CHCl3). IR spectrum, ν, cm−1: 730, 826, 852, 908, 998, 1056, 1066, 1267, 1395, 1406, 1453, 1516, 1599, 2052, 2214, 2972, 2987, 3147, 3246. 1H NMR (400 MHz, CDCl3) δ 0.86 (3H, s, 4-CH3), 0.91 (3H, s, 4-CH3), 1.00 (3H, s, 10-CH3), 1.03–1.14 (2H, m, CH2), 1.15 (1H, dd, J = 12.6, 2.0 Hz, H-5), 1.34–1.59 (4H, m, 2CH2), 1.67 (3H, s, 8-CH3), 1.72–1.88 (2H, m, CH2), 2.11–2.23 (2H, m, H-7), 2.36 (3H, s, 4′-CH3), 3.71 (2H, s, H-11), 7.22 (2H, d, J = 8.4 Hz, H-3′ and H-5′), 7.50 (2H, d, J = 8.4 Hz, H-2′ and H-6′), 9.03 (1H, s, NH). 13C NMR (100 MHz, CDCl3) δ 18.7 (C-2), 18.8 (C-6), 20.1 (C-20), 20.2 (C-17), 21.1 (4′-CH3), 21.6 (C-18), 33.2 (C-19), 33.4 (C-4), 33.6 (C-7), 36.2 (C-1), 39.2 (C-10), 41.6 (C-3), 47.8 (C-11), 52.5 (C-5), 123.8 (C-2′ and C-6′), 129.5 (C-3′ and C-5′), 134.2 (C-8), 136.2 (C-4′), 136.8 (C-9), 136.9 (C-1′), 200.9 (C = S). HRMS (ESI) calculated for C23H33NS [M + H]+, 355.2334. Found: 355.2419.
Compound 27. (176 mg, 52%), white solid, mp 103–105 °C. +155.73 (c 0.83, CHCl3). IR spectrum, ν, cm−1: 817, 1033, 1097, 1365, 1454, 1502, 1600, 1625, 2917. 1H NMR (400 MHz, CDCl3) δ 0.82 (3H, s, 4-CH3), 0.87 (3H, s, 4-CH3), 0.92 (3H, s, 10-CH3), 1.08–1.40 (2H, m, CH2), 1.46–1.55 (2H, m, CH2), 1.56 (3H, s, 8-CH3), 1.64–1.72 (2H, m, CH2), 1.88 (1H, d, J = 8.2 Hz, H-9), 2.23 (3H, s, H-7′), 3.07 (1H, d, J = 17.5 Hz, H-11), 3.19 (1H, dd, J = 17.5, 8.2 Hz, H-11), 5.62 (1H, dd, J = 10.1, 2.1 Hz, H-6), 5.66 (1H, dd, J = 10.1, 1.2 Hz, H-7), 6.75 (2H, d, J = 8.0 Hz, H-2′ and H-6′), 7.11 (2H, d, J = 8.0 Hz, H-3′ and H-5′). 13C NMR (100 MHz, CDCl3) δ 14.5 (C-20), 18.2 (C-2), 20.9 (C-7′), 21.6 (C-18), 32.5 (C-4), 33.9 (C-19), 33.9 (C-17), 37.6 (C-1), 37.7 (C-10), 40.7 (C-11), 40.9 (C-3), 51.9 (C-5), 58.2 (C-9), 59.2 (C-8), 119.9 (C-2′ and C-6′), 126.8 (C-6), 129.6 (C-3′ and C-5′), 130.6 (C-7), 133.4 (C-4′), 149.8 (C-1′), 175.9 (C-12). 15N NMR (400 MHz, CDCl3) δ 293. HRMS (ESI) calculated for C23H31NS [M + H]+, 353.5677. Found: 353.5748.
Compound 25. To a solution of carbothioamide 24 (355 mg, 1 mmol) dissolved in EtOH (9 mL), 30% NaOH (1 mL, 7.9 mmol) was added. The mixture was diluted with EtOH (20 mL) to give 10% NaOH. Portions of this mixture were added to a stirred solution of K3[Fe(CN)6] (1.3 g, 3.9 mmol) in H2O (2 mL) at 85 °C. The resultant mixture was further heated at 85 °C for 5 h and filtered to isolate a light yellow solid (720 mg) K4[Fe(CN)6]·3H2O. Then, the solvent was removed in vacuo from the filtrate. To the residue, H2O (20 mL) was added and the obtained mixture was extracted with CH2Cl2 (2 × 30 mL). The combined extracts were washed with H2O (2 × 20 mL), dried over MgSO4, and the solvent was removed to afford an orange oil. The crude reaction product was purified by flash column chromatography (SiO2, elution CH2Cl2) to give compound 25.
Compound 25. (145 mg, 41%), yellow oil. −92.68 (c 2.0, CHCl3). IR spectrum, ν, cm−1: 730, 824, 851, 909, 1004, 1127, 1147, 1169, 1201, 1249, 1306, 1379, 1451, 1504, 1594, 1618, 2868, 2927. 1H NMR (400 MHz, CDCl3) δ 0.89 (3H, s, 4-CH3), 0.91 (3H, s, 4-CH3), 1.07 (1H, dd, J = 12.5 Hz, J = 2.5 Hz, H-5), 1.19–1.22 (1H, m, CH2), 1.23 (3H, s, 10-CH3), 1.42–1.50 (2H, m, CH2), 1.58–1.65 (2H, m, CH2), 1.72 (3H, s, 8-CH3), 1.78–1.95 (4H, m, 2CH2), 2.22–2.27 (1H, m, CH2), 2.33 (3H, s, 4′-CH3), 6.19 (1H, s, H-11), 6.98 (2H, d, J = 8.0 Hz, H-3′ and H-5′), 7.14 (2H, d, J = 8.0 Hz, H-2′ and H-6′). 13C NMR (100 MHz, CDCl3) δ 18.6 (C-6); 19.6 (C-20), 19.9 (C-2), 21.0 (C-7′), 21.6 (C-18), 29.4 (C-17), 33.3 (C-19), 34.0 (C-4), 39.0 (C-1), 41.2 (C-10), 41.7 (C-3), 43.2 (C-7), 55.2 (C-5), 62.9 (C-8), 120.5 (C-2′ and C-6′), 123.6 (C-11), 129.6 (C-3′ and C-5′), 134.0 (C-4′), 148.9 (C-1′), 170.4 (C-9), 176.6 (C-12). 15N NMR (400 MHz, CDCl3) δ 191. HRMS (ESI) calculated for C23H31NS [M + H]+, 353.5677. Found: 353.5725.
3.2. Antifungal and Antibacterial Activity Assay
Pure cultures of the fungi Aspergillus niger, Fusarium, Penicillium chrysogenum, Penicillium frequentans, and Alternaria alternata and bacteria Pseudomonas aeruginosa and Bacillus sp. were obtained from the American Type Culture Collection (ATCC). Suspensions of microorganisms in DMSO were prepared according to direct colony method and serial dilution procedure. Then, the final concentration of the stock inoculum was 1·10−4 μg/mL. Both antifungal and antibacterial activity assay were performed by applying a mixture of a microorganism suspension and a solution of the target compound in a ratio 1:1 to Petri dishes with a solid medium—Merck Sabouraud agar or agar-agar. The DMSO did not have any inhibitory effect on the tested organisms.
4. Conclusions
A series of 14 novel hybrid terpeno-heterocyclic compounds containing homodrimane and 2-substituted 1,3-thiadiazole, N-substituted 2-amino-1,3-benzothiazole and N-p-toluidyl units were designed, synthesized, and assessed as antimicrobial agents. Several of them showed higher antifungal and antibacterial activities than reference drugs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27165082/s1.
Author Contributions
Conceptualization, A.A.; experimental work, L.L., C.C. and S.B.; evaluated biological activity, N.V.; GC-HRMS analysis, I.D. and E.-I.G.; NMR data acquisition, A.B.; data analysis, A.C.; writing, review and revision of the manuscript, A.C., I.I.M. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Agency for Research and Development (ANCD), Republic of Moldova, project PLANTERAS 20.80009.8007.03.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Authors are thankful to the Romanian Ministry of Research, Innovation and Digitization, within Program 1—Development of the national RD system, Subprogram 1.2—Institutional Performance—RDI excellence funding projects, Contract no.11PFE/30.12.2021, for financial support towards Article Processing Charge (APC).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Singh, M.; Pal, M.; Sharma, R.P. Biological Activity of the Labdane Diterpenes. Planta Med. 1999, 65, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, B.J.M.; de Groot, A. Occurrence, Biological Activity and Synthesis of Drimane Sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A Comprehensive Review in Current Developments of Benzothiazole-Based Molecules in Medicinal Chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Kumar, S.A.; Mishra, A.K. Advancement in Pharmacological Activities of Benzothiazole and its Derivatives: An Up to Date Review. Mini Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar] [CrossRef]
- Banerjee, S.; Payra, S.; Saha, A. A Review on Synthesis of Benzothiazole Derivatives. Curr. Organocatal. 2018, 4, 164–181. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Z.-B. A Review on Domino Condensation/Cyclization Reactions for the Synthesis of 2-Substituted 1,3-Benzothiazole Derivatives. Eur. J. Org. Chem. 2020, 2020, 408–419. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Zuo, X.; Feng, X.; Gao, Y. Recent Advances in Synthesis of Benzothiazole Compounds Related to Green Chemistry. Molecules 2020, 25, 1675. [Google Scholar] [CrossRef] [Green Version]
- Coelho, F.L.; Campo, L.F. Synthesis of 2-Arylbenzothiazoles via Direct Condensation Between in situ Generated 2-Aminothiophenol from Disulfide Cleavage and Carboxylic Acids. Tetrahedron Lett. 2017, 58, 2330–2333. [Google Scholar] [CrossRef]
- Jesberger, M.; Davis, T.P.; Barner, L. Applications of Lawesson’s Reagent in Organic and Organometallic Syntheses. Synthesis 2003, 13, 1929–1958. [Google Scholar] [CrossRef]
- Kuchkova, K.; Aricu, A.; Barba, A.; Ungur, N.; Tuchilus, C.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I. An Efficient and Straightforward Method to New Organic Compounds: Homodrimane Sesquiterpenoids with Diazine Units. Synlett 2013, 24, 697–700. [Google Scholar] [CrossRef]
- Kuchkova, K.; Aricu, A.; Secara, E.; Barba, A.; Vlad, P.; Ungur, N.; Tuchilus, C.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I. Design, Synthesis, and Antimicrobial Activity of Some Novel Homodrimane Sesquiterpenoids with Diazine Skeleton. Med. Chem. Res. 2014, 23, 1559–1568. [Google Scholar] [CrossRef]
- Kuchkova, K.I.; Arycu, A.N.; Sekara, E.S.; Barba, A.N.; Vlad, P.F.; Makaev, F.Z.; Mel’nik, E.; Kravtsov, V.K. Synthesis and Structure of Homodrimane Sesquiterpenoids Containing 1,2,4-Triazole and Carbazole Rings. Chem. Nat. Compd. 2015, 51, 684–688. [Google Scholar] [CrossRef]
- Duca, G.; Aricu, A.; Lungu, L.; Tenu, N.; Ciocarlan, A.; Gutu, Y.; Dragalin, I.; Barba, A. Synthesis of New Homodrimane Sesquiterpenoids Containing Diazine, 1,2,4-Triazole and Carbazole Rings. Chem. J. Mold. 2018, 13, 69–73. [Google Scholar] [CrossRef]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I.; D’Ambrosio, M.; Vornicu, N. Synthesis, Antibacterial, and Antifungal Activities of New Drimane Sesquiterpenoids with Azaheterocyclic Units. Med. Chem. Res. 2016, 25, 2316–2323. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Aricu, A.; Lungu, L.; Edu, C.; Barba, A.; Shova, S.; Mangalagiu, I.I.; D’Ambrosio, M.; Nicolescu, A.; Deleanu, C.; et al. Synthesis of Novel Tetranorlabdane Derivatives with Unprecedented Carbon Skeleton. Synlett 2017, 28, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Lungu, L.; Ciocarlan, A.; Barba, A.; Shova, S.; Pogrebnoi, S.; Mangalagiu, I.I.; Moldoveanu, C.; Vornicu, N.; D’Ambrosio, M.; Babak, M.V.; et al. Synthesis and Evaluation of Biological Activity of Homodrimane Sesquiterpenoids Bearing Hydrazinecarbothioamide or 1,2,4-Triazole Unit. Chem. Heterocycl. Compd. 2019, 55, 716–724. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Smigon, C.; Ozer, I.; Shova, S.; Gutu, I.; Vornicu, N.; Mangalagiu, I.I.; D’Ambrosio, M.; Aricu, A. Synthesis and Evaluation of Biological Activity of Homodrimane Sesquiterpenoids Bearing 1,3,4-Oxadiazole or 1,3,4-Thiadiazole Units. Chem. Heterocycl. Compd. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Blaja, S.P.; Lungu, L.V.; Kuchkova, K.I.; Ciocarlan, A.G.; Barba, A.N.; Vornicu, N.; Aricu, A.N. Norlabdane Compounds Containing Thiosemicarbazone or 1,3-Thiazole Fragments: Synthesis and Antimicrobial Activity. Chem. Nat. Compd. 2021, 57, 101–110. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lungu, L.; Blaja, S.; Dragalin, I.; Aricu, A. The Use of Some Non-Conventional Methods in Chemistry of Bicyclohomofarnesenic Methyl Esters. Chem. J. Mold. 2020, 15, 69–77. [Google Scholar] [CrossRef]
- Aricu, A.N.; Kuchkova, K.I.; Barba, A.N.; Dragalin, I.P.; Shova, S.G.; Vornicu, N.; Gorincioi, E.K.; Secara, E.S.; Lungu, L.V.; Niculaua, M.; et al. Synthesis from Norambreinolide, Structure, and Antimicrobial Activity of Dihomodrimane Sesquiterpenoids with Azine, Hydrazide, and Dihydrazide Fragments. Chem. Nat. Compd. 2016, 52, 1029–1036. [Google Scholar] [CrossRef]
- Ge, F.; Wang, Z.; Wan, W.; Lua, W.; Hao, J. One-Pot Synthesis of 2-Trifluoromethyl and 2-Difluoromethyl Substituted Benzo-1,3-diazoles. Tetrahedron Lett. 2007, 48, 3251–3254. [Google Scholar] [CrossRef]
- Ojwach, S.O.; Westman, G.; Darkwa, J. Substituted (Pyridinyl)benzoazole Palladium Complexes: Synthesis and Application as Heck Coupling Catalysts. Polyhedron 2007, 26, 5544–5552. [Google Scholar] [CrossRef]
- Standard M02; Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2018.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).