Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Preparation
2.1.1. Raw Material
2.1.2. Compression-Cooking Process
2.2. Reagents and Sorbents
2.3. Evaluation of VOC Emissions
2.3.1. Gaseous VOC Sampling
2.3.2. TD-GC-FID/MS
- SER, area-specific emission rate (μg·m−2·h−1);
- m, mass of VOC in the sorbent tube (μg);
- m0, reference value of the glass cell (μg);
- V, sampled volume (m3);
- Q; air flow (m3·h−1);
- S, sample surface (m2).
2.3.3. Pyr-GC-MS
2.3.4. HPLC-DAD
2.3.5. Quality Assurance
2.4. Statistical Analysis
3. Results and Discussion
3.1. Hypothesis of Identification of Emitted Compounds
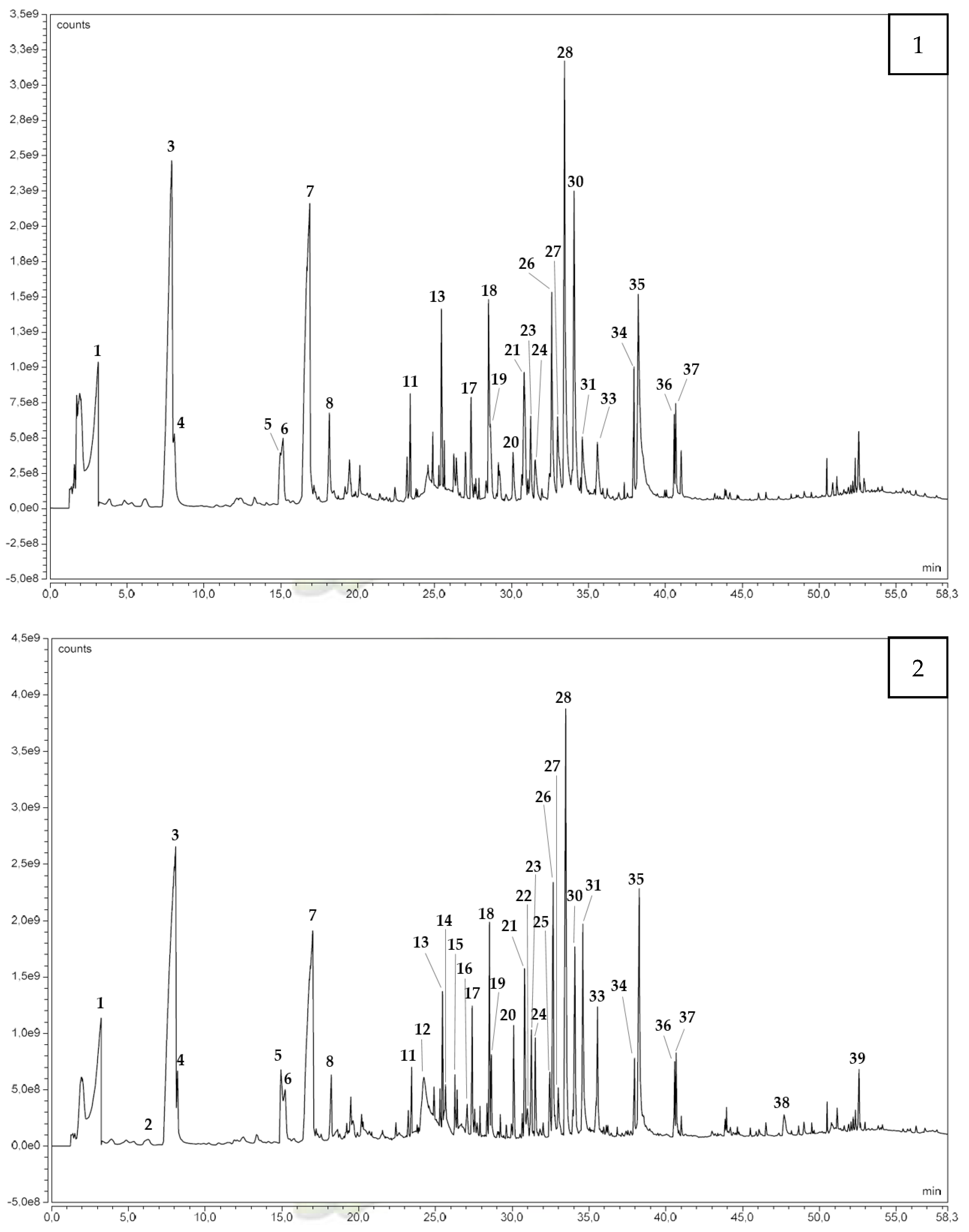
3.1.1. Gas Chromatography Analysis
3.1.2. Liquid Chromatography Analysis
3.2. Compression-Cooking Parameters and VOCs
3.2.1. Area-Specific Emission Rates of Carbonyl Compounds and TVOCs
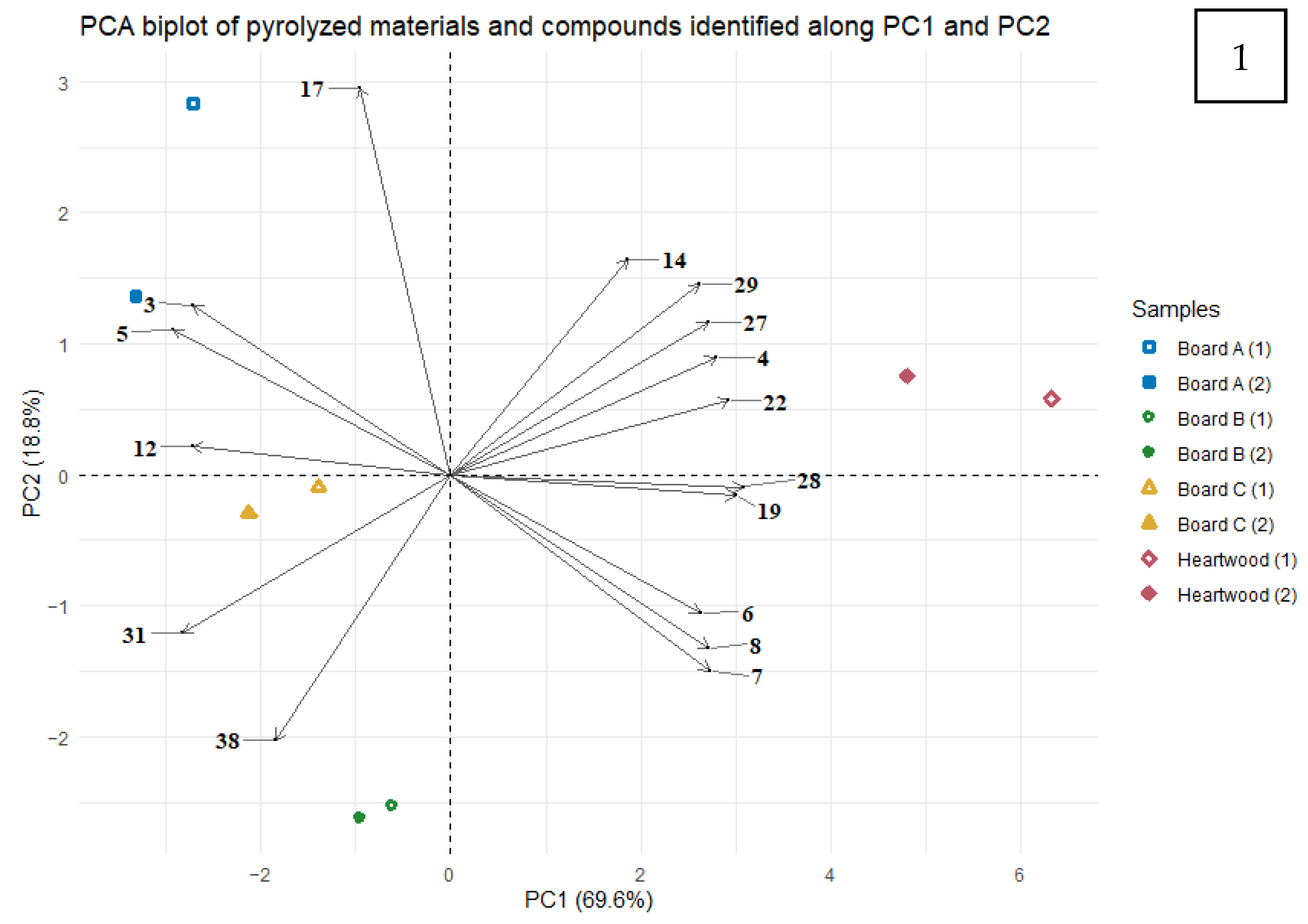
3.2.2. Pyrolysis as a Tool to Evaluate Wood-Panel Released Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Indoor Air Quality: Organic Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 1989; p. 70. [Google Scholar]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral Scent Variation in Two Antirrhinum Majus Subspecies Influences the Choice of Naïve Bumblebees. Behav. Ecol. Sociobiol. 2011, 13, 1015–1027. [Google Scholar] [CrossRef]
- Halios, C.H.; Landeg-Cox, C.; Lowther, S.D.; Middleton, A.; Marczylo, T.; Dimitroulopoulou, S. Chemicals in European Residences—Part I: A Review of Emissions, Concentrations and Health Effects of Volatile Organic Compounds (VOCs). Sci. Total Environ. 2022, 839, 156201. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Milhem, S.; Verriele, M.; Nicolas, M.; Thevenet, F. Indoor Use of Essential Oils: Emission Rates, Exposure Time and Impact on Air Quality. Atmos. Environ. 2021, 244, 117863. [Google Scholar] [CrossRef]
- Hien, T.T.; Huy, D.H.; Dominutti, P.A.; Thien Chi, N.D.; Hopkins, J.R.; Shaw, M.; Forster, G.; Mills, G.; Le, H.A.; Oram, D. Comprehensive Volatile Organic Compound Measurements and Their Implications for Ground-Level Ozone Formation in the Two Main Urban Areas of Vietnam. Atmos. Environ. 2022, 269, 118872. [Google Scholar] [CrossRef]
- EPA. Report to Congress on Indoor Air Quality: Volume II—Assessment and Control of Indoor Air Pollution; United States Environmental Protection Agency: Washington, DC, USA, 1989; p. 250. [Google Scholar]
- Jones, A.P. Indoor Air Quality and Health. Atmos. Environ. 1999, 30, 4535–4564. [Google Scholar] [CrossRef]
- Commission of the European Communities. Sick Building Syndrome—A Practical Guide; European concerted action. Indoor air quality and its impact on man. COST Project 613. Report No. 4; Office for Official Publications of the European Communities: Luxembourg, 1989. [Google Scholar]
- EPA. Indoor Air Facts No. 4: Sick Building Syndrome; United States Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Assessment of Exposure to Indoor Air Pollutants; Jantunen, M., Jaakkola, J.J.K., Krzyżanowski, M., Eds.; WHO regional publications; World Health Organization Regional office for Europe: Copenhagen, Denmark, 1997; ISBN 978-92-890-1342-0. [Google Scholar]
- Geiss, O.; Giannopoulos, G.; Tirendi, S.; Barrero-Moreno, J.; Larsen, B.R.; Kotzias, D. The AIRMEX Study—VOC Measurements in Public Buildings and Schools/Kindergartens in Eleven European Cities: Statistical Analysis of the Data. Atmos. Environ. 2011, 45, 3676–3684. [Google Scholar] [CrossRef]
- Mandin, C.; Bonvallot, N.; Kirchner, S.; Keirsbulck, M.; Alary, R.; Cabanes, P.-A.; Dor, F.; Le Moullec, Y.; Mullot, J.-U.; Peel, A.-E.; et al. Development of French Indoor Air Quality Guidelines. Clean Soil Air Water 2009, 37, 494–499. [Google Scholar] [CrossRef]
- Becerra, J.A.; Lizana, J.; Gil, M.; Barrios-Padura, A.; Blondeau, P.; Chacartegui, R. Identification of Potential Indoor Air Pollutants in Schools. J. Clean. Prod. 2020, 242, 118420. [Google Scholar] [CrossRef]
- Yan, M.; Zhai, Y.; Shi, P.; Hu, Y.; Yang, H.; Zhao, H. Emission of Volatile Organic Compounds from New Furniture Products and Its Impact on Human Health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1886–1906. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Lin, C.-C.; Hsu, S.-C. Comparison of Conventional and Green Building Materials in Respect of VOC Emissions and Ozone Impact on Secondary Carbonyl Emissions. Build. Environ. 2015, 87, 274–282. [Google Scholar] [CrossRef]
- Jiang, C.; Li, D.; Zhang, P.; Li, J.; Wang, J.; Yu, J. Formaldehyde and Volatile Organic Compound (VOC) Emissions from Particleboard: Identification of Odorous Compounds and Effects of Heat Treatment. Build. Environ. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Böhm, M.; Salem, M.Z.M.; Srba, J. Formaldehyde Emission Monitoring from a Variety of Solid Wood, Plywood, Blockboard and Flooring Products Manufactured for Building and Furnishing Materials. J. Hazard. Mater. 2012, 221–222, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; Böhm, M.; Srba, J.; Beránková, J. Evaluation of Formaldehyde Emission from Different Types of Wood-Based Panels and Flooring Materials Using Different Standard Test Methods. Build. Environ. 2012, 49, 86–96. [Google Scholar] [CrossRef]
- Bourdin, D.; Mocho, P.; Desauziers, V.; Plaisance, H. Formaldehyde Emission Behavior of Building Materials: On-Site Measurements and Modeling Approach to Predict Indoor Air Pollution. J. Hazard. Mater. 2014, 280, 164–173. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, S.; Yang, X. Long-Term Formaldehyde Emissions from Medium-Density Fiberboard in a Full-Scale Experimental Room: Emission Characteristics and the Effects of Temperature and Humidity. Environ. Sci. Technol. 2015, 49, 10349–10356. [Google Scholar] [CrossRef]
- Makowski, M.; Ohlmeyer, M.; Meier, D. Long-Term Development of VOC Emissions from OSB after Hot-Pressing. Holzforschung 2005, 59, 519–523. [Google Scholar] [CrossRef]
- Roffael, E. Volatile Organic Compounds and Formaldehyde in Nature, Wood and Wood Based Panels. Holz. Roh. Werkst. 2006, 64, 144–149. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.-M.; Wan, H.; Brunette, G. Binderless Panels Made with Black Spruce Bark. BioResources 2011, 6, 3960–3972. [Google Scholar]
- Uitterhaegen, E.; Labonne, L.; Merah, O.; Talou, T.; Ballas, S.; Véronèse, T.; Evon, P. Optimization of Thermopressing Conditions for the Production of Binderless Boards from a Coriander Twin-Screw Extrusion Cake. J. Appl. Polym. Sci. 2017, 134, 44650. [Google Scholar] [CrossRef]
- Baskaran, M.; Hashim, R.; Sulaiman, O.; Hiziroglu, S.; Sato, M.; Sugimoto, T. Optimization of Press Temperature and Time for Binderless Particleboard Manufactured from Oil Palm Trunk Biomass at Different Thickness Levels. Mater. Today Commun. 2015, 3, 87–95. [Google Scholar] [CrossRef]
- Hidayat, H.; Keijsers, E.R.P.; Prijanto, U.; van Dam, J.E.G.; Heeres, H.J. Preparation and Properties of Binderless Boards from Jatropha Curcas L. Seed Cake. Ind. Crops Prod. 2014, 52, 245–254. [Google Scholar] [CrossRef]
- Mobarak, F.; Fahmy, Y.; Augustin, H. Binderless Lignocellulose Composite from Bagasse and Mechanism of Self-Bonding. Holzforschung 1982, 36, 131–136. [Google Scholar] [CrossRef]
- Simon, V.; Uitterhaegen, E.; Robillard, A.; Ballas, S.; Véronèse, T.; Vilarem, G.; Merah, O.; Talou, T.; Evon, P. VOC and Carbonyl Compound Emissions of a Fiberboard Resulting from a Coriander Biorefinery: Comparison with Two Commercial Wood-Based Building Materials. Environ. Sci. Pollut. Res. 2020, 27, 16121–16133. [Google Scholar] [CrossRef] [PubMed]
- Murat, P.; Harohalli Puttaswamy, S.; Ferret, P.-J.; Coslédan, S.; Simon, V. Identification of Potential Extractables and Leachables in Cosmetic Plastic Packaging by Microchambers-Thermal Extraction and Pyrolysis-Gas Chromatography-Mass Spectrometry. Molecules 2020, 25, 2115. [Google Scholar] [CrossRef]
- Liang, W.; Lv, M.; Yang, X. The Combined Effects of Temperature and Humidity on Initial Emittable Formaldehyde Concentration of a Medium-Density Fiberboard. Build. Environ. 2016, 98, 80–88. [Google Scholar] [CrossRef]
- Souillier, A.; Plaisance, H.; Desauziers, V. New SPME-Based Method for on-Site Measurement of Gas-Phase Concentration of Phthalates and Alternatives at the Surface of PVC Floorings. Green Anal. Chem. 2022, 1, 100012. [Google Scholar] [CrossRef]
- Nicolle, J.; Desauziers, V.; Mocho, P.; Ramalho, O. Optimization of FLEC®-SPME for Field Passive Sampling of VOCs Emitted from Solid Building Materials. Talanta 2009, 80, 730–737. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Burianová, K.; Ballas, S.; Véronèse, T.; Merah, O.; Talou, T.; Stevens, C.V.; Evon, P.; Simon, V. Characterization of Volatile Organic Compound Emissions from Self-Bonded Boards Resulting from a Coriander Biorefinery. Ind. Crops Prod. 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Pánek, M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers 2020, 12, 2289. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E.; Simon, V. BTEX Abatement by Photocatalytic TiO2-Bearing Coatings Applied to Cement Mortars. Build. Environ. 2014, 71, 186–192. [Google Scholar] [CrossRef]
- Harb, P.; Locoge, N.; Thevenet, F. Emissions and Treatment of VOCs Emitted from Wood-Based Construction Materials: Impact on Indoor Air Quality. Chem. Eng. J. 2018, 354, 641–652. [Google Scholar] [CrossRef]
- Yrieix, C.; Dulaurent, A.; Laffargue, C.; Maupetit, F.; Pacary, T.; Uhde, E. Characterization of VOC and Formaldehyde Emissions from a Wood Based Panel: Results from an Inter-Laboratory Comparison. Chemosphere 2010, 79, 414–419. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and Prediction of Biomass Pyrolysis Products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Nonier, M.F.; Vivas, N.; Vivas de Gaulejac, N.; Absalon, C.; Soulié, P.; Fouquet, E. Pyrolysis–Gas Chromatography/Mass Spectrometry of Quercus Sp. Wood. J. Anal. Appl. Pyrolysis 2006, 75, 181–193. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, S.-M.; Park, B.-D.; Lim, J.-Y.; Jang, S.-G.; Kim, S. Effect of Surface Laminate Type on the Emission of Volatile Organic Compounds from Wood-Based Composite Panels. J. Adhes. Sci. Technol. 2013, 27, 620–631. [Google Scholar] [CrossRef]
- Sebio-Puñal, T.; Naya, S.; López-Beceiro, J.; Tarrío-Saavedra, J.; Artiaga, R. Thermogravimetric Analysis of Wood, Holocellulose, and Lignin from Five Wood Species. J. Anal. Calorim. 2012, 109, 1163–1167. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Bridgwater, A.V.; Fang, M.X. Kinetic Study on Thermal Decomposition of Woods in Oxidative Environment. Fuel 2009, 88, 1024–1030. [Google Scholar] [CrossRef]
- Gašparovič, L.; Koreňová, Z.; Jelemenský, Ľ. Kinetic Study of Wood Chips Decomposition by TGA. Chem. Pap. 2010, 64, 174–181. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric Analysis and Kinetic Study of Poplar Wood Pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- TranVan, L.; Legrand, V.; Jacquemin, F. Thermal Decomposition Kinetics of Balsa Wood: Kinetics and Degradation Mechanisms Comparison between Dry and Moisturized Materials. Polym. Degrad. Stab. 2014, 110, 208–215. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- NF ISO 16000-9; Indoor Air—Part 9: Determination of the Emission of Volatile Organic Compounds from Building Products and Furnishing—Emission Test Chamber Method. Afnor: Paris, France, 2006.
- Simon, V.; Riba, M.-L.; Waldhart, A.; Torres, L. Breakthrough Volume of Monoterpenes on Tenax TA: Influence of Temperature and Concentration for α-Pinene. J. Chromatogr. A 1995, 704, 465–471. [Google Scholar] [CrossRef]
- Pohleven, J.; Burnard, M.; Kutnar, A. Volatile Organic Compounds Emitted from Untreated and Thermally Modified Wood—A Review. WFS 2019, 51, 231–254. [Google Scholar] [CrossRef]
- Risholm-Sundman, M.; Lundgren, M.; Vestin, E.; Herder, P. Emissions of Acetic Acid and Other Volatile Organic Compounds from Different Species of Solid Wood. Holz Als Roh Werkst. 1998, 56, 125–129. [Google Scholar] [CrossRef]
- Peters, J.; Fischer, K.; Fischer, S. Characterization of Emissions from Thermally Modified Wood and Their Reduction by Chemical Treatment. BioResources 2008, 3, 491–502. [Google Scholar]
- Hyttinen, M.; Masalin-Weijo, M.; Kalliokoski, P.; Pasanen, P. Comparison of VOC Emissions between Air-Dried and Heat-Treated Norway Spruce (Picea Abies), Scots Pine (Pinus Sylvesteris) and European Aspen (Populus Tremula) Wood. Atmos. Environ. 2010, 44, 5028–5033. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, Y.; Sharma, A.; Bai, X. Lignin Valorization through Thermochemical Conversion: Comparison of Hardwood, Softwood and Herbaceous Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6608–6617. [Google Scholar] [CrossRef]
- Choi, Y.S.; Johnston, P.A.; Brown, R.C.; Shanks, B.H.; Lee, K.-H. Detailed Characterization of Red Oak-Derived Pyrolysis Oil: Integrated Use of GC, HPLC, IC, GPC and Karl-Fischer. J. Anal. Appl. Pyrolysis 2014, 110, 147–154. [Google Scholar] [CrossRef]
- Nonier, M.F.; Vivas, N.; Vivas de Gaulejac, N.; Vitry, C. Effect of Different Chemical Treatments on the Pyrolysis Products of Quercus Petraea L. Heartwood. J. Anal. Appl. Pyrolysis 2011, 91, 134–139. [Google Scholar] [CrossRef]
- Vichi, S.; Santini, C.; Natali, N.; Riponi, C.; López-Tamames, E.; Buxaderas, S. Volatile and Semi-Volatile Components of Oak Wood Chips Analysed by Accelerated Solvent Extraction (ASE) Coupled to Gas Chromatography–Mass Spectrometry (GC–MS). Food Chem. 2007, 102, 1260–1269. [Google Scholar] [CrossRef]
- Omrani, P.; Masson, E.; Pizzi, A.; Mansouri, H.R. Emission Gases in Linear Vibration Welding of Wood. J. Adhes. Sci. Technol. 2009, 23, 85–94. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; Taylor & Francis: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-5428-6. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- National Institute of Standards and Technology. NIST Chemistry WebBook, Retention Indices. Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 June 2022).
- Salem, M.Z.M.; Böhm, M. Understanding of Formaldehyde Emissions from Solid Wood: An Overview. BioResources 2013, 8, 4775–4790. [Google Scholar] [CrossRef]
- Schäfer, M.; Roffael, E. On the Formaldehyde Release of Wood. Holz Als Roh-Werkst. 2000, 58, 259–264. [Google Scholar] [CrossRef]
- Jensen, L.K.; Larsen, A.; Mølhave, L.; Hansen, M.K.; Knudsen, B. Health Evaluation of Volatile Organic Compound (VOC) Emissions from Wood and Wood-Based Materials. Arch. Environ. Health Int. J. 2001, 56, 419–432. [Google Scholar] [CrossRef]
- Seaman, V.Y.; Bennett, D.H.; Cahill, T.M. Origin, Occurrence, and Source Emission Rate of Acrolein in Residential Indoor Air. Environ. Sci. Technol. 2007, 41, 6940–6946. [Google Scholar] [CrossRef]
- Que, Z.-L.; Wang, F.-B.; Li, J.-Z.; Furuno, T. Assessment on Emission of Volatile Organic Compounds and Formaldehyde from Building Materials. Compos. Part B: Eng. 2013, 49, 36–42. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, S.; Kim, H.-J.; Park, J.C. Formaldehyde and TVOC Emission Behaviors According to Finishing Treatment with Surface Materials Using 20 L Chamber and FLEC. J. Hazard. Mater. 2010, 177, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-S.; Lim, B.-A.; Park, H.-J.; Kim, J.-C. Characteristics of Volatile Organic Compounds (VOCs) Emitted from Building Materials to Improve Indoor Air Quality: Focused on Natural VOCs. Air Qual. Atmos. Health 2013, 6, 737–746. [Google Scholar] [CrossRef]
- Baumann, M.; Lorenz, L.; Batterman, S.; Zhang, G. Aldehyde Emissions from Particleboard and Medium Density Fiberboard Products. For. Prod. J. 2000, 50, 75–82. [Google Scholar]
- Xue, L.; Zhao, Z.; Zhang, Y.; Chu, D.; Mu, J. Analysis of Gas Chromatography-Mass Spectrometry Coupled with Dynamic Headspace Sampling on Volatile Organic Compounds of Heat-Treated Poplar at High Temperatures. BioResources 2016, 11, 3550–3560. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC Emissions between Air-Dried and Heat-Treated Scots Pine Wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Čabalová, I.; Kačík, F.; Lagaňa, R.; Výbohová, E.; Bubeníková, T.; Čaňová, I.; Ďurkovič, J. Effect of Thermal Treatment on the Chemical, Physical, and Mechanical Properties of Pedunculate Oak (Quercus Robur L.) Wood. BioResources 2017, 13, 157–170. [Google Scholar] [CrossRef]
- Wikberg, H.; Liisamaunu, S. Characterisation of Thermally Modified Hard- and Softwoods by C CPMAS NMR. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
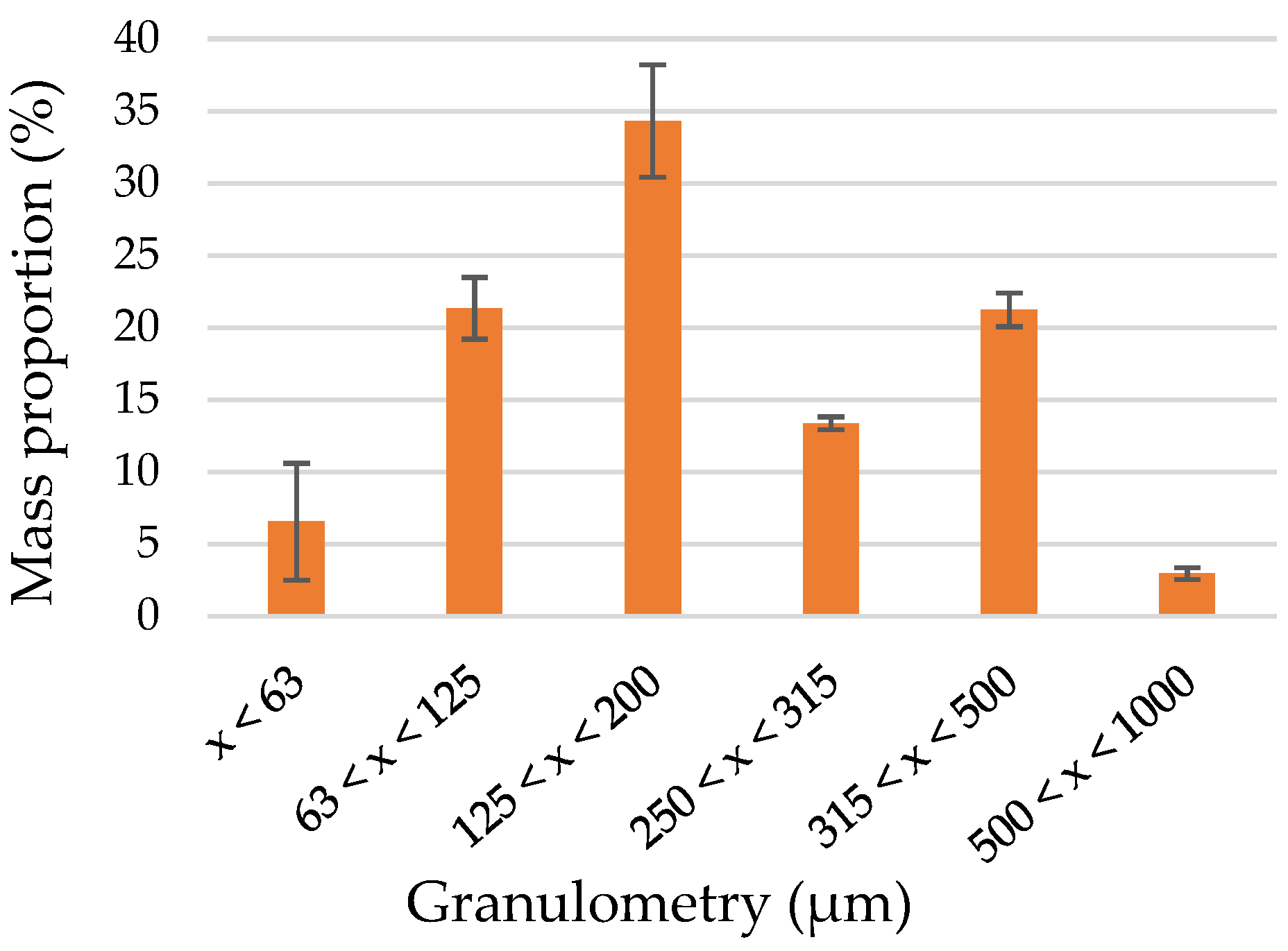
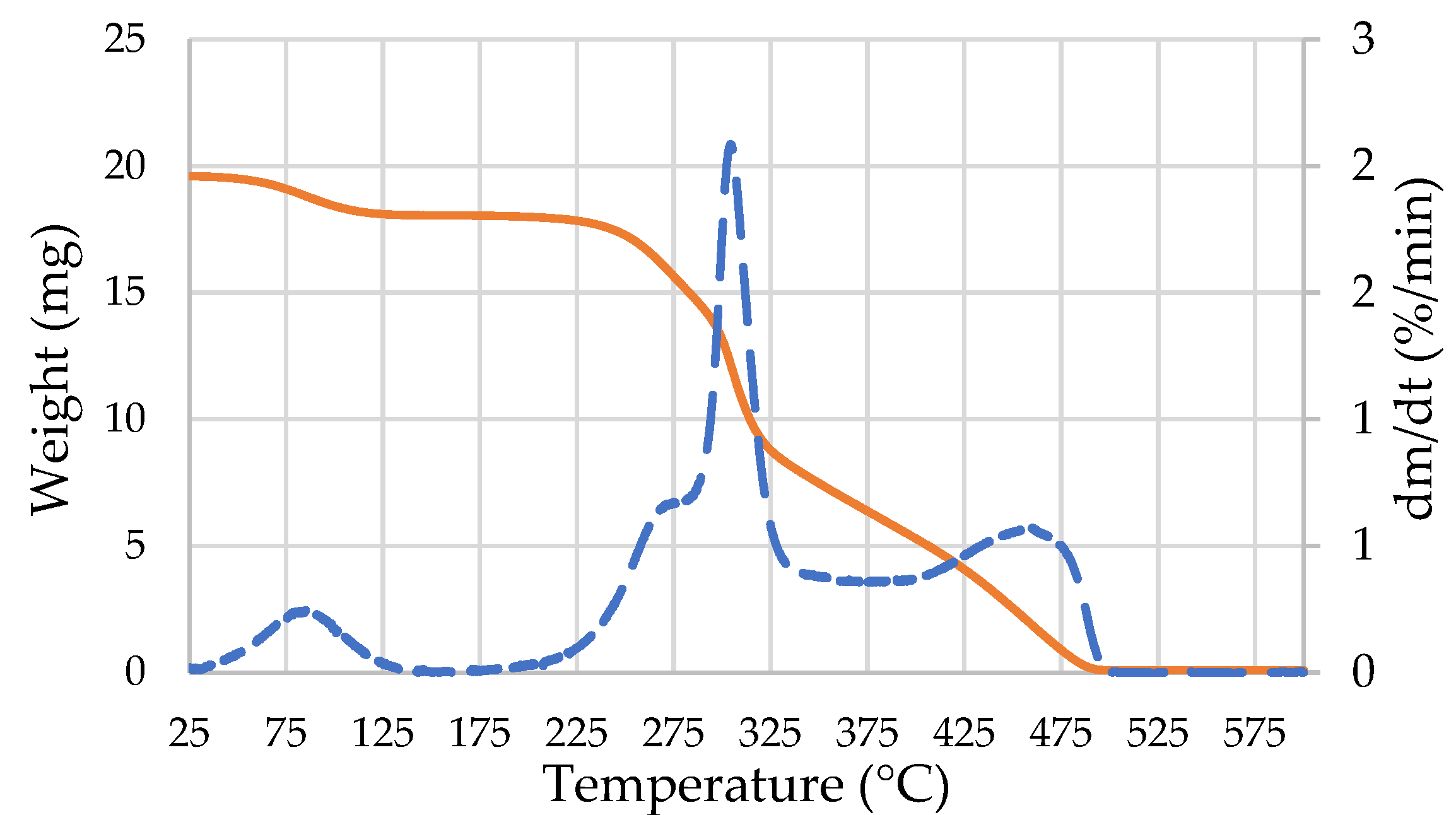

| Pressing Parameters | Conditions A | Conditions B | Conditions C |
|---|---|---|---|
| Pressing temperature (°C) | 170 | 129 | 170 |
| Pressing time (min) | 6 | 6 | 1 |
| Pressing pressure (MPa) | 30 | 30 | 30 |
| Compound | LOD(ng) | LOQ(ng) | LODSER (a) | LOQSER (a) | ||
|---|---|---|---|---|---|---|
| (µg·m−2·h−1) | (ng·gmaterial−1·h−1) | (µg·m−2·h−1) | (ng·gmaterial−1·h−1) | |||
| Formaldehyde | 0.7 | 1.0 | 0.1 | 0.3 | 0.2 | 0.4 |
| Acetaldehyde | 0.9 | 1.2 | 0.1 | 0.4 | 0.2 | 0.5 |
| Acrolein | 1.0 | 1.7 | 0.1 | 0.4 | 0.2 | 0.7 |
| Acetone | 1.0 | 1.6 | 0.1 | 0.4 | 0.2 | 0.7 |
| Propanal | 0.8 | 1.3 | 0.1 | 0.3 | 0.2 | 0.5 |
| Butanal | 0.9 | 1.3 | 0.1 | 0.4 | 0.2 | 0.5 |
| Benzaldehyde | 2.1 | 3.6 | 0.3 | 0.8 | 0.5 | 1.5 |
| Toluene | 0.6 | 1.2 | 0.6 | 1.8 | 1.2 | 3.7 |
| Compound Family | No. | Compounds | Chemical Formula | CAS | RIth (DB-5MS-Like) | Boiling Points (°C) [62] | Identification Method | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Heartwood | Board A | Board B | Board C | |||||||
| Aldehydes (linear) | 41 | Acetaldehyde | C2H4O | 75-07-0 | 21 | LC | LC | LC | LC | |
| 42 | Acrolein | C3H4O | 107-02-8 | 52 | LC | LC | LC | LC | ||
| 44 | Butanal | C4H8O | 123-72-8 | 75 | LC | LC | LC | LC | ||
| 40 | Formaldehyde | CH2O | 50-00-0 | -19 | LC | LC | LC | LC | ||
| 43 | Propanal | C3H6O | 123-38-6 | 48 | LC | LC | LC | LC | ||
| Aromatics | 29 | Acetosyringone | C10H12O4 | 2478-38-8 | 1739 (b) | Pyr | Pyr | |||
| 24 | 4-Allyl-2,6-dimethoxyphenol | C11H14O3 | 6627-88-9 | 1608 (b) | Pyr | Pyr | Pyr | Pyr | ||
| 45 | Benzaldehyde | C7H6O | 100-52-7 | 179 | LC | LC | LC | LC | ||
| 23 | Butyrovanillone | C11H14O3 | 64142-23-0 | 1593 (b) | Pyr | Pyr | Pyr | Pyr | ||
| 30 | Coniferaldehyde | C10H10O3 | 458-36-6 | 1728 (a) | Pyr | Pyr | Pyr | Pyr | ||
| 21 | 2,6-Dimethoxy-4-vinylphenol | C10H12O3 | 28343-22-8 | 1573 (c) | Pyr | Pyr | Pyr | Pyr | ||
| 20 | Guaiacylacetone | C10H12O3 | 2503-46-0 | 1571 (b) | Pyr | Pyr | Pyr | Pyr | ||
| 18 | Isoeugenol | C10H12O2 | 97-54-1 | 1448 (a) | 264 | Pyr | Pyr | Pyr | Pyr | |
| 19 | 2-Methoxy-4-propylphenol | C10H14O2 | 2785-87-7 | 1366 (a) | 121 | Pyr | Pyr | Pyr | Pyr | |
| 13 | 2-Methoxy-4-vinylphenol | C9H10O2 | 7786-61-0 | 1309 (a) | Pyr | Pyr | Pyr | Pyr | ||
| 28 | 4-Propenyl-2,6-dimethoxyphenol | C11H14O3 | 20675-95-0 | 1704 (c) | Pyr | Pyr | Pyr | Pyr | ||
| 33 | Propiosyringone | C11H14O4 | 5650-43-1 | 1827 (b) | Pyr | Pyr | Pyr | Pyr | ||
| 32 | Sinapyl alcohol | C11H14O4 | 537-33-7 | 1998 (b) | Pyr | Pyr | ||||
| 35 | Sinapaldehyde | C11H12O4 | 4206-58-0 | 1989 (b) | Pyr | Pyr | Pyr | Pyr | ||
| 26 | Syringaldehyde | C9H19O4 | 134-96-3 | 1655 (a) | 192 | Pyr | Pyr | Pyr | Pyr | |
| 31 | Syringylacetone | C12H16O4 | 112468-41-4 | 1746 (c) | Pyr | Pyr | Pyr | Pyr | ||
| 15 | Syringol | C8H10O3 | 91-10-1 | 1346 (a) | 261 | Pyr | Pyr | Pyr | ||
| 17 | Vanillin | C8H8O3 | 121-33-5 | 1393 (a) | 285 | Pyr | Pyr | Pyr | Pyr | |
| Furans and derivatives | 4 | 3-Furaldehyde | C5H4O2 | 498-60-2 | 832 (c) | 145 | Pyr | Pyr | Pyr | Pyr |
| 3 | Furfural | C5H4O2 | 98-01-1 | 828 (a) | 162 | Pyr, Th | Pyr, Th | Pyr, Th | Pyr, Th | |
| 9 | 4-Hydroxy-5-methylfuran-3(2H)-one | C5H6O3 | 19322-27-1 | 1042 (c) | Pyr | Pyr | ||||
| 12 | 5-Hydroxymethylfurfural | C6H6O3 | 67-47-0 | 1215 (b) | 115 | Pyr | Pyr | Pyr | ||
| 5 | 5-Methylfurfural | C6H6O2 | 620-02-0 | 957 (a) | 186 | Pyr, Th | Pyr, Th | Pyr, Th | Pyr, Th | |
| Lactones | 14 | cis-Oak lactone | C9H16O2 | 55013-32-6 | 1327 (b) | Pyr | ||||
| Lipids | 37 | Oleic acid | C18H34O2 | 112-80-1 | 2142 (a) | 360 | Pyr | Pyr | Pyr | Pyr |
| 34 | Palmitic acid | C16H32O2 | 57-10-3 | 1959 (a) | 351 | Pyr | Pyr | Pyr | Pyr | |
| Others | 1 | Acetic acid | C2H4O2 | 64-19-7 | 606 (a) | 118 | Pyr, Th | Pyr, Th | Pyr, Th | Pyr, Th |
| 43 | Acetone | C3H6O | 67-64-1 | 56 | LC | LC | ||||
| 38 | Lignostilbene | C16H16O4 | 7329-69-3 | Pyr | Pyr | Pyr | ||||
| 11 | 6-Propyl-5,6-dihydro-2H-pyran-2-one | C8H12O2 | 16400-69-4 | 1268 (c) | Pyr | Pyr | Pyr | Pyr | ||
| 2 | N.I. 1 (84, 55, 85) * | - | - | - | - | Pyr | Pyr | |||
| 6 | N.I. 2 (110, 109, 53) * | - | - | - | - | Pyr | Pyr | Pyr | Pyr | |
| 7 | N.I. 3 (114, 58, 57) * | - | - | - | - | Pyr | Pyr | Pyr | Pyr | |
| 8 | N.I. 4 (113, 123, 58) * | - | - | - | - | Pyr | Pyr | Pyr | Pyr | |
| 10 | N.I. 5 (128, 129, 42) * | - | - | - | - | Pyr | ||||
| 16 | N.I. 6 (43, 172, 29) * | - | - | - | - | Pyr | Pyr | |||
| 22 | N.I. 7 (137, 124, 180) * | - | - | - | - | Pyr | Pyr | Pyr | ||
| 25 | N.I. 8 (194, 91, 119) * | - | - | - | - | Pyr | Pyr | Pyr | ||
| 27 | N.I. 9 (195, 131, 132) * | - | - | - | - | Pyr | Pyr | Pyr | Pyr | |
| 36 | N.I. 10 (81, 67, 95) * | - | - | - | - | Pyr | Pyr | Pyr | Pyr | |
| 39 | N.I. 11 (147, 145, 105) * | - | - | - | - | Pyr | Pyr | Pyr | ||
| Compounds | Heartwood (ng·g−1·h−1) | Board A | Board B | Board C |
|---|---|---|---|---|
| (µg·m−2·h−1) | ||||
| Formaldehyde | 186.9 ± 9.0 | 60.5 ± 9.4 | 30.7 ± 2.0 | 44.2 ± 3.1 |
| Acetaldehyde | 313 ± 73 | 782 ± 97 | 87.1 ± 4.0 | 109.8 ± 9.8 |
| Acrolein | (108 ± 33)·101 | 187 ± 57 | 15.7 ± 4.2 | 25.8 ± 2.6 |
| Acetone | <0.4 * | 6.5 ± 1.2 | 4.1 ± 0.6 | <0.2 * |
| Propanal | 13.4 ± 1.1 | 11.0 ± 2.7 | 4.8 ± 1.2 | 2.1 ± 1.0 |
| Butanal | 5.1 ± 4.0 | 13.8 ± 3.8 | 7.2 ± 0.3 | 1.3 ± 0.7 |
| Benzaldehyde | <1.5 ** | <0.5 ** | <0.5 ** | <0.5 ** |
| TCCs | (160 ± 41)·101 | (106 ± 17)·101 | 150 ± 12 | 183 ± 17 |
| Acetic acid | (106 ± 15)·104 (a) | 387 ± 68 (b) | 84 ± 61 (b) | 180 ± 29 (b) |
| Furfural | (360 ± 50)·102 (a) | 337 ± 58 (b) | 27 ± 11 (b) | 126 ± 18 (b) |
| TVOCs | (1235 ± 53)·103 (a) | (838 ± 15)·101 (b) | 111 ± 46 (b) | 301 ± 50 (b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertheau, E.; Simon, V.; Delgado Raynaud, C. Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions. Molecules 2022, 27, 8260. https://doi.org/10.3390/molecules27238260
Bertheau E, Simon V, Delgado Raynaud C. Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions. Molecules. 2022; 27(23):8260. https://doi.org/10.3390/molecules27238260
Chicago/Turabian StyleBertheau, Elise, Valérie Simon, and Christine Delgado Raynaud. 2022. "Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions" Molecules 27, no. 23: 8260. https://doi.org/10.3390/molecules27238260
APA StyleBertheau, E., Simon, V., & Delgado Raynaud, C. (2022). Microchamber Extraction and Analytical Pyrolysis to Explore Volatile Organic Compounds from Compression-Cooking Wood Materials Obtained under Different Conditions. Molecules, 27(23), 8260. https://doi.org/10.3390/molecules27238260









