SnCl4 Promoted Efficient Cleavage of Acetal/Ketal Groups with the Assistance of Water in CH2Cl2
Abstract
1. Introduction
2. Results
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.-Y.; Blaszczyk, S.A.; Xiao, G.-Z.; Tang, W.-P. Chiral reagents in glycosylation and modification of carbohydrates. Chem. Soc. Rev. 2018, 47, 681–701. [Google Scholar] [CrossRef] [PubMed]
- Dimakos, V.; Taylor, M.S. Site-Selective Functionalization of Hydroxyl Groups in Carbohydrate Derivatives. Chem. Rev. 2018, 118, 11457–11517. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, C.-Y.; Guo, Y.-F.; Feng, G.-J.; Dong, H. SnCl2-Catalyzed Acetalation/Selective Benzoylation Sequence for the Synthesis of Orthogonally Protected Glycosyl Acceptors. Eur. J. Org. Chem. 2022, 2022, e202101565. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Luo, T.; Feng, G.-J.; Liu, C.-Y.; Dong, H. Efficient Synthesis of 2-OH Thioglycosides from Glycals Based on the Reduction of Aryl Disulfides by NaBH4. Molecules 2022, 27, 5980. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yu, J.-C.; Feng, G.-J.; Luo, T.; Dong, H. Stannous chloride as a low toxicity and extremely cheap catalyst for regio-/site-selective acylation with unusually broad substrate scope. Green Chem. 2020, 22, 6936–6942. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, F.-L.; Luo, T.; Pei, Z.; Dong, H. Regio/Stereoselective Glycosylation of Diol and Polyol Acceptors in Efficient Synthesis of Neu5Ac-α-2,3-LacNPhth Trisaccharide. Chem. Asian J. 2019, 14, 223–234. [Google Scholar] [CrossRef]
- Ramadan, S.; Yang, W.-Z.; Zhang, Z.-R.; Huang, X.-F. Synthesis of Chondroitin Sulfate A Bearing Syndecan 1 Glycopeptide. Org. Lett. 2017, 19, 4838–4841. [Google Scholar] [CrossRef]
- Lv, J.; Zhu, J.-J.; Liu, Y.; Dong, H. Regioselective Sulfonylation/Acylation of Carbohydrates Catalyzed by FeCl3 Combined with Benzoyltrifluoroacetone and Its Mechanism Study. J. Org. Chem. 2020, 85, 3307–3319. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Zhu, J.-J.; Zou, D.; Dong, H. Regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with ferric chloride and dipivaloylmethane as the catalytic system. Green Chem. 2020, 22, 1139–1144. [Google Scholar] [CrossRef]
- Lv, J.; Ge, J.-T.; Luo, T.; Dong, H. An inexpensive catalyst, Fe(acac)3, for regio/siteselective acylation of diols and carbohydrates containing a 1,2-cis-diol. Green Chem. 2018, 20, 1987–1991. [Google Scholar] [CrossRef]
- Xu, H.-F.; Ren, B.; Zhao, W.; Xin, X.-T.; Lu, Y.-C.; Pei, Y.-X.; Dong, H.; Pei, Z.-C. Regioselective mono and multiple alkylation of diols and polyols catalyzed by organotin and its applications on the synthesis of value-added carbohydrate intermediates. Tetrahedron 2016, 72, 3490–3499. [Google Scholar] [CrossRef]
- Traboni, S.; Bedini, E.; Giordano, M.; Iadonisi, A. Three Solvent-Free Catalytic Approaches to the Acetal Functionalization of Carbohydrates and Their Applicability to One-Pot Generation of Orthogonally Protected Building Blocks. Adv. Synth. Catal. 2015, 357, 3562–3572. [Google Scholar] [CrossRef]
- Tran, A.T.; Jones, R.A.; Pastor, J.; Boisson, J.; Smith, N.; Galan, M.C. Copper(II) Triflate: A Versatile Catalyst for the One-Pot Preparation of Orthogonally Protected Glycosides. Adv. Synth. Catal. 2011, 353, 2593–2598. [Google Scholar] [CrossRef]
- Jones, R.A.; Davidson, R.; Tran, A.T.; Smith, N.; Galan, M.C. Iodine-catalyzed one-pot acetalation-esterification reaction for the preparation of orthogonally protected glycosides. Carbohydr. Res. 2010, 345, 1842–1845. [Google Scholar] [CrossRef]
- Vohra, Y.; Vasan, M.; Venot, A.; Boons, G.J. One-Pot Synthesis of Oligosaccharides by Combining Reductive Openings of Benzylidene Acetals and Glycosylations. Org. Lett. 2008, 10, 3247–3250. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Kulkarni, S.S.; Huang, Y.-W.; Lee, C.-C.; Chang, K.-L.; Hung, S.-C. Regioselective one-pot protection of carbohydrates. Nature 2007, 446, 896–899. [Google Scholar] [CrossRef]
- Shie, C.-R.; Tzeng, Z.-H.; Kulkarni, S.S.; Uang, B.-J.; Hsu, C.-Y.; Hung, S.-C. Cu(OTf)2 as an Efficient and Dual-Purpose Catalyst in the Regioselective Reductive Ring Opening of Benzylidene Acetals. Angew. Chem. Int. Ed. 2005, 44, 1665–1668. [Google Scholar] [CrossRef]
- Garegg, P.J.; Kvarnstrom, I.; Niklasson, A.; Niklasson, G.; Svensson, S.C.T. Partial Substitution of Thioglycosides by Phase Transfer Catalyzed Benzoylation and Benzylation. J. Carbohydr. Chem. 1993, 12, 933–953. [Google Scholar] [CrossRef]
- Abronina, P.I.; Malysheva, N.N.; Litvinenko, V.V.; Zinin, A.I.; Kolotyrkina, N.G.; Kononov, L.O. A Ring Contraction of 2,3-Di-O-Silylated Thiopyranosides To Give Thiofuranosides under Mildly Acidic Conditions. Org. Lett. 2018, 20, 6051–6054. [Google Scholar] [CrossRef]
- Yan, M.-C.; Chen, Y.-N.; Wu, H.-T.; Lin, C.-C.; Chen, C.-T.; Lin, C.-C. Removal of Acid-Labile Protecting Groups on Carbohydrates Using Water-Tolerant and Recoverable Vanadyl Triflate Catalyst. J. Org. Chem. 2007, 72, 299–302. [Google Scholar] [CrossRef]
- Procopio, A.; Dalpozzo, R.; Nino, A.D.; Maiuolo, L.; Nardi, M.; Romeo, G. Mild and efficient method for the cleavage of benzylidene acetals by using erbium (III) triflate. Org. Biomol. Chem. 2005, 3, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hui, Y.-Z. A Convenient Method for Highly Selective Deprotection of Benzylidene Acetals from Sugars. Synth. Commun. 1996, 26, 881–886. [Google Scholar] [CrossRef]
- Michigami, K.; Terauchi, M.; Hayashi, M. Cleavage of 4,6-O-Benzylidene Acetal Using Sodium Hydrogen Sulfate Monohydrate. Synthesis 2013, 45, 1519–1523. [Google Scholar]
- Szarek, W.A.; Zamojski, A.; Tiwari, K.N.; Ison, E.R. A new, facile method for cleavage of acetals and dithioacetals in carbohydrate derivatives. Tetrahedron Lett. 1986, 27, 3827–3830. [Google Scholar] [CrossRef]
- Chen, C.-T.; Lin, Y.-D.; Liu, C.-Y. Catalytic carbon–sulfur bond formation by amphoteric vanadyl triflate: Exploring with thia-Michael addition, thioacetalization, and transthioacetalization reactions. Tetrahedron 2009, 65, 10470–10476. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, J.; Sun, J.-C.; Cai, L.; Zhao, Y.-Q.; Fang, J.; Hu, B.; Shu, P.-H.; Meng, L.-K.; Wan, Q. 1,4-Dithiothreitol mediated cleavage of the acetal and ketal type of diol protecting groups. Org. Chem. Front. 2018, 5, 2427–2431. [Google Scholar] [CrossRef]
- Kim, K.-S.; Song, Y.-H.; Lee, B.-H.; Hahn, C.-S. Efficient and Selective Cleavage of Acetals and Ketals Using Ferric Chloride Adsorbed on Silica Gel. J. Org. Chem. 1986, 51, 404–407. [Google Scholar] [CrossRef]
- Niu, Y.-H.; Wang, N.; Cao, X.-P.; Ye, X.-S. Efficient Formation and Cleavage of Benzylidene Acetals by Sodium Hydrogen Sulfate Supported on Silica Gel. Synlett 2007, 13, 2116–2120. [Google Scholar] [CrossRef]
- Agnihotri, G.; Misra, A.K. Mild and efficient method for the cleavage of benzylidene acetals using HClO4-SiO2 and direct conversion of acetals to acetates. Tetrahedron Lett. 2006, 47, 3653–3658. [Google Scholar] [CrossRef]
- Roy, B.; Verma, P.; Mukhopadhyay, B. H2SO4-silica-promoted ‘on-column’ removal of benzylidene, isopropylidene, trityl and tert-butyldimethylsilyl groups. Carbohydr. Res. 2009, 344, 145–148. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, G.D.K.; Baskaran, S. Truly Catalytic and Chemoselective Cleavage of Benzylidene Acetal with Phosphomolybdic Acid Supported on Silica Gel. Eur. J. Org. Chem. 2008, 2008, 6063–6067. [Google Scholar] [CrossRef]
- Couri, M.R.C.; Evangelista, E.A.; Alves, R.B.; Prado, M.A.F.; Gil, R.P.F.; De Almeida, M.V.; Raslan, D.S. Microwave-Assisted Rapid Deacetalation of Carbohydrates. Synth. Commun. 2005, 35, 2025–2031. [Google Scholar] [CrossRef]
- Santra, A.; Ghosh, T.; Misra, A.K. Removal of benzylidene acetal and benzyl ether in carbohydrate derivatives using triethylsilane and Pd/C. Beilstein J. Org. Chem. 2013, 9, 74–78. [Google Scholar] [CrossRef]
- Hori, H.; Nishida, Y.; Ohrui, H.; Meguro, H. Regioselective De-O-benzylation with Lewis Acids. J. Org. Chem. 1989, 54, 1346–1353. [Google Scholar] [CrossRef]
- Doyle, L.M.; O’Sullivan, S.; Di Salvo, C.; McKinney, M.; McArdle, P.; Murphy, P.V. Stereoselective Epimerizations of Glycosyl Thiols. Org. Lett. 2017, 19, 5802–5805. [Google Scholar] [CrossRef] [PubMed]
- Kartha, K.P.R.; Kiso, M.; Hasegawa, A.; Jennings, H.J. Novel Selectivity in Carbohydrate Reactions III. Selective Deprotection of p-Methoxybenzyl (PMBn) Ethers of Carbohydrates by Tin(IV) Chloride. J. Carbohydr. Chem. 1998, 17, 811–817. [Google Scholar] [CrossRef]
- Sawama, Y.; Masuda, M.; Asai, S.; Goto, R.; Nagata, S.; Nishimura, S.; Monguchi, Y.; Sajiki, H. FeCl3-Catalyzed Self-Cleaving Deprotection of Methoxyphenylmethyl-Protected Alcohols. Org. Lett. 2015, 17, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Dombray, T.; Blanc, A.; Weibel, J.M.; Pale, P. Silver(I)-Catalyzed Deprotection of p-Methoxybenzyl Ethers: A Mild and Chemoselective Method. J. Org. Chem. 2012, 77, 9227–9235. [Google Scholar] [CrossRef]
- Ren, B.; Gan, L.; Zhang, L.; Yan, N.-N.; Dong, H. Diisopropylethylamine-triggered, highly efficient, self-catalyzed regioselective acylation of carbohydrates and diols. Org. Biomol. Chem. 2018, 16, 5591–5597. [Google Scholar] [CrossRef]
- Matwiejuk, M.; Thiem, J. Defining oxyanion reactivities in base-promoted glycosylations. Chem. Commun. 2011, 47, 8379–8381. [Google Scholar] [CrossRef]
- Rocheleau, S.; Pottel, J.; Huskić, I.; Moitessier, N. Highly Regioselective Monoacylation of Unprotected Glucopyranoside Using Transient Directing-Protecting Groups. Eur. J. Org. Chem. 2017, 2017, 646–656. [Google Scholar] [CrossRef]
- Medgyes, G.; Jerkovich, G.; Kuszmann, J.; Fügedi, P. Synthesis of sorbistin analogues. Carbohydr. Res. 1989, 186, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Moen, A.R.; Anthonsen, T. Screening of the regioselectivity of acetyl xylan esterase from Bacillus pumilus as a catalyst for the deacetylation of glycoside acetates. Biocatal. Biotransformation 2009, 27, 226–236. [Google Scholar] [CrossRef]
- Attouche, A.; Urban, D.; Beau, J.M. A Tin-Free Regioselective Radical De-O-benzylation by an Intramolecular Hydrogen Atom Transfer on Carbohydrate Templates. Angew. Chem. Int. Ed. 2013, 52, 9572–9575. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-Z.; Wang, K.-X.; Ma, K.-R.; Zhao, W.; Zhang, G.-Q. Preparation of rare L-idose derivatives from D-glucofuranose via neighboring acyl group assistance. Tetrahedron Lett. 2021, 73, 153135–153139. [Google Scholar] [CrossRef]
- Kajihara, Y.; Kodam, H.; Endo, T.; Hashimoto, H. Novel features of acceptor recognition by β-(1→4)-galactosyltransferase. Carbohydr. Res. 1998, 306, 361–378. [Google Scholar] [CrossRef]
- Fujiki, K.; Tanaka, K. Exploration of the Fluoride Reactivity of Aryltrifluoroborate on Selective Cleavage of Diphenylmethylsilyl Groups. Eur. J. Org. Chem. 2020, 29, 4616–4620. [Google Scholar] [CrossRef]
- Cavedon, C.; Sletten, E.T.; Madani, A.; Niemeyer, O.; Seeberger, P.H.; Pieber, B. Visible-Light-Mediated Oxidative Debenzylation Enables the Use of Benzyl Ethers as Temporary Protecting Groups. Org. Lett. 2021, 23, 514–518. [Google Scholar] [CrossRef]
- Johnsson, R.; Ohlin, M.; Ellervik, U. Reductive Openings of Benzylidene Acetals Revisited: A Mechanistic Scheme for Regio- and Stereoselectivity. J. Org. Chem. 2010, 75, 8003–8011. [Google Scholar] [CrossRef]
- Borén, H.B.; Garegg, P.J.; Pilotti, Å.; Swahn, C.-G. NMR Spectra of Some Glycoside Acetates in the Presence of Tris(dipivaloylmethanato)europium. Acta Chem. Scand. 1972, 26, 3261–3268. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Ramstrom, O.; Dong, H. Organosilicon-mediated regioselective acetylation of carbohydrates. Chem. Commun. 2012, 48, 5370–5372. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Senthilkumar, S.; Baskaran, S. Benzylidene acetal protecting group as a carboxylic acid surrogate: Synthesis of functionalized uronic acids and sugar amino acids. Chem. Eur. J. 2016, 22, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Dayoub, W.; Chen, G.-R.; Lemaire, M. TMDS as a Dual-Purpose Reductant in the Regioselective Ring Cleavage of Hexopyranosyl Acetals to Ethers. Eur. J. Org. Chem. 2012, 2012, 1960–1966. [Google Scholar] [CrossRef]
- Seitz, A.; Wende, R.C.; Roesner, E.; Niedek, D.; Topp, C.; Colgan, A.C. McGarrigle, E.M.; Schreiner, P.R. Site-Selective Acylation of Pyranosides with Oligopeptide Catalysts. J. Org. Chem. 2021, 86, 3907–3922. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, S.; Hansen, T.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. The Influence of Acceptor Nucleophilicity on the Glycosylation Reaction Mechanism. Chem. Sci. 2017, 8, 1867–1875. [Google Scholar] [CrossRef]
- Maki, Y.; Nomura, K.; Okamoto, R.; Izumi, M.; Mizutani, Y.; Kajihara, Y. Acceleration and Deceleration Factors on the Hydrolysis Reaction of 4,6-O-Benzylidene Acetal Group. J. Org. Chem. 2020, 85, 15849–15856. [Google Scholar] [CrossRef]
- Emmadi, M.; Kulkarni, S.S. Synthesis of Rare Deoxy Amino Sugar Building Blocks Enabled the Total Synthesis of a Polysaccharide Repeating Unit Analogue from the LPS of Psychrobacter cryohalolentis K5T. J. Org. Chem. 2018, 83, 14323–14337. [Google Scholar] [CrossRef]
- Ye, D.-F.; Liu, Z.-Y.; Chen, H.; Sessler, J.L.; Lei, C.-H. Cesium Carbonate Catalyzed Esterification of N-Benzyl-N-Bocamides under Ambient Conditions. Org. Lett. 2019, 21, 6888–6892. [Google Scholar] [CrossRef]
- Bauder, C. A Convenient synthesis of orthogonally protected 2-deoxystreptamine (2-DOS) as an aminocyclitol scaffold for the development of novel aminoglycoside antibiotic derivatives against bacterial resistance. Org. Biomol. Chem. 2008, 6, 2952–2960. [Google Scholar] [CrossRef]
- Babu, R.B.R.; Sørensen, M.D. Parmar, V.S.; Harrit, N.H.; Wengel, J. Oligodeoxynucleotides containing α-L-ribo confifigured LNA-type C-aryl nucleotides. Org. Biomol. Chem. 2004, 2, 80–89. [Google Scholar]
- Viuffa, A.H.; Heuckendorfa, M.; Jensen, H.H. p-Chlorobenzyl Ether: A p-Methoxybenzyl Ether in Disguise. Org. Lett. 2016, 18, 5773–5775. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Banerjee, A.; Yao, Q.-J. Direct Chemical Synthesis of the β-d-Mannans: The β-(1→2) and β-(1→4) Series. J. Am. Chem. Soc. 2004, 126, 14930–14934. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Kawada, T.; Rosenau, T.; Kosma, P. Synthesis of methyl 4′-O-methyl-13C12-β-D-cellobioside from 13C6-D-glucose. Part 1: Reaction optimization and synthesis. Carbohydr. Res. 2005, 340, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
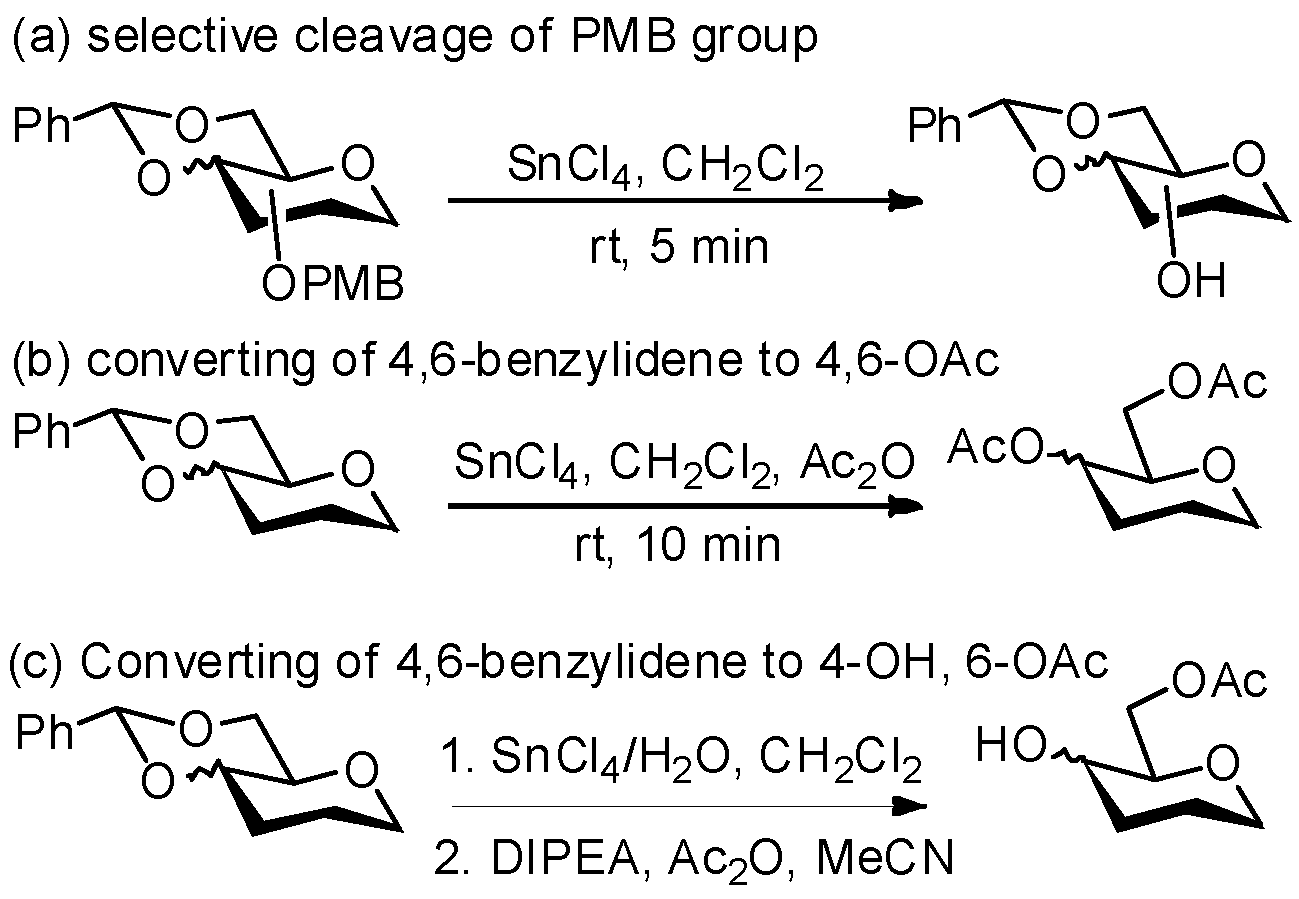




| Entry | Reaction Conditions | Lit. |
|---|---|---|
| 1 | AcOH/H2O, 100 °C, 0.5–1 h | [18,19] |
| 2 | TFA/H2O, CH2Cl2, rt, 0.5–1 h | [18,19] |
| 3 | VO(OTf)2, MeOH/CH2Cl2, 55 °C, 16–18 h | [20] |
| 4 | Er(OTf)3, CH3CN, rt, 2–24 h | [21] |
| 5 | SnCl2, CH2Cl2, rt, 12 h | [22] |
| 6 | NaHSO4, MeOH/CH2Cl2, rt, 1–24 h | [23] |
| 7 | I2, CH3OH, rt/reflux, 24/2.5 h | [24] |
| 8 | VO(OTf)2/HS(CH2)3SH, CH3CN/CH2Cl2, rt, 1 h | [25] |
| 9 | CSA/DTT, CH2Cl2, rt, 2 h | [26] |
| 10 | FeCl3-SiO2, CHCl3, rt, 10 h | [27] |
| 11 | NaHSO4-SiO2, MeOH/CH2Cl2, rt, 10–20 h | [28] |
| 12 | HClO4-SiO2, CH3CN, rt, 0.5 h | [29] |
| 13 | H2SO4-SiO2, CH2Cl2, rt, 0.5 h | [30] |
| 14 | PMA-SiO2, CH2Cl2, 0.5–5 h | [31] |
| 15 | AcOH/H2O-SiO2, microwave, 10 min | [32] |
| 16 | Et3SiH, Pd/C, CH3OH, rt, 0.5–2 h | [33] |

| Entry | Additives (equiv) | Reaction Conditions | Yield of 2 (%) |
|---|---|---|---|
| 1 | SnCl4 (0.2\0.5) | DCM, rt. 0.5 h | 19\47 |
| 2 | SnCl4 (1.0\1.5) | DCM, rt. 0.5 h | 69\79 |
| 3 | SnCl4 (2.0\2.5) | DCM, rt. 0.5 h | 93\95 |
| 4 | SnCl4 (2.5) | MeOH\MeCN, rt. 0.5 h | 20\40 |
| 5 b | SnCl4 (1.2), H2O (0.5\1.0) | DCM, rt. 0.5 h | 85\92 |
| 6 | SnCl4 (1.2), H2O (0.5\1.0) | DCM, rt. 0.5 h | 80\91 |
| 7 | SnCl4 (0.5\1.0), H2O (1.0) | DCM, rt. 0.5 h | 47\75 |
| 8 | SnCl4 (1.2\1.5), H2O (1.5\1.0) | DCM, rt. 10 min | 93\95 |
| 9 | SnCl4 (1.5), MeOH (2.0\3.0) | DCM, rt. 0.5 h | 37\41 |
| 10 | HCl (36%, 0.3) | DCM, rt. 0.5 h | 32 |
| 11 | SnCl2\FeCl3 (1.2), H2O (1.0) | DCM, rt. 0.5 h | 63\71 |
| 12 | CuCl2\Cu(OTf)2 (1.2), H2O (1.0) | DCM, rt. 0.5 h | 0\10 |
| 13 | SnCl4 (1.2), AcCl (1.2\2.2) | DCM, rt. 0.5 h | - |
| 14 | SnCl4 (1.2), Ac2O (1.2\2.2) | DCM, rt. 0.5 h | 2a: 30\78 |
| Entry | Substrate | Product | Yield |
|---|---|---|---|
| 1 |  | 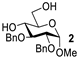 | 92% from 1b 90% from 1c |
| 2 | 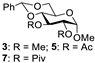 |  | 4: 91% 6: 98% 8: 97% |
| 3 |  |  | 95% |
| 4 |  | 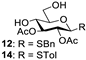 | 12: 90% 14: 94% |
| 5 | 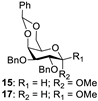 |  | 16: 89% 18: 90% |
| 6 |  |  | 93% |
| 7 |  | 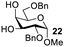 | 97% |
| 8 |  |  | 25: 94% 27: 95% |
| 9 b | 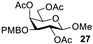 |  | 92% |
| 10 b |  | 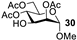 | 98% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Xu, T.-T.; Guo, Y.-F.; Dong, H. SnCl4 Promoted Efficient Cleavage of Acetal/Ketal Groups with the Assistance of Water in CH2Cl2. Molecules 2022, 27, 8258. https://doi.org/10.3390/molecules27238258
Luo T, Xu T-T, Guo Y-F, Dong H. SnCl4 Promoted Efficient Cleavage of Acetal/Ketal Groups with the Assistance of Water in CH2Cl2. Molecules. 2022; 27(23):8258. https://doi.org/10.3390/molecules27238258
Chicago/Turabian StyleLuo, Tao, Tian-Tian Xu, Yang-Fan Guo, and Hai Dong. 2022. "SnCl4 Promoted Efficient Cleavage of Acetal/Ketal Groups with the Assistance of Water in CH2Cl2" Molecules 27, no. 23: 8258. https://doi.org/10.3390/molecules27238258
APA StyleLuo, T., Xu, T.-T., Guo, Y.-F., & Dong, H. (2022). SnCl4 Promoted Efficient Cleavage of Acetal/Ketal Groups with the Assistance of Water in CH2Cl2. Molecules, 27(23), 8258. https://doi.org/10.3390/molecules27238258





