Abstract
Anchusa strigosa is a widespread weed in Greece, Syria, Turkey, Lebanon, Israel, Jordan, and Iran. The purpose of this study was to identify the phytochemicals of Anchusa strigose and estimate the pro-wound healing (pro-WH) and antimicrobial activities of its active compounds. An identification of volatile compounds was performed by GC/MS analysis; HPLC, LC-ESI-MS, and MALDI-TOF-MS were also applied. Our results demonstrate that two specific combinations of compounds from A. strigosa extract significantly enhanced WH (p < 0.001). Several flavonoids of the plant extract, including quercetin 3-O-rutinoside, kaempferol, kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside, and kaempferol 3-O-α-rhamnopyranosyl(1→6)-β-galactopyranoside, were effective against drug-resistant microorganisms. In addition, all the above-mentioned compounds had antibiofilm activity against Escherichia coli and Salmonella enteritidis.
1. Introduction
Anchusa strigosa (strigose bugloss or prickly alkanet) is a widespread weed, with a rosette of leaves at its base and an inflorescence stem reaching one meter or more [1]. This plant belongs to the Boraginaceae family, and it is native to Greece, Syria, Turkey, Lebanon, Israel, Jordan, and Iran. The specimens used in this research were collected in the Judea region (Israel). This region has a peculiar geography that influences local herbal populations [1]. The plants from the Judea region are exposed to permanent stress, which stimulates production of high quantities of various phytochemicals with promising therapeutic properties [2,3,4,5,6,7,8,9,10].
Although many publications are devoted to the plants of this region, to the best of our knowledge, A. strigosa medicinal properties have not been reported. In general, some studies demonstrated medicinal properties of A. strigosa from other regions including anti-ulcer [11], pro-wound healing (WH) [12] and antioxidant activities [13,14]. Yet, it is still unknown which compounds are responsible for their therapeutic effects.
In this publication, we focus on investigating pro-WH properties since WH is one of the most important public health problems worldwide. For example, chronic wounds annually affect 6.5 million patients in the United States [15]. Indeed, the number of chronic wounds has a tendency to increase due to the rise in age-related conditions and pathologies, such as diabetes, obesity, and cardiovascular diseases [15]. The calling card of WH is inadequate efficacy and a number of serious adverse effects associated with the widespread use of common therapeutic agents. For example, glucocorticoids are effective in many cases, but they have many side effects, including the promotion of wound infections [15]. In addition, deviations from regular WH could lead to diverse pathological conditions, from slow or ineffective repair (e.g., diabetic, pressure and ischemic skin ulcers, mainly in older people) to fibrosis (e.g., hypertrophic scars, keloids, scleroderma, mainly in young and middle-aged people [16,17].
As suggested by us and others [10,13,18,19], another factor that retards the process of WH are infections, mainly of the bacterial origin.
With regard to bacterial infections, the combined influence of environmental factors [20,21] and overuse of antibiotics [21] can cause the widespread appearance of antibiotic-resistant microorganisms. Some modern approaches against antibiotic-resistant microorganisms include a reduction in antibiotic consumption, preservation of existing therapeutics, and development of new antibiotics [21]. Unfortunately, the above-mentioned measures have not been effective so far.
Gram-negative bacteria are more widespread in hospital patients than Gram-positive bacteria [22]. Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Serratia marcescens and Salmonella enteritidis are Gram-negative species that are responsible for major health problems worldwide.
In addition, many microorganisms become more dangerous because of quorum sensing (QS) [23]. QS is one of the major signaling mechanisms that directly contributes to biofilm formation [24]. Biofilm formation is one of the challenging problems in treating infections as bacteria in biofilms are extremely resistant to the antibacterial agents.
Thus, there is an urgent demand for novel anti-microbial compounds that must be effective, non-toxic and able to suppress QS. Medicinal plants hold enormous potential in this context as A. strigosa is a rich reservoir of such compounds.
In summary, this study aimed to identify active compounds of A. strigosa with pro-WH and antimicrobial activities.
2. Results
2.1. Identification of Active Compounds
The following compounds of A. strigosa were identified (Table 1). Bioassay-guided fractionation of the extract allowed us to determine that only one fraction eluted with 80%-MeOH had pro-wound healing and anti-microbial properties. Different analytical methods used in this study confirmed each other, and the comprehensive use of various analytical methods was very helpful for increasing the probability of compound identification. The Supplementary Materials includes the GC-MS and LC-ESI-MS chromatograms of extracts of Anchusa strigosa.

Table 1.
Identification of phytochemicals of A. strigosa.
2.2. Pro-Wound Healing (WH) Properties
Taking into consideration an important role of dermal fibroblasts in skin WH, we used HDF (human dermal fibroblasts) cell lines in the current study. The crude extract was not toxic at a concentration below 900 µg/mL for HDF cells; quercetin 3-O-rutinoside and ellagic acid also had no cytotoxicity at this concentration. The other identified compounds were toxic for the HDF cells at concentrations of more than 100 µg/mL. The ethanol at the used concentrations was not toxic.
The crude extract significantly stimulated pro-WH (p < 0.001), when it was added at a concentration of 50 µg/mL (Figure 1). It is interesting to note that, at a concentration of 50 µg/mL, each separate compound did not significantly change the gap closure. Then, the compounds were tested in various combinations. Figure 1 demonstrates that both combinations of the tested compounds, combination 1 and combination 2, had a significant pro-WH effect (p < 0.001). Combination 1 of the tested compounds included quercetin 3-O-rutinoside at a concentration of 50 µg/mL, ellagic acid at a concentration of 10 µg/mL, and kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside at a concentration of 10 µg/mL. Combination 2 of the tested compounds included kaempferol at a concentration of 10 µg/mL and ellagic acid at a concentration of 1 µg/mL. In addition, both mixtures of compounds were not toxic for cells (unpresented data).
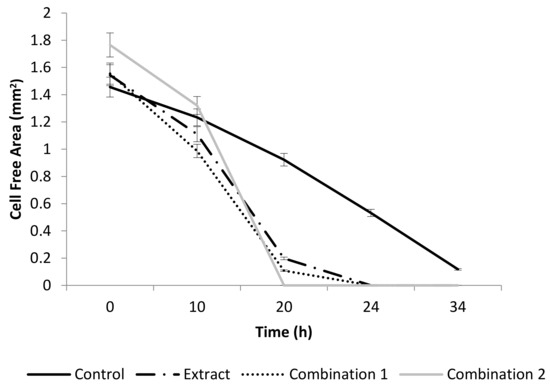
Figure 1.
The effect of A. strigosa extract and compounds on wound healing in vitro. The rate of gap closure in cultured human dermal fibroblasts (scratch assay: in vitro model of wound healing) at 0, 10, 20, 24, 34 h after wound generation. Control was untreated fibroblasts. Crude extract was added at a concentration of 50 µg/mL. The combination 1 of tested compounds included: quercetin 3-O-rutinoside at a concentration of 50 µg/mL, ellagic acid at a concentration of 10 µg/mL and kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside at a concentration of 10 µg/mL. Combination 2 of the tested compounds included: kaempferol at a concentration of 10 µg/mL and ellagic acid at a concentration of 1 µg/mL. Data from three independent experiments are shown (mean ± SD).
2.3. Estimation of Anti-Microbial Properties
The ethanolic diluted extract of A. strigosa and its identified compounds (Table 1) were applied for the estimation of their anti-microbial properties in vitro. The concentration of the initial extract was 2.5 mg/mL in PBS, and the diluted concentration was 0.1 mg/mL. The use of a positive control was not possible in this experiment because antibiotics do not influence these strains. The negative control was PBS. The phytochemicals were tested at a concentration of 2 µM. Although every identified compound was examined, only effective compounds are presented in Figure 2. We found that quercetin 3-O-rutinoside was less effective than the crude extract, while kaempferol and its glycoside derivatives significantly inhibited all tested microorganisms (p < 0.001).
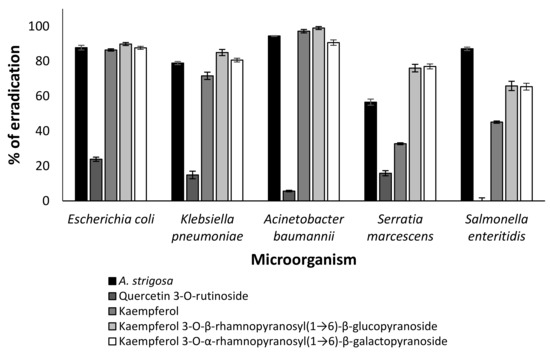
Figure 2.
Effect of A. strigosa extract and its phytochemicals on eradication of drug-resistant microorganisms (Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Serratia marcescens and Salmonella enteritidis). The phytochemicals were applied at concentration of 2 µM. Data from three independent experiments are shown.
In order to estimate an effect of all identified compounds on biofilm formation, a crystal violet assay was used [25]. Only quercitin 3-O-rutinoside, kaempferol, kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside and kaempferol 3-O-α-rhamnopyranosyl(1→6)-β-galactopyranoside had antibiofilm activity (Figure 3). The effect of these compounds was significant against Escherichia coli and Salmonella enteritidis (p < 0.001) (Figure 3), while streptomycin (positive control) was less effective against Salmonella enteritidis than quercitin 3-O-rutinoside, kaempferol, kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside and kaempferol 3-O-α-rhamnopyranosyl(1→6)-β-galactopyranoside (Figure 3).
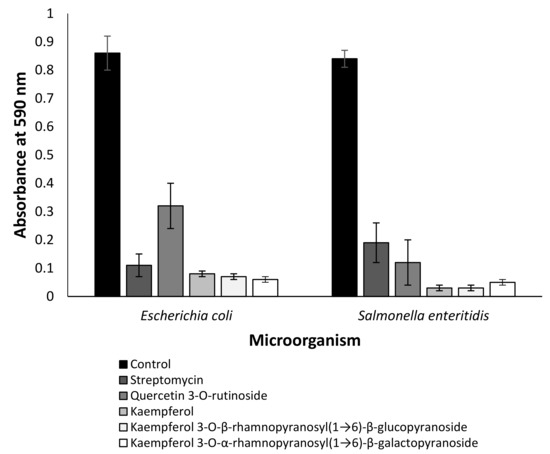
Figure 3.
Effect of quercitin 3-O-rutinoside, kaempferol and kaempferol glycoside derivatives on biofilm formation. The results are presented as the mean ± SE of the absorbance at 590 nm. The identified compounds and streptomycin were used at a concentration of 2 µM. Data from three independent experiments are shown.
3. Discussion
Our previous publications [6,10] showed that plants growing in the Judea region (Israel) have unique chemotypes. For example, when we studied another plant from the same area, Phlomis viscosa Poiret, we expected to identify β-Caryophyllene, germacrene D, alloaromadendrene and humulene as bioactive compounds since these compounds were reported to have medicinal activities in plants growing in Turkey [26]. Surprisingly, these compounds were not identified by us, but other compounds were found to possess medicinal properties in the Israeli plants [6,10].
Although MALDI-TOF-MS is more often used for the analysis of polypeptides, the structural heterogeneity of plant polyphenols requires more detailed investigation [27,28]. The different analytical methods used in this study confirmed each other, and the comprehensive use of various analytical methods was very helpful for increasing the probability of compound identification.
The presence of pyrrolizidine alkaloid was confirmed in Anchusa strigosa (Table 1) and its presence is also mentioned in the literature [29], but to the best of our knowledge, the rest of the identified compounds (Table 1) were not reported to be present in the investigated weed. We showed that the crude extract was not toxic at concentrations below 900 µg/mL for HDF cells. Its concentration of 50 µg/mL was sufficient for effective pro-wound healing activity (Figure 1), while each identified compound on its own is not active. We succeeded in finding some effective combinations of compounds (Figure 1), proving that the extract has a pro-wound healing activity because of the synergistic interactions between its components. It is interesting that the absence of the desired activity in individual identified compounds of plant extracts is mentioned in the literature [10,30,31]. Further research to identify other compounds of the extract is necessary.
Quercetin 3-O-rutinoside, ellagic acid, kaempferol and kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside were identified as the pro-WH components of the extract (Figure 1). Although the pro-WH properties of quercetin 3-O-rutinoside, ellagic acid and kaempferol were previously mentioned [15,31,32,33], kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside was not reported as a pro-WH compound. The molecular mechanisms of their actions are unknown. The modes of actions behind the synergistic enhancement of WH by our combinations of compounds are yet to be investigated.
We demonstrated that several flavonoids of Anchusa strigosa were effective against drug-resistant microorganisms (Figure 2). The antibacterial activity of kaempferol against drug-resistant Staphylococcus aureus was reported [34], but no information is available on the antibacterial activity of kaempferol 3-O-β-rhamnopyranosyl(1→6)-β-glucopyranoside and kaempferol 3-O-α-rhamnopyranosyl(1→6)-β-galactopyranoside. Although the mode of action of some antibacterial flavonoids was partially revealed [35], the mechanism of action of kaempferol and its glycoside derivatives should be investigated in the future. In addition, all of these compounds demonstrated anti-QS properties (Figure 3).
Altogether, our results indicate the beneficial effect of extracts of A. strigosa and its phytochemicals on WH, as well as their anti-microbial properties, including anti-QS abilities and efficacy against drug-resistant bacteria. Thus, there is a need for further and more detailed research on the mechanisms of action of the tested compounds.
4. Materials and Methods
4.1. Preparation of Plant Material
Leaves and flowers of A. strigosa were grounded for gas chromatography/mass spectrometry (GC/MS) analysis. Leaves and flowers of the plant were used for the preparation of the extract as described in our previous publications [10,32,35]. Fractionation of the extract was performed with assistance of reverse phase RP-C18 Sepack column (Supelco, St. Louis, MO, USA); methanol gradients were 0% (v/v), 20% (v/v), 40% (v/v), 60% (v/v), 80% (v/v) and 100% (v/v) [10,36].
4.2. Identification of Plant Compounds
An identification of volatile compounds was performed with assistance of GC/MS analysis [6,10,36]. High-performance liquid chromatography (HPLC) (HEKAtech GmbH, Wegberg, Germany), liquid chromatography–electrospray ionization–mass spectrometry (LC-ESI-MS) (Bruker Daltonik GmbH, Bremen, Germany) and Matrix-assisted laser desorption/ionization—time-of-flight—mass spectrometry (MALDI-TOF-MS) (Bruker, Berlin, Germany) were used.
4.3. Materials and Bacterial Strains
Lyophilized powders of Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Serratia marcescens and Salmonella enteritidis were obtained from ATCC, and drug-resistant bacteria were treated as described in our previous publication [10].
4.4. Biofilm Formation Estimation
The crystal violet assay was performed as previously described [37]. The biofilm-producing bacteria were cultured with or without the tested compounds or streptomycin for 48 h at 37 °C. The nonadherent bacteria were removed by washing with sterile PBS, and adherent bacteria were stained for 10 min with a 1% crystal violet solution. A crystal violet assay was performed in 96-well polystyrene plates.
4.5. Anti-Bacterial Activity
An equal number of cells were seeded in 96-well plates in 100 μL of appropriate medium with or without the tested compounds. Desired concentration of each chemical was achieved by addition of calculated volume of stock solutions. Plates were incubated at 37 °C and in 5% CO2 for up to 5 days. Toxicity was determined either by cell counting once every 24 h or by WST method on the final day, as described by manufacturer [38].
4.6. In Vitro Wound Healing Assay
Pro-wound-healing effects were studied in an in vivo, as described in our previous publication [10].
4.7. Statistical Analysis
Statistica Version 13.6 for Windows software (StatSoft, Inc., Tulsa, OK, USA) was chosen for statistical data processing. Numbers represent the mean ± standard error from at least three independent experiments, each conducted as duplicates. Mean values were compared using Student’s t-test, and the difference between the results was considered significant when the p-value was less than 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238239/s1, Figure S1: GC-MS and LC-ESI-MS chromatograms of extracts of Anchusa strigosa.
Author Contributions
Conceptualization, S.B.-S., A.B., L.Y. (Ludmila Yarmolinsky) and B.K.; methodology, B.K. and L.Y. (Ludmila Yarmolinsky); software, B.K. and L.Y. (Ludmila Yarmolinsky); validation, L.Y. (Ludmila Yarmolinsky); formal analysis, A.B.; investigation, A.B., B.K., L.Y. (Ludmila Yarmolinsky) and L.Y. (Leonid Yarmolinsky); resources, S.B.-S.; data curation, B.K.; writing—original draft preparation, L.Y. (Ludmila Yarmolinsky) and A.B.; writing—review and editing, S.B.-S.; visualization, B.K.; supervision, S.B.-S.; project administration, S.B.-S.; funding acquisition, L.Y. (Ludmila Yarmolinsky). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the extracts and compounds are available at Eastern R&D Center, Kiryat Arba 9010000, Israel.
References
- Russell, A.; Russell, P. The Natural History of Aleppo: Containing a Description of the City, and the Principal Natural Productions in Its Neighbourhood: Together with an Account of the Climate, Inhabitants, and Diseases, Particularly of the Plague, 2nd ed.; Gregg International Publishers Limited, Westmead: London, UK, 1794; p. 1. Available online: https://www.biodiversitylibrary.org/item/133807#page/9/mode/1up (accessed on 20 September 2022).
- Budovsky, A.; Fraifeld, V.E. Medicinal plants growing in the Judea region: Network approach for searching potential therapeutic targets. Netw. Biol. 2012, 2, 84–94. [Google Scholar] [CrossRef]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000, 72, 191–205. [Google Scholar] [CrossRef]
- Gorelick, J.; Kitron, A.; Pen, S.; Rosenzweig, T.; Madar, Z. Anti-diabetic activity of Chiliadenus iphionoides. J. Ethnopharmacol. 2011, 137, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Tamir, H.; Satovic, Z.; Gorelick, J.; Danin, A.; Fischer, R.; Chaimovitsh, D.; Dudai, N. Intraspecific variation of Chiliadenus iphionoides essential oil in Israel. Chem. Biodivers. 2011, 8, 1065–1082. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Budovsky, A.; Ben-Shabat, S.; Khalfin, B.; Gorelick, J.; Bishitz, Y.; Miloslavski, R.; Yarmolinsky, L. Recent Updates on the Phytochemistry and Pharmacological Properties of Phlomis viscosa Poiret. Rejuvenation Res. 2019, 22, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boakye, Y.D.; Bekoe, E.O.; Hensel, A.; Dapaah, S.O.; Appiah, T. Review: African medicinal plants with wound healing properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Budovsky, A.; Shteinberg, A.; Maor, H.; Duman, O.; Yanai, H.; Wolfson, M.; Fraifeld, V.E. Uncovering the geroprotective potential of medicinal plants from the Judea region of Israel. Rejuvenation Res. 2014, 17, 134–139. [Google Scholar] [CrossRef]
- Yarmolinsky, L.L.; Budovsky, A.; Yarmolinsky, L.L.; Khalfin, B.; Glukhman, V.; Ben-Shabat, S. Effect of bioactive phytochemicals from phlomis viscosa poiret on wound healing. Plants 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Disi, A.M.; Tamimi, S.O.; Abuereish, G.M. Effects of Anchusa strigosa root aqueous extract on gastric ethanol-induced ulcer in laboratory animals. J. Ethnopharmacol. 1998, 60, 189–198. [Google Scholar] [CrossRef]
- Aburjai, T.; Hudaib, M.; Tayyem, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294–304. [Google Scholar] [CrossRef]
- Al-Khateeb, E.H.; Al-Assi, G.A.; Shakya, A.K.; Al-Rawi, N.S.N. Antioxidant Potential of Pistacia Vera L. Fruit Hull, Anchusa Strigosa Flowers and Ilex Paraguariensis A. St.-Hil. leaves Extracte. Orient. J. Chem. 2019, 35, 982. [Google Scholar]
- Al-Snafi, A.E. The pharmacology of Anchusa Italica and Anchusa strigosa. A review. Int. J. Pharm. Pharm. Sci. 2014, 64, 7–10. [Google Scholar]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Ghatnekar, G.S.; Palatinus, J.A.; O’Quinn, M.; Yost, M.J.; Gourdie, R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008, 26, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Valentine, K.P.; Viacheslav, K.M. Bacterial flora of combat wounds from eastern Ukraine and time-specified changes of bacterial recovery during treatment in Ukrainian military hospital. BMC Res. Notes 2017, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Mohr, C.; Lügger, K.; Heller, C.; Siemens, J.; Mulder, I. Widespread occurrence of quaternary alkylammonium disinfectants in soils of Hesse, Germany. Sci. Total Environ. 2022, 857, 159228. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.-P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Bronstein, M.; Gorelick, J. Review: Inhibition of bacterial quorum sensing by plant extracts. Isr. J. Plant Sci. 2015, 62, 294–297. [Google Scholar] [CrossRef]
- Wagh, M.S.; Osborne, W.J.; Sivarajan, S. Volatile Organic Compounds for Enhancement of Plant Growth through Plant Growth Promoting Rhizobacteria; Kumar, A., Singh, J., Samuel, J., Eds.; Academic Press: Warsaw, Poland, 2021; Chaper 16; pp. 325–347. ISBN 978-0-12-824523-1. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; ComlekcIog, N. Essential Oil Composition of Nepeta cilicia Boiss. Apud Bentham and Phlomis viscosa Poiret from Turke. Int. J. Bot. 2006, 3, 122–124. [Google Scholar] [CrossRef][Green Version]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef]
- Qaâdan, F.; Nahrstedt, A.; Schmidt, M.; Mansoor, K. Polyphenols from Ginkgo biloba. Sci. Pharm. 2010, 78, 897–907. [Google Scholar] [CrossRef]
- Siciliano, T.; De Leo, M.; Bader, A.; De Tommasi, N.; Vrieling, K.; Braca, A.; Morelli, I. Pyrrolizidine alkaloids from Anchusa strigosa and their antifeedant activity. Phytochemistry 2005, 66, 1593–1600. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Saleem, U.; Khalid, S.; Zaib, S.; Anwar, F.; Ahmad, B.; Ullah, I.; Zeb, A.; Ayaz, M. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wallr. J. Ethnopharmacol. 2020, 249, 112401. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound healing activities of standardized pomegranate rind extract and its major antioxidant ellagic acid in rat dermal wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef]
- Mouzié, C.M.; Guefack, M.-G.F.; Kianfé, B.Y.; Serondo, H.U.; Ponou, B.K.; Siwe-Noundou, X.; Teponno, R.B.; Krause, R.W.M.; Kuete, V.; Tapondjou, L.A. A New Chalcone and Antimicrobial Chemical Constituents of Dracaena stedneuri. Pharmaceuticals (Basel) 2022, 15, 725. [Google Scholar] [CrossRef] [PubMed]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Process 2021, 9, 2089. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Chaudhary, S.K.; Sharma, A.; Yadav, K.K.; Nema, N.K.; Sekhoacha, M.; Karmakar, S.; Braga, F.C.; Matsabisa, M.G.; Mukherjee, P.K.; et al. Anti-biofilm activity of Marula-a study with the standardized bark extract. J. Ethnopharmacol. 2014, 154, 170–175. [Google Scholar] [CrossRef]
- Joo, K.-M.; Kim, S.; Koo, Y.J.; Lee, M.; Lee, S.-H.; Choi, D.; Lim, K.-M. Development and validation of UPLC method for WST-1 cell viability assay and its application to MCTT HCETM eye irritation test for colorful substances. Toxicol. Vitr. Int. J. Public Assoc. BIBRA 2019, 60, 412–419. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).